Evaluating the Assembly Strategy of a Fungal Genome from Metagenomic Data: Solorina crocea (Peltigerales, Ascomycota) as a Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Solorina crocea
2.2. Fungal Material
2.3. DNA Extraction and Quantification
2.4. Library Preparation and Sequencing
2.5. Quality Control and Reads Filter
2.6. Benchmarking of Strategies for Genome Assembly
2.6.1. Assembly Strategy 1: Hybrid Metagenome Assembly Using Short and Long Reads
2.6.2. Assembly Strategy 2: Genome Assembly Using Long Reads and Scaffolding Using Short Reads
2.6.3. Assembly Strategy 3: Hybrid Assembly Based on Mycobiont Reads Previously Filtered
2.7. Scaffolding and Polishing Mycobiont Assemblies
2.8. Quality Assessment of Mycobiont Assemblies
2.9. Repetitive Element Library Construction
2.10. Gene Prediction and Functional Annotation
3. Results
3.1. Mycobiont Assembly Resulting from Strategy 1
3.2. Mycobiont Assembly Resulting from Strategy 2
3.3. Mycobiont Assembly Resulting from Strategy 3
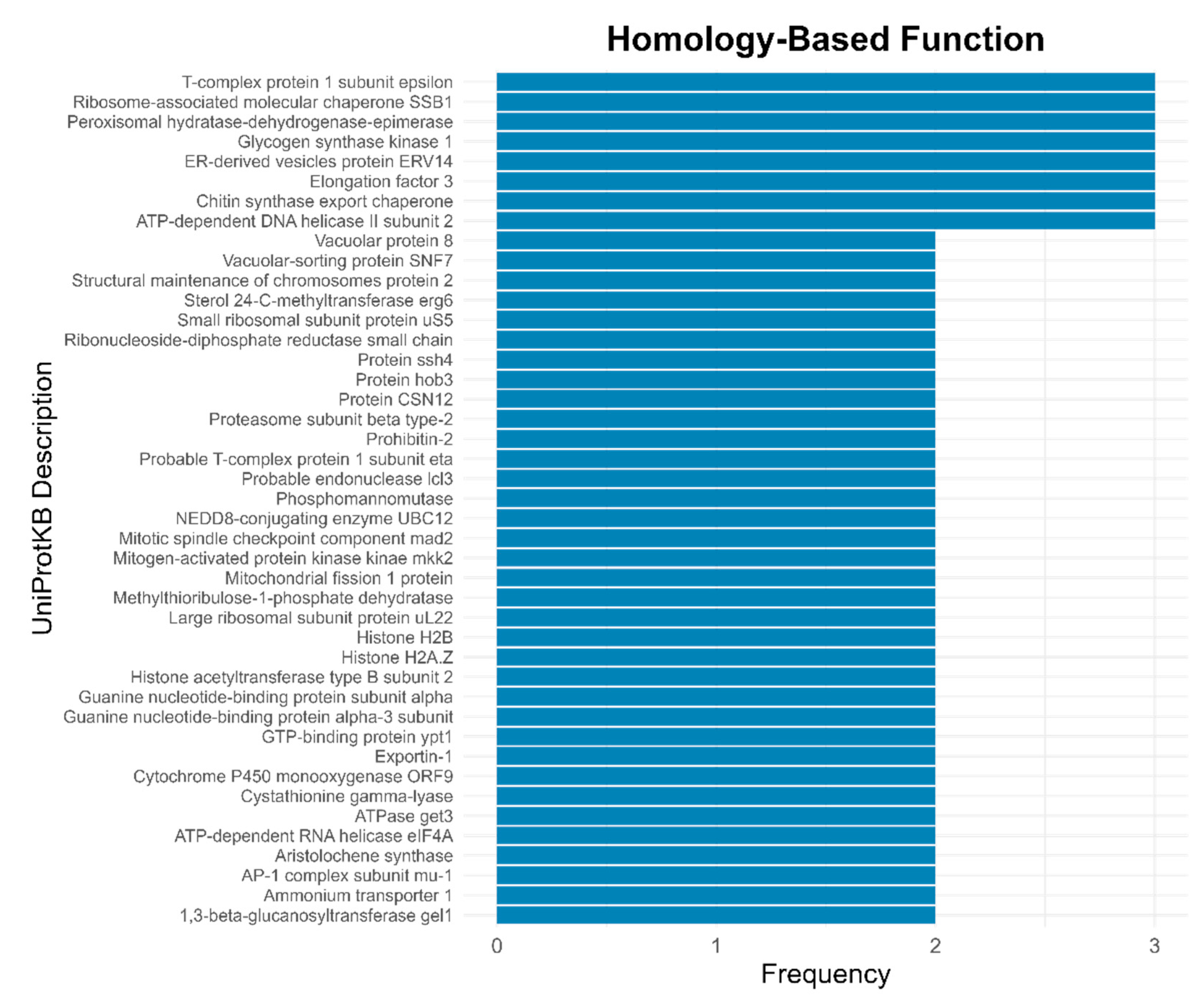
3.4. Functional Features of the Solorina crocea Genome
4. Discussion
4.1. Assessing Different Assembly Strategies and Other Considerations
4.2. Genomic Features of Solorina crocea
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGC | Biosynthetic Gene Cluster |
| BUSCO | Benchmarking Universal Single-Copy Orthologs |
| PKS | Polyketide synthase |
| NRPS | Non-ribosomal peptide synthetase |
References
- Honneger, R. Lichen-forming fungi and their photobionts. In Plant Relationships, 2nd ed.; Deising, H.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5, pp. 307–333. [Google Scholar]
- Hawksworth, D.L.; Grube, M. Lichens Redefined as Complex Ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Hodkinson, B.P.; Lutzoni, F. A Microbiotic Survey of Lichen-Associated Bacteria Reveals a New Lineage from the Rhizobiales. Symbiosis 2009, 49, 163–180. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; Berg, G. 17 Bacteria and the Lichen Symbiosis. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9, pp. 363–372. [Google Scholar]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and Geographic Structure of Endophytic and Endolichenic Fungi at a Continental Scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete Yeasts in the Cortex of Ascomycete Macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Cometto, A.; Leavitt, S.D.; Millanes, A.M.; Wedin, M.; Grube, M.; Muggia, L. The Yeast Lichenosphere: High Diversity of Basidiomycetes from the Lichens Tephromela atra and Rhizoplaca melanophthalma. Fungal Biol. 2022, 126, 587–608. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yang, J.; Zou, Y.; Zhao, X.-Q. Diversity of Endophytic Bacterial and Fungal Microbiota Associated with the Medicinal Lichen Usnea longissima at High Altitudes. Front. Microbiol. 2022, 13, 958917. [Google Scholar] [CrossRef]
- Dědková, K.; Vančurová, L.; Muggia, L.; Steinová, J. The Plurality of Photobionts within Single Lichen Thalli. Symbiosis 2025, 95, 35–63. [Google Scholar] [CrossRef]
- McDonald, T.R.; Gaya, E.; Lutzoni, F. Twenty-Five Cultures of Lichenizing Fungi Available for Experimental Studies on Symbiotic Systems. Symbiosis 2013, 59, 165–171. [Google Scholar] [CrossRef]
- Rosabal, D.; Pino-Bodas, R. A Review of Laboratory Requirements to Culture Lichen Mycobiont Species. J. Fungi 2024, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Greshake, B.; Zehr, S.; Dal Grande, F.; Meiser, A.; Schmitt, I.; Ebersberger, I. Potential and Pitfalls of Eukaryotic Metagenome Skimming: A Test Case for Lichens. Mol. Ecol. Resour. 2016, 16, 511–523. [Google Scholar] [CrossRef]
- Meiser, A.; Otte, J.; Schmitt, I.; Grande, F.D. Sequencing Genomes from Mixed DNA Samples—Evaluating the Metagenome Skimming Approach in Lichenized Fungi. Sci. Rep. 2017, 7, 14881. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, D.; Divakar, P.K.; Grewe, F.; Leavitt, S.D.; Huang, J.-P.; Dal Grande, F.; Schmitt, I.; Wedin, M.; Crespo, A.; Lumbsch, H.T. Phylogenomic Analysis of 2556 Single-Copy Protein-Coding Genes Resolves Most Evolutionary Relationships for the Major Clades in the Most Diverse Group of Lichen-Forming Fungi. Fungal Divers. 2018, 92, 31–41. [Google Scholar] [CrossRef]
- McKenzie, S.K.; Walston, R.F.; Allen, J.L. Complete, High-Quality Genomes from Long-Read Metagenomic Sequencing of Two Wolf Lichen Thalli Reveals Enigmatic Genome Architecture. Genomics 2020, 112, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Tagirdzhanova, G.; Saary, P.; Tingley, J.P.; Díaz-Escandón, D.; Abbott, D.W.; Finn, R.D.; Spribille, T. Predicted Input of Uncultured Fungal Symbionts to a Lichen Symbiosis from Metagenome-Assembled Genomes. Genome Biol. Evol. 2021, 13, evab047. [Google Scholar]
- Resl, P.; Bujold, A.R.; Tagirdzhanova, G.; Meidl, P.; Freire Rallo, S.; Kono, M.; Fernández-Brime, S.; Guðmundsson, H.; Andrésson, Ó.S.; Muggia, L.; et al. Large Differences in Carbohydrate Degradation and Transport Potential among Lichen Fungal Symbionts. Nat. Commun. 2022, 13, 2634. [Google Scholar] [CrossRef]
- Llewellyn, T.; Mian, S.; Hill, R.; Leitch, I.J.; Gaya, E. First Whole Genome Sequence and Flow Cytometry Genome Size Data for the Lichen-Forming Fungus Ramalina farinacea (Ascomycota). Genome Biol. Evol. 2023, 15, evad074. [Google Scholar] [CrossRef]
- Llewellyn, T.; Nowell, R.W.; Aptroot, A.; Temina, M.; Prescott, T.A.K.; Barraclough, T.G.; Gaya, E. Metagenomics Shines Light on the Evolution of “Sunscreen” Pigment Metabolism in the Teloschistales (lichen-Forming Ascomycota). Genome Biol. Evol. 2023, 15, evad002. [Google Scholar] [CrossRef] [PubMed]
- Ghurye, J.S.; Cepeda-Espinoza, V.; Pop, M. Metagenomic Assembly: Overview, Challenges and Applications. Yale J. Biol. Med. 2016, 89, 353–362. [Google Scholar]
- Uritskiy, G.V.; DiRuggiero, J.; Taylor, J. MetaWRAP-a Flexible Pipeline for Genome-Resolved Metagenomic Data Analysis. Microbiome 2018, 6, 158. [Google Scholar] [CrossRef]
- Bai, D.; Chen, T.; Xun, J.; Ma, C.; Luo, H.; Yang, H.; Cao, C.; Cao, X.; Cui, J.; Deng, Y.-P.; et al. EasyMetagenome: A User-Friendly and Flexible Pipeline for Shotgun Metagenomic Analysis in Microbiome Research. Imeta 2025, 4, e70001. [Google Scholar] [CrossRef]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and Challenges in Long-Read Sequencing Data Analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef]
- Kim, C.; Pongpanich, M.; Porntaveetus, T. Unraveling Metagenomics through Long-Read Sequencing: A Comprehensive Review. J. Transl. Med. 2024, 22, 111. [Google Scholar] [CrossRef]
- Adams, J.N.; Escalona, M.; Marimuthu, M.P.A.; Fairbairn, C.W.; Beraut, E.; Seligmann, W.; Nguyen, O.; Chumchim, N.; Stajich, J.E. The Reference Genome Assembly of the Bright Cobblestone Lichen, Acarospora socialis. J. Hered. 2023, 114, 707–714. [Google Scholar] [CrossRef]
- Cho, M.; Lee, S.J.; Choi, E.; Kim, J.; Choi, S.; Lee, J.H.; Park, H. An Antarctic Lichen Isolate (Cladonia borealis) Genome Reveals Potential Adaptation to Extreme Environments. Sci. Rep. 2024, 14, 1342. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, S.D.; DeBolt, A.; McQuhae, E.; Allen, J.L. Genomic Resources for the First Federally Endangered Lichen: The Florida Perforate Cladonia (Cladonia perforata). J. Fungi 2023, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, A.L.; Korobeynikov, A.I. Metagenomic Data Assembly—The Way of Decoding Unknown Microorganisms. Front. Microbiol. 2021, 12, 613791. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chowdhury, D.; Zhang, Z.; Cheung, W.K.; Lu, A.; Bian, Z.; Zhang, L. A Review of Computational Tools for Generating Metagenome-Assembled Genomes from Metagenomic Sequencing Data. Comput. Struct. Biotechnol. J. 2021, 19, 6301–6314. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; Liu, X.; Guan, F.; Xu, Y.; Yue, H.; Huang, J.-Q.; Chen, J.; Wu, N.; Tian, J. Combined Assembly of Long and Short Sequencing Reads Improve the Efficiency of Exploring the Soil Metagenome. BMC Genom. 2022, 23, 37. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Nesme, J.; Santos-Bay, L.; Koziol, A.; Sørensen, S.J.; Alberdi, A.; Aizpurua, O. A Comparison of Short-Read, HiFi Long-Read, and Hybrid Strategies for Genome-Resolved Metagenomics. Microbiol. Spectr. 2024, 12, e0359023. [Google Scholar] [CrossRef]
- Goldstein, S.; Beka, L.; Graf, J.; Klassen, J.L. Evaluation of Strategies for the Assembly of Diverse Bacterial Genomes Using MinION Long-Read Sequencing. BMC Genom. 2019, 20, 23. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, D.; Wang, Y.; Yao, X.; Luo, Y.; Yang, Z. Comparison of De Novo Assembly Strategies for Bacterial Genomes. Int. J. Mol. Sci. 2021, 22, 7668. [Google Scholar] [CrossRef]
- Ekblom, R.; Wolf, J.B.W. A Field Guide to Whole-Genome Sequencing, Assembly and Annotation. Evol. Appl. 2014, 7, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Greshake Tzovaras, B.; Segers, F.H.I.D.; Bicker, A.; Dal Grande, F.; Otte, J.; Anvar, S.Y.; Hankeln, T.; Schmitt, I.; Ebersberger, I. What Is in Umbilicaria pustulata? A Metagenomic Approach to Reconstruct the Holo-Genome of a Lichen. Genome Biol. Evol. 2020, 12, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 Classification of Lichenized Fungi in the Ascomycota and Basidiomycota—Approaching One Thousand Genera. Bryologist 2017, 119, 361. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Lutzoni, F. Phylogenetic Revision of the genus Peltigera (lichen-forming Ascomycota) Based on Morphological, Chemical, and Large Subunit Nuclear Ribosomal DNA Data. Int. J. Plant Sci. 2000, 161, 925–958. [Google Scholar] [CrossRef]
- Cannon, P.; Magain, N.; Sérusiaux, E.; Yahr, R.; Coppins, B.; Sanderson, N.; Simkin, J. Peltigerales: Peltigeraceae Including the Genera Crocodia, Lobaria, Lobarina, Nephroma, Peltigera, Pseudocyphellaria, Ricasolia, Solorina and Sticta. In Revisions of Lichen British Society; Lichen British Society: London, UK, 2021. [Google Scholar]
- Galloway, D. The Lichen Genus Solorina Ach. (Peltigeraceae, Lichenized Ascomycotina) in New Zealand. Cryptogamie. Bryol. Lichénologie 1998, 19, 137–146. [Google Scholar]
- Smith, C.W. The Lichens of Great Britain and Ireland; Smith, C.W., Ed.; Lichen British Society: London, UK, 2009. [Google Scholar]
- Zhurbenko, M. Clypeococcum Lenae (Dothideomycetes), a New Lichenicolous Species from the Arctic, with a Key to Species of Lichenicolous Fungi on Solorina. Opusc. Philolichenum 2020, 19, 199–207. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, L.; Ai, M.; Gan, Y.; Fan, R.; Zhang, Y.; Worthy, F.R.; Jin, J.; Meng, W.; Zhang, S.; et al. Taxonomic Revision of Solorina (Peltigeraceae, Ascomycota), Reveals a New Genus and Three New Species. J. Fungi 2025, 11, 169. [Google Scholar] [CrossRef]
- Burgaz, A.R.; Martínez, I. Estudio Del Género Solorina Ach. (Ascomicetes liquenizados) En La Península Ibérica. Bot. Complut. 1998, 55, 0214–4565. [Google Scholar]
- Blanco, E.; Múgica, F.; Ollero, H.S. Encuadre Geobotánico de La Sierra de Guadarrama. Ambient. Rev. Minist. Medio Ambiente 2013, 103, 50–67. [Google Scholar]
- Luceño, M.; Vargas, P. Guía Botánica del Sistema Central Español; Piramide Ediciones: Madrid, Spain, 1991. [Google Scholar]
- Martinez, M.I.; Aragon, R.G. Epiphytic Lichens from the North Face of Puerto de La Quesera, Macizo de Ayllon (Central Spain). Cryptogam. Bryol. Lichenol. 1996, 17, 143–156. [Google Scholar]
- Ruiz-Labourdette, D.; Martínez, F.; Martín-López, B.; Montes, C.; Pineda, F.D. Equilibrium of Vegetation and Climate at the European Rear Edge. A Reference for Climate Change Planning in Mountainous Mediterranean Regions. Int. J. Biometeorol. 2011, 55, 285–301. [Google Scholar] [CrossRef]
- Orange, A.; James, P.; White, F.; Orange, G. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- van Burik, J.-A.H.; Schreckhise, R.W.; White, T.C.; Bowden, R.A.; Myerson, D. Comparison of Six Extraction Techniques for Isolation of DNA from Filamentous Fungi. Med. Mycol. 1998, 36, 299–303. [Google Scholar] [CrossRef]
- Pino-Bodas, R.; Ahti, T.; Stenroos, S.; Martín, M.P.; Burgaz, A.R. Multilocus Approach to Species Recognition in the Cladonia humilis Complex (Cladoniaceae, Ascomycota). Am. J. Bot. 2013, 100, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Ryberg, M. Improved Software Detection and Extraction of ITS1 and ITS 2 from Ribosomal ITS Sequences of Fungi and Other Eukaryotes for Analysis of Environmental Sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Madden, T.L. The BLAST Sequence Analysis Tool. NCBI Handb. 2013, 2, 425–436. [Google Scholar]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. metaSPAdes: A New Versatile Metagenomic Assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Suzek, B.E.; Huang, H.; McGarvey, P.; Mazumder, R.; Wu, C.H. UniRef: Comprehensive and Non-Redundant UniProt Reference Clusters. Bioinformatics 2007, 23, 1282–1288. [Google Scholar] [CrossRef]
- Laetsch, D.; Blaxter, M. BlobTools: Interrogation of Genome Assemblies. F1000Research 2017, 6, 1287. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise Alignment for Nucleotide Sequences. Bioinformatics 2017, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Le, S.; Li, Y.; Hu, F. SeqKit: A Cross-Platform and Ultrafast Toolkit for FASTA/Q File Manipulation. PLoS ONE 2016, 11, e0163962. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptive K-Mer Weighting and Repeat Separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Bickhart, D.M.; Behsaz, B.; Gurevich, A.; Rayko, M.; Shin, S.B.; Kuhn, K.; Yuan, J.; Polevikov, E.; Smith, T.P.L.; et al. metaFlye: Scalable Long-Read Metagenome Assembly Using Repeat Graphs. Nat. Methods 2020, 17, 1103–1110. [Google Scholar] [CrossRef]
- Cheng, H.; Concepcion, G.T.; Feng, X.; Zhang, H.; Li, H. Haplotype-Resolved de Novo Assembly Using Phased Assembly Graphs with Hifiasm. Nat. Methods 2021, 18, 170–175. [Google Scholar] [CrossRef]
- Chakraborty, M.; Baldwin-Brown, J.G.; Long, A.D.; Emerson, J.J. Contiguous and Accurate de Novo Assembly of Metazoan Genomes with Modest Long Read Coverage. Nucleic Acids Res. 2016, 44, e147. [Google Scholar]
- Gurevich, A.A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar]
- Antipov, D.; Korobeynikov, A.; McLean, J.S.; Pevzner, P.A. hybridSPAdes: An Algorithm for Hybrid Assembly of Short and Long Reads. Bioinformatics 2016, 32, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2014, 31, 1674–1676. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014. [Google Scholar]
- Alneberg, J.; Bjarnason, B.S.; de Bruijn, I.; Schirmer, M.; Quick, J.; Ijaz, U.Z.; Loman, N.J.; Andersson, A.F.; Quince, C. CONCOCT: Clustering cONtigs on COverage and ComposiTion. arXiv 2013, arXiv:1312.4038. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Wickham, H. Getting Started with Qplot. In ggplot2; Springer: New York, NY, USA, 2009; pp. 9–26. ISBN 9780387981406. [Google Scholar]
- Pryszcz, L.P.; Gabaldón, T. Redundans: An Assembly Pipeline for Highly Heterozygous Genomes. Nucleic Acids Res. 2016, 44, e113. [Google Scholar] [CrossRef]
- Vaser, R.; Sovic, I.; Nagarajan, N.; Šikić, M. Racon-Rapid Consensus Module for Raw de Novo Genome Assembly of Long Uncorrected Reads. In Proceedings of the London Calling Conference, Amsterdam, The Netherlands, 28 October 2016. [Google Scholar]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Levy Karin, E.; Mirdita, M.; Söding, J. MetaEuk-Sensitive, High-Throughput Gene Discovery, and Annotation for Large-Scale Eukaryotic Metagenomics. Microbiome 2020, 8, 48. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing Genomic Data Quality and beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Hubley, R.; Goubert, C.; Rosen, J.; Clark, A.G.; Feschotte, C.; Smit, A.F. RepeatModeler2 for Automated Genomic Discovery of Transposable Element Families. Proc. Natl. Acad. Sci. USA 2020, 117, 9451–9457. [Google Scholar] [CrossRef] [PubMed]
- Crescente, J.M.; Zavallo, D.; Helguera, M.; Vanzetti, L.S. MITE Tracker: An Accurate Approach to Identify Miniature Inverted-Repeat Transposable Elements in Large Genomes. BMC Bioinform. 2018, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Ou, S.; Hufford, M.B.; Peterson, T. A Tutorial of EDTA: Extensive De Novo TE Annotator. Methods Mol. Biol. 2021, 2250, 55–67. [Google Scholar]
- Xiong, W.; He, L.; Lai, J.; Dooner, H.K.; Du, C. HelitronScanner Uncovers a Large Overlooked Cache of Helitron Transposons in Many Plant Genomes. Proc. Natl. Acad. Sci. USA 2014, 111, 10263–10268. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gu, X.; Peterson, T. TIR-Learner, a New Ensemble Method for TIR Transposable Element Annotation, Provides Evidence for Abundant New Transposable Elements in the Maize Genome. Mol. Plant 2019, 12, 447–460. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Kurtz, S.; Willhoeft, U. LTRharvest, an Efficient and Flexible Software for de Novo Detection of LTR Retrotransposons. BMC Bioinform. 2008, 9, 18. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H. LTR_FINDER: An Efficient Tool for the Prediction of Full-Length LTR Retrotransposons. Nucleic Acids Res. 2007, 35, W265–W268. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Nishimura, D. RepeatMasker. Biotech Softw. Internet Rep. 2000, 1, 36–39. [Google Scholar] [CrossRef]
- Chen, N. Using RepeatMasker to Identify Repetitive Elements in Genomic Sequences. Curr. Protoc. Bioinform. 2004, 5, 4–10. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Yandell, M. MAKER2: An Annotation Pipeline and Genome-Database Management Tool for Second-Generation Genome Projects. BMC Bioinform. 2011, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.S.C.; Birney, E. Automated Generation of Heuristics for Biological Sequence Comparison. BMC Bioinform. 2005, 6, 31. [Google Scholar] [CrossRef]
- Campbell, M.S.; Holt, C.; Moore, B.; Yandell, M. Genome Annotation and Curation Using MAKER and MAKER-P. Curr. Protoc. Bioinform. 2014, 48, 4–11. [Google Scholar] [CrossRef]
- Korf, I. Gene Finding in Novel Genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef]
- Stanke, M.; Morgenstern, B. AUGUSTUS: A Web Server for Gene Prediction in Eukaryotes That Allows User-Defined Constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef]
- Palmer, J.M.; Stajich, J.E. Funannotate v1. 8.1: Eukaryotic Genome Annotation. 2020. Available online: https://github.com/nextgenusfs/funannotate (accessed on 29 June 2025).
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The Peptidase Database. Nucleic Acids Res. 2009, 38, D227–D233. [Google Scholar] [CrossRef]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A Web Resource for Automated Carbohydrate-Active Enzyme Annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; Von, M.C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Medema, M.; Blin, K.; Cimermancic, P.; Jager, V.D.; Zakrzewski, P.; Fischbach, M.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Cao, L.; Liao, L.; Su, C.; Mo, T.; Zhu, F.; Qin, R.; Li, R. Metagenomic Analysis Revealed the Microbiota and Metabolic Function during Co-Composting of Food Waste and Residual Sludge for Nitrogen and Phosphorus Transformation. Sci. Total Environ. 2021, 773, 145561. [Google Scholar] [CrossRef]
- Mavromatis, K.; Land, M.L.; Brettin, T.S.; Quest, D.J.; Copeland, A.; Clum, A.; Goodwin, L.; Woyke, T.; Lapidus, A.; Klenk, H.P.; et al. The Fast Changing Landscape of Sequencing Technologies and Their Impact on Microbial Genome Assemblies and Annotation. PLoS ONE 2012, 7, e48837. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Zhou, P.; Mudge, J.; Gurtowski, J.; Lee, H.; Ramaraj, T.; Walenz, B.P.; Liu, J.; Stupar, R.M.; Denny, R.; et al. Hybrid Assembly with Long and Short Reads Improves Discovery of Gene Family Expansions. BMC Genom. 2017, 18, 541. [Google Scholar] [CrossRef] [PubMed]
- Gorman, Z.; Chen, J.; de Leon, A.A.P.; Wallis, C.M. Comparison of Assembly Platforms for the Assembly of the Nuclear Genome of Trichoderma Harzianum Strain PAR3. BMC Genom. 2023, 24, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.-G.; Yang, S.-H.; Wang, X.; Bai, F.-W.; Wang, Z. Benchmarking of Long-Read Sequencing, Assemblers and Polishers for Yeast Genome. Brief. Bioinform. 2022, 23, bbac146. [Google Scholar] [CrossRef]
- Al Kawam, A.; Khatri, S.; Datta, A. A Survey of Software and Hardware Approaches to Performing Read Alignment in next Generation Sequencing. IEEE/ACM Trans. Comput. Biol. Bioinform. 2017, 14, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Murigneux, V.; Rai, S.K.; Furtado, A.; Bruxner, T.J.C.; Tian, W.; Harliwong, I.; Wei, H.; Yang, B.; Ye, Q.; Anderson, E.; et al. Comparison of Long-Read Methods for Sequencing and Assembly of a Plant Genome. Gigascience 2020, 9, giaa146. [Google Scholar] [CrossRef]
- Jung, H.; Jeon, M.-S.; Hodgett, M.; Waterhouse, P.; Eyun, S.-I. Comparative Evaluation of Genome Assemblers from Long-Read Sequencing for Plants and Crops. J. Agric. Food Chem. 2020, 68, 7670–7677. [Google Scholar] [CrossRef]
- Feng, X.; Cheng, H.; Portik, D.; Li, H. Metagenome Assembly of High-Fidelity Long Reads with Hifiasm-Meta. Nat. Methods 2022, 19, 671–674. [Google Scholar] [CrossRef]
- Purayil, G.P.; Almarzooqi, A.Y.; El-Tarabily, K.A.; You, F.M.; AbuQamar, S.F. Fully Resolved Assembly of Fusarium Proliferatum DSM106835 Genome. Sci. Data 2023, 10, 705. [Google Scholar] [CrossRef]
- Yao, G.; Chen, W.; Sun, J.; Wang, X.; Wang, H.; Meng, T.; Zhang, L.; Guo, L. Gapless Genome Assembly of Fusarium Verticillioides, a Filamentous Fungus Threatening Plant and Human Health. Sci. Data 2023, 10, 229. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, M.; Liu, Y.-Y.; Li, M.; Xie, X.; Qi, J. Haplotype-Phased Chromosome-Level Genome Assembly of Cryptoporus Qinlingensis, a Typical Traditional Chinese Medicine Fungus. J. Fungi 2025, 11, 163. [Google Scholar] [CrossRef]
- Yu, W.; Luo, H.; Yang, J.; Zhang, S.; Jiang, H.; Zhao, X.; Hui, X.; Sun, D.; Li, L.; Wei, X.-Q.; et al. Comprehensive Assessment of 11 de Novo HiFi Assemblers on Complex Eukaryotic Genomes and Metagenomes. Genome Res. 2024, 34, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Huang, H.; Qi, Z.; Dou, H.-M.; Liu, X.-Y.; Han, T.-F.; Chen, Y.; Song, X.-J.; Zhang, Y.-H.; Tu, J. Evaluating Metagenomics Tools for Genome Binning with Real Metagenomic Datasets and CAMI Datasets. BMC Bioinform. 2020, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Cornet, L.; Baurain, D. Contamination Detection in Genomic Data: More Is Not Enough. Genome Biol. 2022, 23, 60. [Google Scholar] [CrossRef]
- Vecherskii, M.V.; Khayrullin, D.R.; Shadrin, A.M.; Lisov, A.V.; Zavarzina, A.G.; Zavarzin, A.A.; Leontievsky, A.A. Metagenomes of Lichens Solorina Crocea and Peltigera Canina. Microbiol. Resour. Announc. 2022, 11, e0100021. [Google Scholar] [CrossRef]
- Tagirdzhanova, G.; Saary, P.; Cameron, E.S.; Allen, C.C.G.; Garber, A.I.; Escandón, D.D.; Cook, A.T.; Goyette, S.; Nogerius, V.T.; Passo, A.; et al. Microbial Occurrence and Symbiont Detection in a Global Sample of Lichen Metagenomes. PLoS Biol. 2024, 22, e3002862. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, B.; Zhang, X.-Y.; Zhou, Q.-M.; Zhang, T.; Li, H.; Yu, Y.-F.; Zhang, X.-L.; Hao, X.-Y.; Wang, M.; et al. Genome Characteristics Reveal the Impact of Lichenization on Lichen-Forming Fungus Endocarpon pusillum Hedwig (Verrucariales, Ascomycota). BMC Genom. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Urban, M.; Hammond-Kosack, M.C.U.; Hassani-Pak, K.; Hammond-Kosack, K.E. The Completed Genome Sequence of the Pathogenic Ascomycete Fungus Fusarium Graminearum. BMC Genom. 2015, 16, 544. [Google Scholar] [CrossRef]
- Prasad, P.; Varshney, D.; Adholeya, A. Whole Genome Annotation and Comparative Genomic Analyses of Bio-Control Fungus Purpureocillium lilacinum. BMC Genom. 2015, 16, 1004. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, F.; Si, J.; Zhao, Y.-F.; Dai, Y.-C. Whole Genome Sequence of Auricularia heimuer (Basidiomycota, Fungi), the Third Most Important Cultivated Mushroom Worldwide. Genomics 2019, 111, 50–58. [Google Scholar] [CrossRef]
- Mao, Z.; Yang, P.; Liu, H.; Mao, Y.; Lei, Y.; Hou, D.; Ma, H.; Liao, X.; Jiang, W. Whole-Genome Sequencing and Analysis of the White-Rot Fungus Ceriporia lacerata Reveals Its Phylogenetic Status and the Genetic Basis of Lignocellulose Degradation and Terpenoid Synthesis. Front. Microbiol. 2022, 13, 880946. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, K.-T.; Park, S.-Y.; Lee, G.-W.; Choi, J.; Jeon, J.; Cheong, K.-C.; Choi, G.; Hur, J.; Lee, Y.-H. A Comparative Genomic Analysis of Lichen-Forming Fungi Reveals New Insights into Fungal Lifestyles. Sci. Rep. 2022, 12, 10724. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H., Jr. The Major Facilitator Superfamily (MFS) Revisited: The Major Facilitator Family Revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Müller, A.; Mӓkelӓ, M.R.; de Vries, R.P. Aldo-Keto Reductases, Short Chain Dehydrogenases/reductases, and Zinc-Binding Dehydrogenases Are Key Players in Fungal Carbon Metabolism. Adv. Appl. Microbiol. 2025, 130, 123–157. [Google Scholar]
- Bowman, S.M.; Free, S.J. The Structure and Synthesis of the Fungal Cell Wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Li, M.; Jiang, C.; Wang, Q.; Zhao, Z.; Jin, Q.; Xu, J.-R.; Liu, H. Evolution and Functional Insights of Different Ancestral Orthologous Clades of Chitin Synthase Genes in the Fungal Tree of Life. Front. Plant Sci. 2016, 7, 37. [Google Scholar] [CrossRef]
- Crešnar, B.; Petrič, S. Cytochrome P450 Enzymes in the Fungal Kingdom. Biochim. Biophys. Acta 2011, 1814, 29–35. [Google Scholar] [CrossRef]
- Mlambo, G.; Padayachee, T.; Nelson, D.R.; Syed, K. Genome-Wide Analysis of the Cytochrome P450 Monooxygenases in the Lichenized Fungi of the Class Lecanoromycetes. Microorganisms 2023, 11, 2590. [Google Scholar] [CrossRef]
- Hagiwara, D.; Sakamoto, K.; Abe, K.; Gomi, K. Signaling Pathways for Stress Responses and Adaptation in Aspergillus Species: Stress Biology in the Post-Genomic Era. Biosci. Biotechnol. Biochem. 2016, 80, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, R.; Wang, D.; Qian, B.; Bian, Z.; Wei, J.; Wei, X.; Xu, J.-R. Regulation of Symbiotic Interactions and Primitive Lichen Differentiation by UMP1 MAP Kinase in Umbilicaria muhlenbergii. Nat. Commun. 2023, 14, 6972. [Google Scholar] [CrossRef]
- Armaleo, D.; Müller, O.; Lutzoni, F.; Andrésson, Ó.S.; Blanc, G.; Bode, H.B.; Collart, F.R.; Dal Grande, F.; Dietrich, F.; Grigoriev, I.V.; et al. The Lichen Symbiosis Re-Viewed through the Genomes of Cladonia grayi and Its Algal Partner Asterochloris glomerata. BMC Genom. 2019, 20, 605. [Google Scholar] [CrossRef] [PubMed]
- Rikkinen, J. Symbiotic Cyanobacteria in Lichens. In Algal and Cyanobacteria Symbioses; Grube, M., Seckbach, J.A., Muggia, L., Eds.; World Scientific (Europe): London, UK, 2017; pp. 147–167. [Google Scholar]
- Palmqvist, K. Cyanolichens: Carbon Metabolism. In Cyanobacteria in Symbiosis; Rai, A.N., Bergman, B., Rasmussen, E., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 73–96. [Google Scholar]
- Castanera, R.; López-Varas, L.; Borgognone, A.; LaButti, K.; Lapidus, A.; Schmutz, J.; Grimwood, J.; Pérez, G.; Pisabarro, A.G.; Grigoriev, I.V.; et al. Transposable Elements versus the Fungal Genome: Impact on Whole-Genome Architecture and Transcriptional Profiles. PLoS Genet. 2016, 12, e1006108. [Google Scholar] [CrossRef]
- Armstrong, E.E.; Prost, S.; Ertz, D.; Westberg, M.; Frisch, A.; Bendiksby, M. Draft Genome Sequence and Annotation of the Lichen-Forming Fungus Arthonia radiata. Genome Announc. 2018, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Dal Grande, F.; Meiser, A.; Greshake Tzovaras, B.; Otte, J.; Ebersberger, I.; Schmitt, I. The Draft Genome of the Lichen-Forming fungus Lasallia hispanica (Frey) Sancho & A. Crespo. Lichenologist 2018, 50, 329–340. [Google Scholar]
- Grande, F.D.; Jamilloux, V.; Choisne, N.; Calchera, A.; Rolshausen, G.; Petersen, M.; Schulz, M.; Nilsson, M.A.; Schmitt, I. Transposable Elements in the Genome of the Lichen-Forming Fungus Umbilicaria pustulata and Their Distribution in Different Climate Zones along Elevation. Biology 2021, 11, 24. [Google Scholar] [CrossRef]
- Santana, M.; Queiroz, M.V. Transposable Elements in Fungi: A Genomic Approach. Sci. J. Genet. Gene Ther. 2015, 1, 10–16. [Google Scholar] [CrossRef]
- Castanera, R.; Borgognone, A.; Pisabarro, A.G.; Ramírez, L. Biology, Dynamics, and Applications of Transposable Elements in Basidiomycete Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Muszewska, A.; Steczkiewicz, K.; Stepniewska-Dziubinska, M.M.; Ginalski, K. Transposable Elements Contribute to Fungal Genes and Impact Fungal Lifestyle. Sci. Rep. 2019, 9, 4307. [Google Scholar] [CrossRef]
- Asplund, J.; Gauslaa, Y. Content of Secondary Compounds Depends on Thallus Size in the Foliose Lichen Lobaria pulmonaria. Lichenologist 2007, 39, 273–278. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic Diversity of Lichen-Forming Ascomycetous Fungi: Culturing, Polyketide and Shikimate Metabolite Production, and PKS Genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Lind, M.; Nybakken, L.; Gauslaa, Y. Possible Functional Roles of Cortical Depsides and Medullary Depsidones in the Foliose Lichen Hypogymnia physodes. Flora 2009, 204, 40–48. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Avalos, M.; Garbeva, P.; Vader, L.; van Wezel, P.G.; Dickschat, J.S.; Ulanova, D. Biosynthesis, Evolution and Ecology of Microbial Terpenoids. Nat. Prod. Rep. 2022, 39, 249–272. [Google Scholar] [CrossRef]
- Abdel-Hameed, M.; Bertrand, R.L.; Piercey-Normore, M.D.; Sorensen, J.L. Putative Identification of the Usnic Acid Biosynthetic Gene Cluster by de Novo Whole-Genome Sequencing of a Lichen-Forming Fungus. Fungal Biol. 2016, 120, 306–316. [Google Scholar] [CrossRef]
- Calchera, A.; Dal Grande, F.; Bode, H.B.; Schmitt, I. Biosynthetic Gene Content of the “Perfume Lichens” Evernia prunastri and Pseudevernia furfuracea. Molecules 2019, 24, 203. [Google Scholar] [CrossRef]
- Singh, G.; Pasinato, A.; Yriarte, A.L.-C.; Pizarro, D.; Divakar, P.K.; Schmitt, I.; Dal Grande, F. Are There Conserved Biosynthetic Genes in Lichens? Genome-Wide Assessment of Terpene Biosynthetic Genes Suggests Ubiquitous Distribution of the Squalene Synthase Cluster. BMC Genom. 2024, 25, 936. [Google Scholar] [CrossRef]
- Pasinato, A.; Singh, G. Lichens Are a Treasure Chest of Bioactive Compounds: Fact or Fake? New Phytol. 2025, 246, 389–395. [Google Scholar] [CrossRef]
- Gagunashvili, A.N.; Davídsson, S.P.; Jónsson, Z.O.; Andrésson, O.S. Cloning and Heterologous Transcription of a Polyketide Synthase Gene from the Lichen Solorina Crocea. Mycol. Res. 2009, 113, 354–363. [Google Scholar] [CrossRef]
- Gerasimova, J.V.; Beck, A.; Scheunert, A.; Kulkarni, O. De Novo Genome Assembly of Toniniopsis dissimilis (Ramalinaceae, Lecanoromycetes) from Long Reads Shows a Comparatively High Composition of Biosynthetic Genes Putatively Involved in Melanin Synthesis. Genes 2024, 15, 1029. [Google Scholar] [CrossRef] [PubMed]
- Pasinato, A.; Singh, G. Bioinformatic Exploration of RiPP Biosynthetic Gene Clusters in Lichens. Fungal Biol. Biotechnol. 2025, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Sorensen, J.L.; Collemare, J. Lichen Fungal Secondary Metabolites: Progress in the Genomic Era toward Ecological Roles in the Interaction. In Plant Relationships: Fungal-Plant Interactions; Scott, B., Mesarich, C., Eds.; Springer: Cham, Switzerland, 2023; Volume 5, pp. 185–208. [Google Scholar]
- Stocker-Wörgötter, E. Biochemical Diversity and Ecology of Lichen-Forming Fungi: Lichen Substances, Chemosyndromic Variation and Origin of Polyketide-Type Metabolites (Biosynthetic Pathways). In Recent Advances in Lichenology; Upreti, D., Divakar, P., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 161–179. [Google Scholar]



| Sequencing Technology | Assembly Tool | No. Contigs | Largest Contig (bp) | Genome Size (Mb) | N50 (kb) | %GC Content | %BUSCO Scores |
|---|---|---|---|---|---|---|---|
| Strategy 1: hybrid assembly | |||||||
| PacBio HiFi + Illumina | metaSPAdes | 22,583 | 347,633 | 121.69 | 41.75 | 39.16 | C: 97.8 [S: 96.2, D: 1.6], F: 0.5, M: 1.7 |
| Strategy 2: long reads + short reads scaffolding | |||||||
| PacBio HiFi | Canu | 4834 | 242,057 | 68.16 | 15.31 | 41.34 | C: 43.0 [S: 42.1, D: 0.9], F: 5.9, M: 51.2 |
| PacBio HiFi | Hifiasm-meta | 4804 | 230,570 | 108.66 | 25.57 | 41.61 | C: 67.3 [S: 65.2, D: 2.1], F: 5.3, M: 27.4 |
| PacBio HiFi | metaFlye | 6089 | 230,570 | 118.01 | 24.83 | 41.56 | C: 68.6 [S: 66.8, D: 1.9], F: 5.3, M: 26.0 |
| PacBio HiFi | Canu + Hifiasm-meta | 4802 | 327,659 | 109.03 | 25.65 | 41.61 | C: 67.4 [S: 65.2, D: 2.1], F: 5.3, M: 27.3 |
| PacBio HiFi | Canu + metaFlye | 3941 | 409,251 | 96.59 | 29.29 | 41.41 | C: 60.8 [S: 59.2, D: 1.6], F: 5.8, M: 34.2 |
| PacBio HiFi | Hifiasm-meta + metaFlye | 4325 | 264,302 | 117.58 | 32.54 | 41.68 | C: 72.6 [S: 70.3, D: 2.3], F: 4.7, M: 22.6 |
| Assembly Strategy | No. Scaffolds | Largest Contig (bp) | Genome Size (Mb) | N50 (kb) | %GC Content | %BUSCO Scores |
|---|---|---|---|---|---|---|
| Strategy 1: hybrid assembly | 4157 | 347,633 | 90.81 | 63.23 | 37.46 | C: 95.4 [S: 94.0, D: 1.4], F: 0.6, M: 4.0 |
| Strategy 2: metagenome & scaffolding with short reads | 519 | 547,588 | 55.50 | 142.84 | 37.24 | C: 96.7 [S: 95.7, D: 0.9], F: 0.7, M: 2.6 |
| Strategy 3: hybrid assembly of filtered long and short reads | 6280 | 140,100 | 33.71 | 16.58 | 42.43 | C: 37.6 [S: 37.5, D: 0.1], F: 1.0, M: 61.4 |
| Strategy 1 | Strategy 2 | Strategy 3 | |
|---|---|---|---|
| No. genes | 7352 | 6151 | 2767 |
| Genes < 0.1 AED score | 56% | 61% | 53% |
| BUSCO (annotated gene set) | C: 89.4% [S: 88.1%, D: 1.3%], F: 2.9%, M: 7.7% | C: 91.6% [S: 90.7%, D: 0.8%], F: 2.8%, M: 5.7% | C: 33.5% [S: 33.5%,D: 0%], F: 1.6%, M: 64.8% |
| No. repetitive elements | 10,136 | 813 | 8739 |
| Genome covered by repetitive elements | 22.53% | 22.18% | 22.04% |
| BGCs | 19 | 18 | 10 |
| Genome covered by BGCs | 0.25% | 1.40% | 0.23% |
| Functional terms | 30,404 | 52,648 | 14,611 |
| Annotated genes | 87.2% | 91.8% | 85.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Muñoz, A.; Pino-Bodas, R. Evaluating the Assembly Strategy of a Fungal Genome from Metagenomic Data: Solorina crocea (Peltigerales, Ascomycota) as a Case Study. J. Fungi 2025, 11, 596. https://doi.org/10.3390/jof11080596
García-Muñoz A, Pino-Bodas R. Evaluating the Assembly Strategy of a Fungal Genome from Metagenomic Data: Solorina crocea (Peltigerales, Ascomycota) as a Case Study. Journal of Fungi. 2025; 11(8):596. https://doi.org/10.3390/jof11080596
Chicago/Turabian StyleGarcía-Muñoz, Ana, and Raquel Pino-Bodas. 2025. "Evaluating the Assembly Strategy of a Fungal Genome from Metagenomic Data: Solorina crocea (Peltigerales, Ascomycota) as a Case Study" Journal of Fungi 11, no. 8: 596. https://doi.org/10.3390/jof11080596
APA StyleGarcía-Muñoz, A., & Pino-Bodas, R. (2025). Evaluating the Assembly Strategy of a Fungal Genome from Metagenomic Data: Solorina crocea (Peltigerales, Ascomycota) as a Case Study. Journal of Fungi, 11(8), 596. https://doi.org/10.3390/jof11080596







