Topographic Habitat Drive the Change of Soil Fungal Community and Vegetation Soil Characteristics in the Rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region
Abstract
1. Introduction
2. Materials and Methods
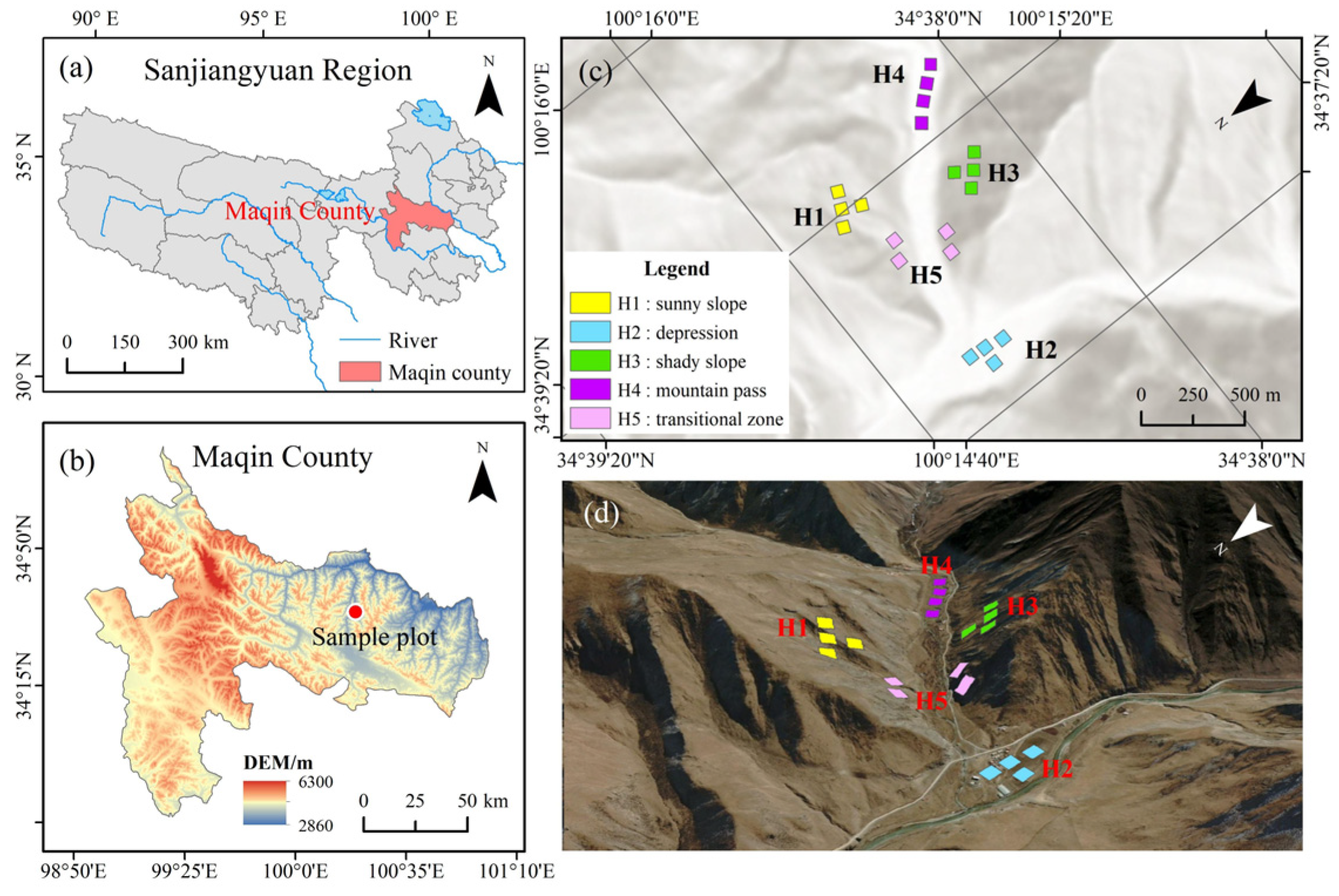
2.1. Study Area Description
2.2. Experimental Design
2.3. Plant Community Investigation and Soil Sample Collection
2.4. Indicator Measurement and Methodology
2.4.1. Vegetation Characterization
2.4.2. Determination of Physical and Chemical Properties of Soil
2.4.3. Soil Fungal DNA Extraction, PCR Amplification
2.5. Data Processing
3. Results
3.1. Composition of Soil Fungi Community in Rhizosphere of Kengyilia thoroldiana in Five Topographic Habitats
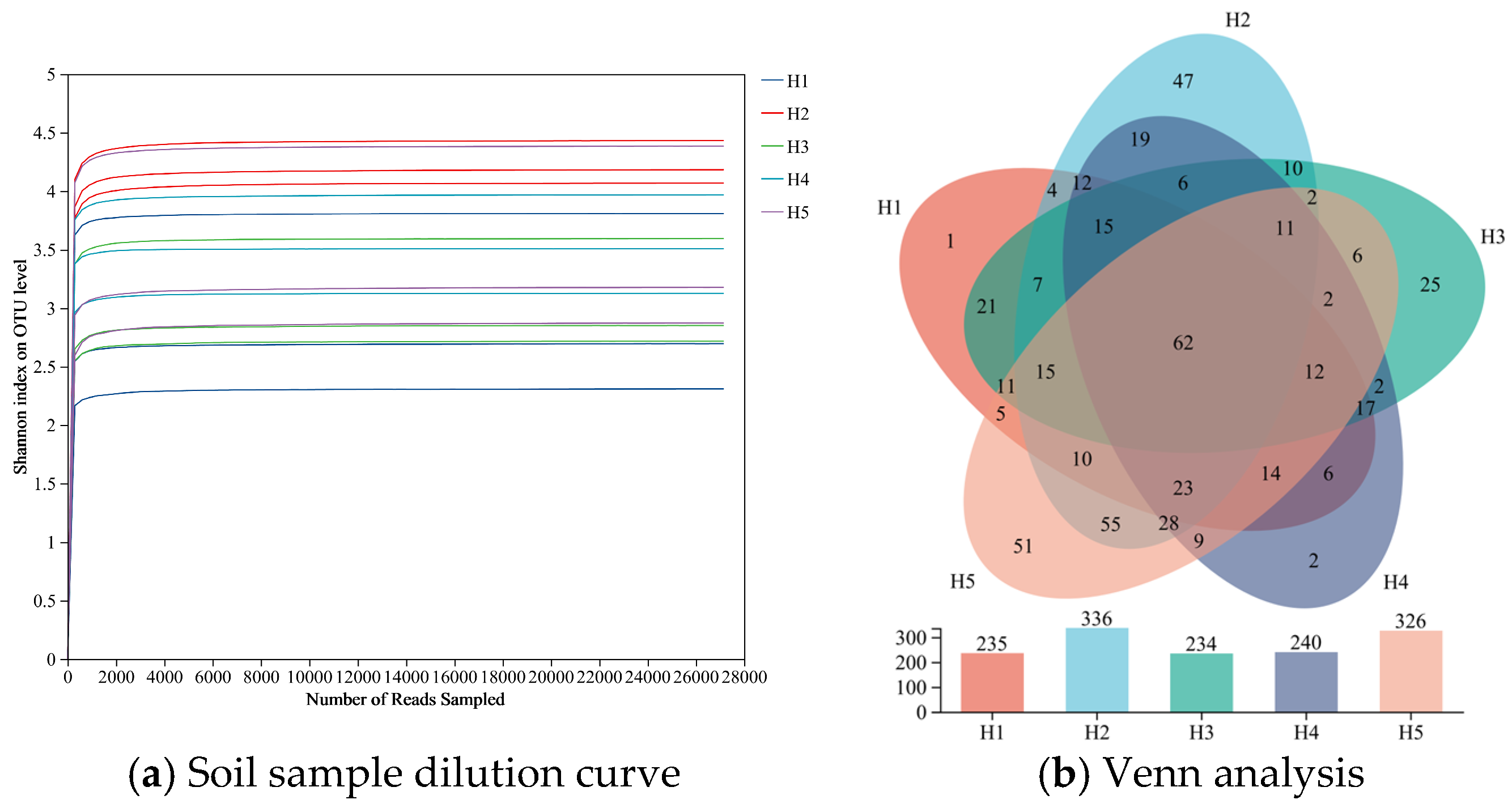
3.1.1. Quality Analysis of ITS Sequencing Results of Soil Fungi and Changes of OUT Quantity
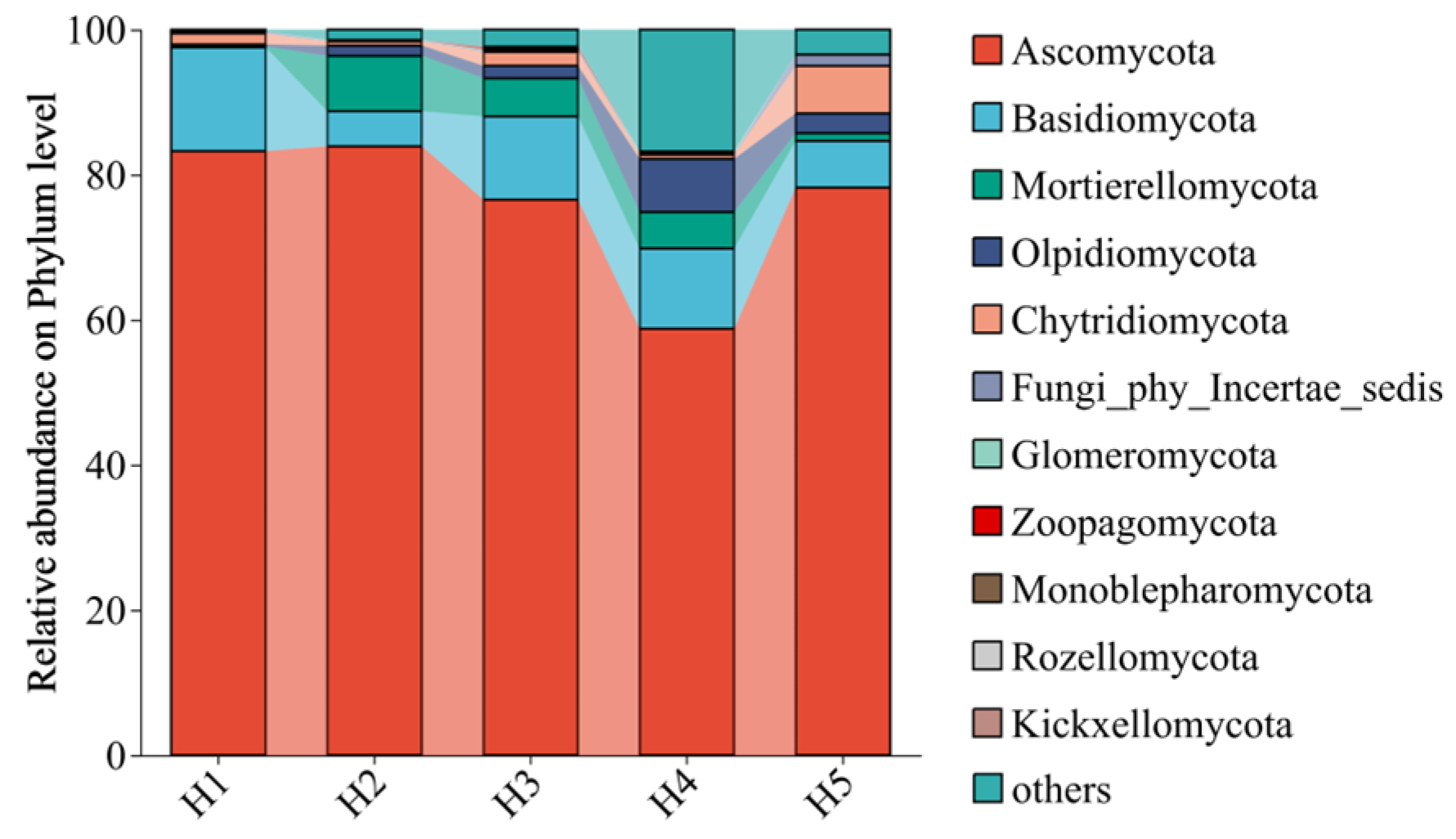
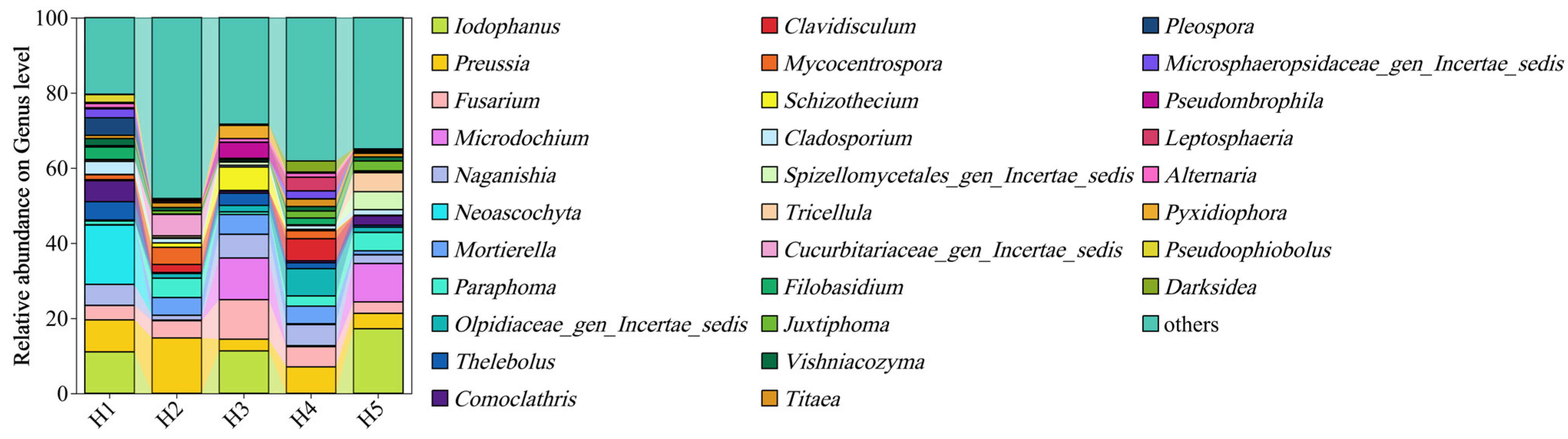
3.1.2. Analysis of Fungal Community Composition and Relative Abundance
3.1.3. LEfSe Analysis of Fungal Community
3.2. Diversity of Soil Fungal Communities in the Rhizosphere of Kengyilia thoroldiana Across Five Topographical Habitats
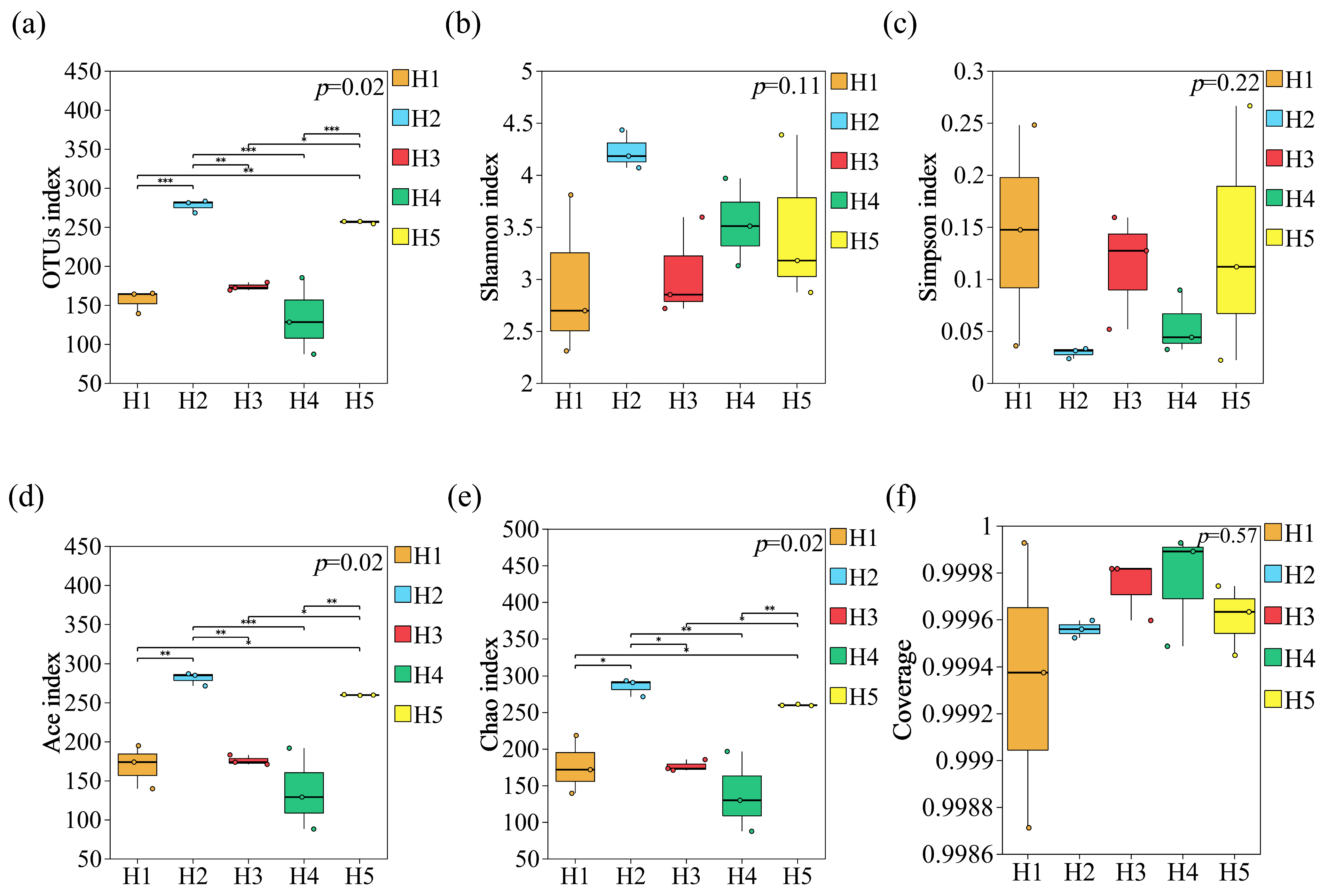
3.2.1. Analysis of α Diversity of Fungal Communities
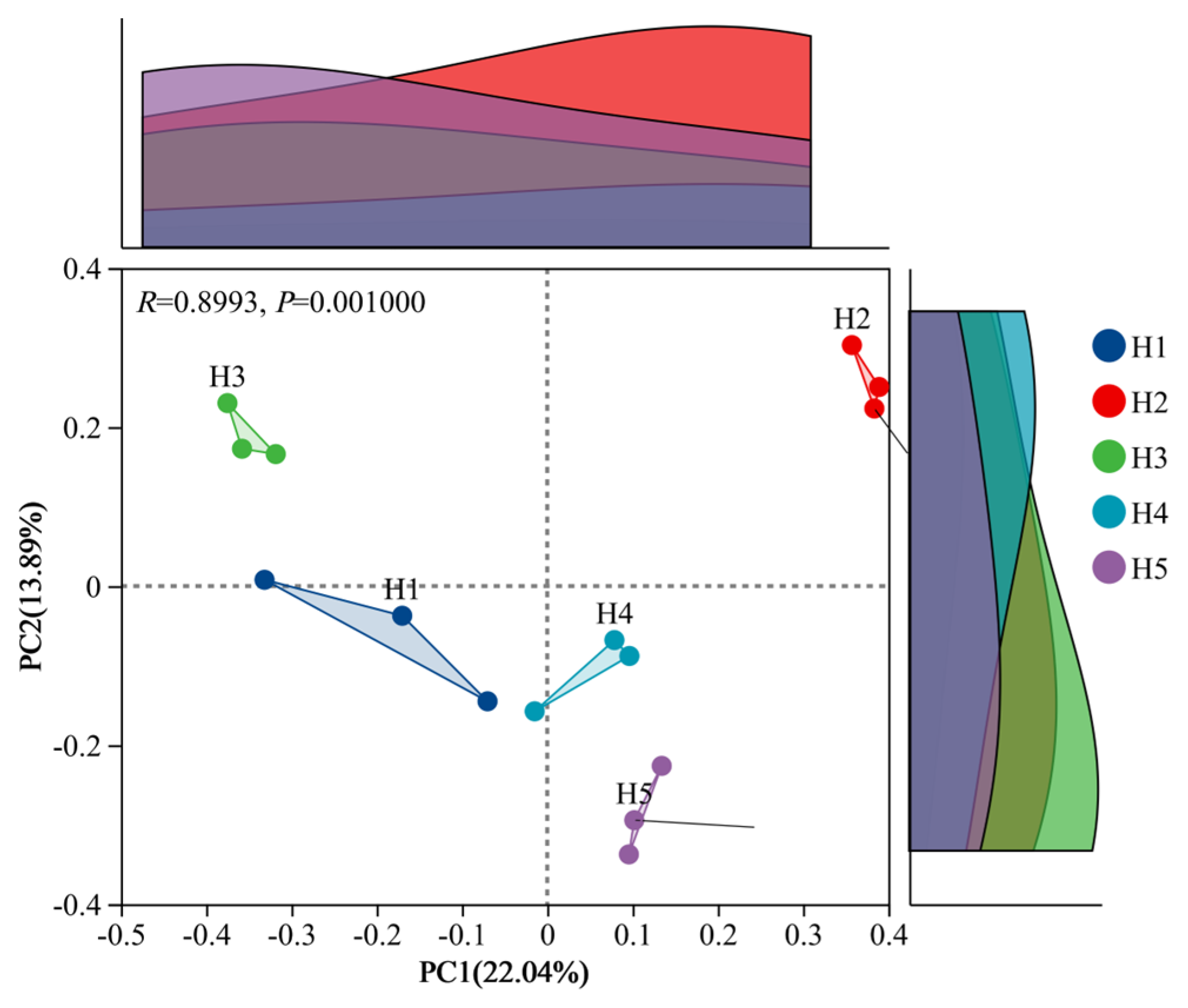
3.2.2. Analysis of β Diversity (PCoA) of Fungal Communities
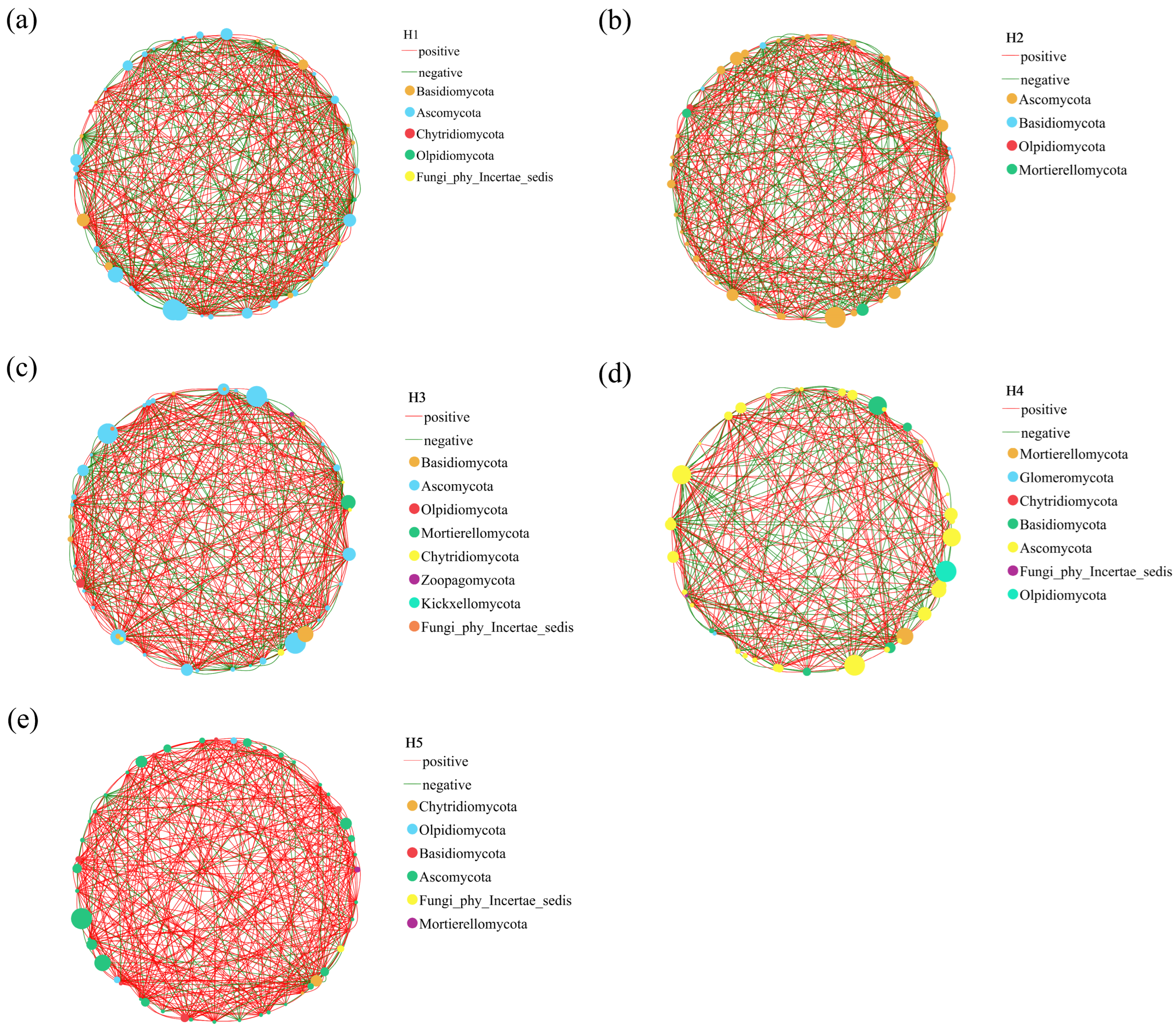
3.3. Changes of Single Factor Correlation Network of Soil Fungi in Rhizosphere of Kengyilia thoroldiana in Five Topographical Habitats
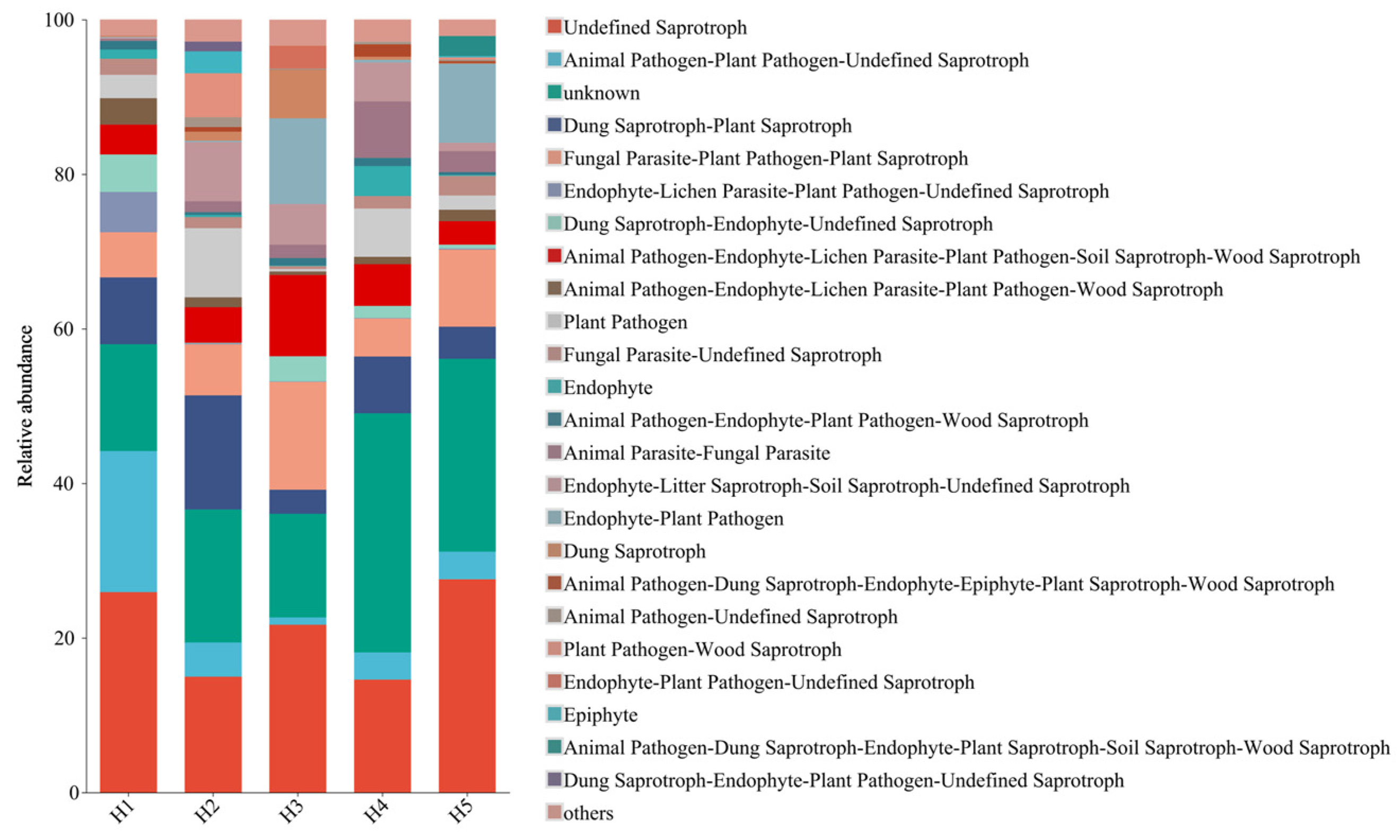
3.4. Prediction on the Function of Soil Fungal Community in Kengyilia thoroldiana Rhizosphere in Five Topographic Habitats
3.5. Cluster Analysis of Soil Fungal Community in Kengyilia thoroldiana Rhizosphere in Five Topographic Habitats
3.6. Changes of Vegetation and Soil Characteristics of Kengyilia thoroldiana Community in Five Topographic Habitats
3.7. Coupling Relationships Between Soil Environmental Factors, Plant Community Characteristics, and the Diversity of Soil Fungal Community in Kengyilia thoroldiana Rhizosphere
4. Discussion
4.1. Changes of Physicochemical Properties of Kengyilia thoroldiana Vegetation and Rhizosphere Soil in Five Topographic Habitats
4.2. Changes of Soil Fungal Community Structure and Function in Rhizosphere of Kengyilia thoroldiana in Five Topographical Habitats
4.3. Correlation Analysis of Soil Fungi Composition in Kengyilia thoroldiana Rhizosphere with Soil Physical and Chemical Properties and Plant Community Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.H.; Xie, Y.; Qiao, J.; Chai, S.J.; Chen, L. Rhizobacteria strain from a hypersaline environment promotes plant growth of Kengyilia thoroldiana. Microbiology 2019, 88, 220–231. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, X.; Ma, Y.S.; Su, X.D.; Yang, Y.Q.; Yang, Y.P. Proteome response of wild wheat relative Kengyilia thoroldiana to drought stress. Can. J. Plant Sci. 2015, 95, 237–249. [Google Scholar] [CrossRef]
- Li, C.H.; Li, S.J.; Zhang, J.; Sun, Q.Z. Observations on spike differentiation of Kengyilia thoroldiana. Grassl. Sci. 2013, 30, 1189–1193. (In Chinese) [Google Scholar]
- Zhang, J.; Caiwen, D.J.; Suonan, C.R.; Wen, X.C.; Wang, S.C.; Yang, M.J.; Renzeng, O.Z.; Sun, H.Q. The correlation and niche of survival community of Kengyilia thoroldiana in Maduo County, Qinghai Province. Grassl. Sci. 2019, 36, 2752–2765. (In Chinese) [Google Scholar] [CrossRef]
- Han, H.M.; Zhang, B.; Liu, H.; Li, Y.; Xu, W.D.; Zhang, L.T.; Cao, H.; Wang, F. Abundant taxa enhanced stability of fungal communities according to reduced nitrogen fertilization. Soil Tillage Res. 2025, 249, 106501. [Google Scholar] [CrossRef]
- Li, A.D.; Mdeidl, P.; Wang, S.H. Atmospheric nitrogen deposition has minor impacts on the abundance and diversity of arbuscular mycorrhizal fungi and their contribution to soil carbon stock in tropical forests. Soil Biol. Biochem. 2025, 204, 109746. [Google Scholar] [CrossRef]
- Shi, X.F.; Zhou, S.Y.; Xu, L.Z.; Nethmini, R.T.; Zhang, Y.; Huang, L.L.; Dong, K.; Zhao, H.X.; Pan, L.H. Shifts in soil fungal community and trophic modes during mangrove ecosystem restoration. J. Fungi 2025, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.O.; Alves, A.; Fidalgo, C.; Freitas, J.G.R.D.; Carvalho, M.A.A.P.D. Variations in the structure and function of the soil fungal communities in the traditional cropping systems from Madeira Island. Front. Microbiol. 2024, 15, 1426957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Y.; Mo, W.H.; Fan, Y.; Wang, Z.H.; Ji, Q.W.; Liao, S.M.; Ou, J. Community structure and diversity of fungi in rhizosphere soil of the rare and endangered plant Woonyoungia septentrionalis. J. Ecol. 2025, 44, 1–12. (In Chinese) [Google Scholar]
- Gao, H.Y.; Zhang, S.G.; Yang, Z.G.; Zhang, L.; Huang, H.G.; Yan, D.R. Structure and function of soil fungal community in Pinus tabuliformis sand-fixing forests in Horqin Sandy Land. Res. Arid Areas 2025, 42, 118–126. (In Chinese) [Google Scholar] [CrossRef]
- Xie, L.L.; Ma, Y.S.; Wang, Y.L.; Ma, Y.; Wang, X.L. Changes in soil bacterial and fungal community composition and functional groups during the artificial restoration of degraded grassland of “black-soil mountain”. Ecol. Evol. 2024, 14, e70361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, L.R.; Liu, Y.F.; Huang, Z.; Shi, J.J.; Wang, Y.L.; Ma, Y.S. Restoration of a hillslope grassland with an ecological grass species (Elymus tangutorum) favors rainfall interception and water infiltration and reduces soil loss on the Qinghai-Tibetan Plateau. Catena 2022, 219, 106632. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Zhao, X.M.; Guo, X.; Zeng, X.L. Study on spatial variation characteristics of cultivated land soil attributes based on improved topographic distribution index. J. Nucl. Agric. 2024, 38, 1595–1604. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Q.Q.; Shi, J.J.; Ma, Y.S.; Wang, Y.L.; Wang, X.L.; Lyu, L.Y.; He, M.H.; Cai, Z.C. Relationship between plant diversity and community stability in alpine mining areas. J. Mt. Sci. 2025, 22, 901–912. [Google Scholar] [CrossRef]
- Liu, M.R.; Liu, S.T.; Li, H.; Ning, Y.N.; Boeck, H.J.D.; Liu, Y.J. An exploration of plant biomass–species richness relationships in three subalpine grasslands on the Qinghai–Tibetan plateau. J. Veg. Sci. 2025, 36, e70008. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Xu, J.J.; Xu, C.L.; Hou, Q.Q.; Yang, C.Y.; Yu, X.J. Short-term effects of restoration measures on vegetation community and soil characteristics of an achnatherum inebrians-type degraded alpine grassland in the eastern Qilian Mountains. Glob. Ecol. Conserv. 2024, 54, e03128. [Google Scholar] [CrossRef]
- He, G.X.; Liu, X.N.; Li, Y.L.; Ji, T. Response of plant life forms and soil physical properties to near-natural restoration measures in alpine grassland, Tibetan plateau. Front. Plant Sci. 2025, 15, 1504754. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000; pp. 39–97. (In Chinese) [Google Scholar]
- Wang, G.X.; Wang, Y.B.; Li, Y.S. Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai–Tibet plateau, China—Science direct. Catena 2007, 70, 506–514. [Google Scholar] [CrossRef]
- Liu, T.S. Study on Community Structure Characteristics of Ulmus Pumila in Southern Edge of Hunshandake Sandy Land. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2019; pp. 28–31. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Lyu, Z.Z.; Qian, Y.; Song, J.; Zhong, X.Y. Soil nutrients under vegetation cover of different desert landscapes in junggar basin, Xinjiang. China Desert 2010, 30, 1367–1373. (In Chinese) [Google Scholar]
- Li, X.W.; Li, X.L.; Shi, Y.; Zhao, S.J.; Liu, J.L.; Lin, Y.Y.; Li, C.L.; Zhang, C.H. Effects of microtopography on soil microbial communities in alpine meadows on the Qinghai-Tibetan Plateau. Catena 2024, 239, 107945. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Hu, L.J.; Liu, Y.T.; Guo, Y.X.; Tang, S.M.; Ren, J. Land use shapes the microbial community structure by altering soil aggregates and dissolved organic matter components. J. Integr. Agric. 2025, 24, 827–844. [Google Scholar] [CrossRef]
- Bai, L.L.; Wang, W.Y.; Chen, Z.; Chen, X.Y.; Xiong, Y.C. The variations in soil microbial communities and their mechanisms along an elevation gradient in the Qilian Mountains, China. Sustainability 2025, 17, 1797. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, W.Q.; Yang, W.S.; Huang, M.; Lu, P.; Hu, D.Y. Green manure (Raphanus stativus L.) alters soil microbial structure and promotes acetochlor degradation. Arab. J. Chem. 2024, 17, 106017. [Google Scholar] [CrossRef]
- Sui, X.; Zeng, X.N.; Li, M.S.; Weng, X.H.; Frey, B.; Yang, L.B.; Li, M.H. Influence of different vegetation types on soil physicochemical parameters and fungal communities. Microorganisms 2022, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Anas, I.; Izhar, A.; Pengli, Y.; Rayyan, K.; He, L.; Wei, S.Q.; Jiang, L.G. Combined application of manure and chemical fertilizers alters soil environmental variables and improves soil fungal community composition and rice grain yield. Front. Microbiol. 2022, 13, 856355. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.J.; Song, D.J.; Dong, S.L.; Wang, B.T.; Du, H.B.; Qu, P.; Pu, T.H.; Shi, D.X.; Lyu, W.S.; Ge, Y. Diversity of endophytic fungi in the root systems of Chinese herbal medicines under rubber forests and their correlation with the physicochemical properties of rhizosphere soil. J. South Agric. 2022, 1–14. (In Chinese) [Google Scholar]
- Nie, J.M.; Zhang, X.F.; Zhang, Q.D.; Guo, X.D.; Cai, S.M.; Dong, Y.W.; Liu, H.Y.; Zhao, H.B. Responses of soil Fungal community structure to seasonal soil layer interactions in typical grassland of inner mongolia. J. Grassl. Sci. 2024, 32, 3408–3416. (In Chinese) [Google Scholar] [CrossRef]
- Dong, W.X.; Ma, J.; He, H.; Zhu, Y.F.; You, Y.N.; Chen, F. Effects of land reclamation on soil microbial community structure and function in the Huang-Huai plain mining area. Coal Sci. Technol. 2023, 51, 223–233. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhao, P.S.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Liu, M.H. Complexity and stability of molecular ecological network of root-associated fungi associated with Pinus syvestris var. mogolica. Chin. J. Ecol. 2024, 44, 7226–7237. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, K.W.; Wang, Y.F.; Liu, C.T.; Li, H.; Li, C.; Tu, R.; Zhang, Q.Q. Responses of soil microbial communities to grazing and their relationship with environmental factors. Res. Arid Reg. 2025, 42, 467–479. (In Chinese) [Google Scholar] [CrossRef]
- Pang, Y.G.; Zhang, M.H.; Jiang, M.; Zhong, H.S.; Ren, X.N.; Chen, X.F.; Zhu, D.Y.; Liang, Z.L.; Dai, J.; Zhang, C. Spatial heterogeneity and quality assessment of physicochemical and microbiological characteristics of arable soils in Gaoyao district, Zhaoqing City, Guangdong Province. J. South China Agric. Univ. 2025, 46, 151–163. (In Chinese) [Google Scholar]
- Ullah, M.R.; Carrillo, Y.; Dijkstra, F.A. Relative contributions of fungi and bacteria to litter decomposition under low and high soil moisture in an Australian grassland. Appl. Soil Ecol. 2023, 182, 104737. [Google Scholar] [CrossRef]
- MacColl, A.K.; Maherali, H. The effect of ecological restoration on mutualistic services provided by arbuscular mycorrhizal fungi depends on site location and host identity. Plant Soil, 2024; prepublish. [Google Scholar] [CrossRef]
- Guang, S.; Ying, Z.; Yang, H.T.; Li, X.R. Fungi in biocrusts facilitate ecosystem restoration during long-term vegetation succession in arid environments. Catena 2025, 252, 108893. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.M.; Fang, Z.; Ji, B.M.; Au, D.R. Effects of seasonal glacier freeze-thaw on funga structure and function in alpine meadow. Chin. J. Grassl. Sci. 2022, 44, 91–101. (In Chinese) [Google Scholar] [CrossRef]
- Gao, P.; Li, X.L.; Zhang, J.; Chai, Y.; Li, C.Y.; Li, X.H.; Wang, Y.; Zhao, X.Y.; Miao, J.H. Effects of microbial inoculants and sheep manure on plant community characteristics and soil bacterial diversity in degraded alpine meadow. Environ. Sci. 2025, 1–20. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.H.; Li, X.L.; Yang, P.N.; Gao, P.; Chai, Y.; She, Y.D.; Zhou, Y.Z. Regulatory effects of multi-component ecosystems in patchily degraded alpine meadow. Chin. Soil Fertil. 2025, 1–13. (In Chinese) [Google Scholar]
- Teng, Q.M.; Fang, T.; Zhang, Q.Q.; Gunina, A.; Zheng, A.Y.; Song, Z.L.; Zhou, J.Y.; Chang, S.X.; Li, Y.C. Successional transition from broadleaf to bamboo forests promotes fungal communities and soil carbon mineralization following the altered litterfall quality. Appl. Soil Ecol. 2025, 209, 106006. [Google Scholar] [CrossRef]
- Barbero, M.F.; Dominchin, F.M.; Verdenelli, A.R.; Frasier, I.; Restovich, B.S.; Mancilla, C.E.J.; Mlewski, C.E.; Labuckas, D.; Gil, V.S.; Meriles, M.J. Impact of land use changes on soil chemical properties, enzyme activities and microbial communities in two contrasting localities of the Argentinian Pampas. Appl. Soil Ecol. 2025, 206, 105836. [Google Scholar] [CrossRef]
- Wang, J.K.; Xu, Y.D.; Ding, F. Research progress on the transformation process and stability mechanism of plant residues into soil organic matter. J. Soil Sci. 2019, 56, 528–540. (In Chinese) [Google Scholar] [CrossRef]


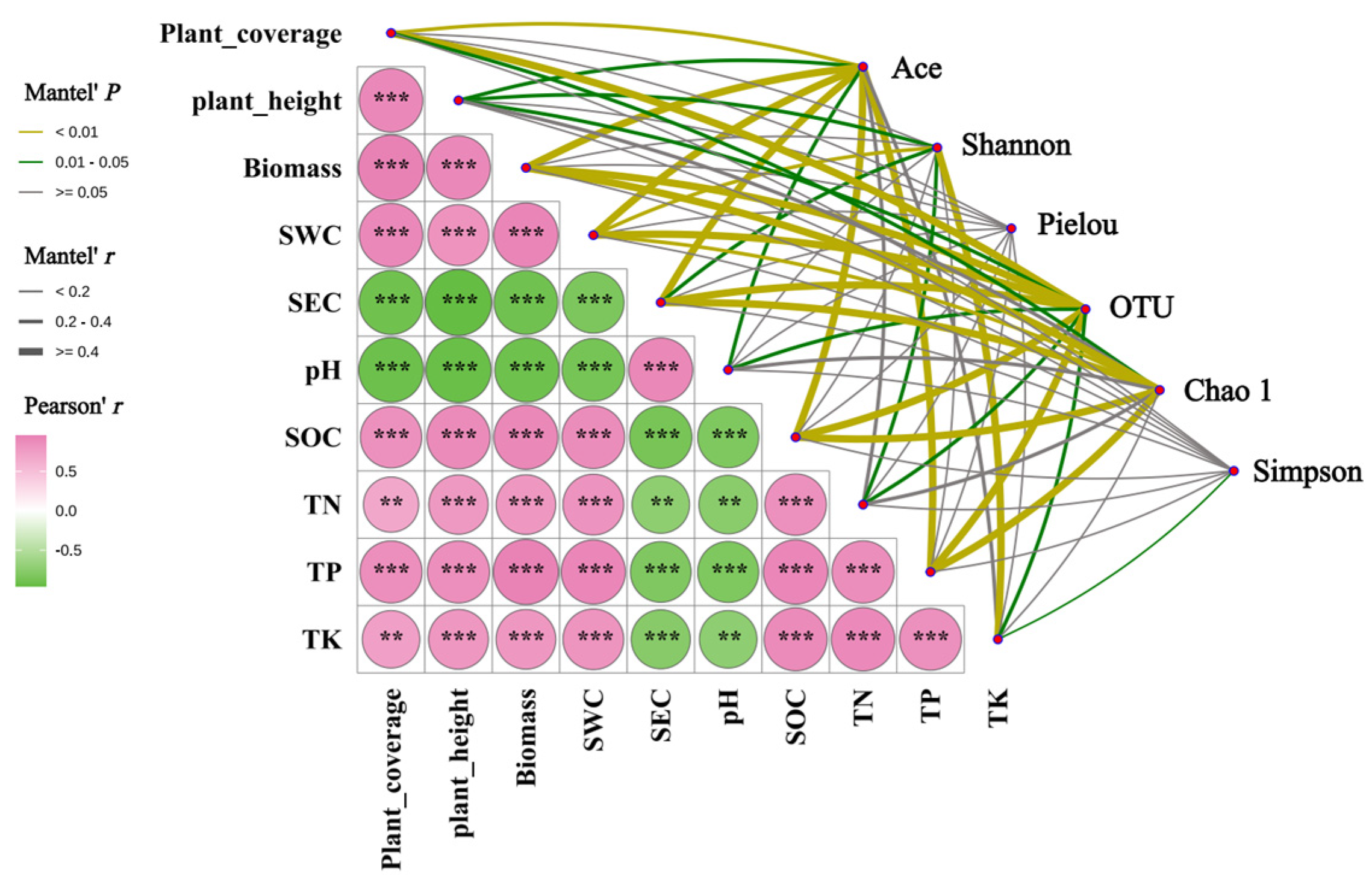
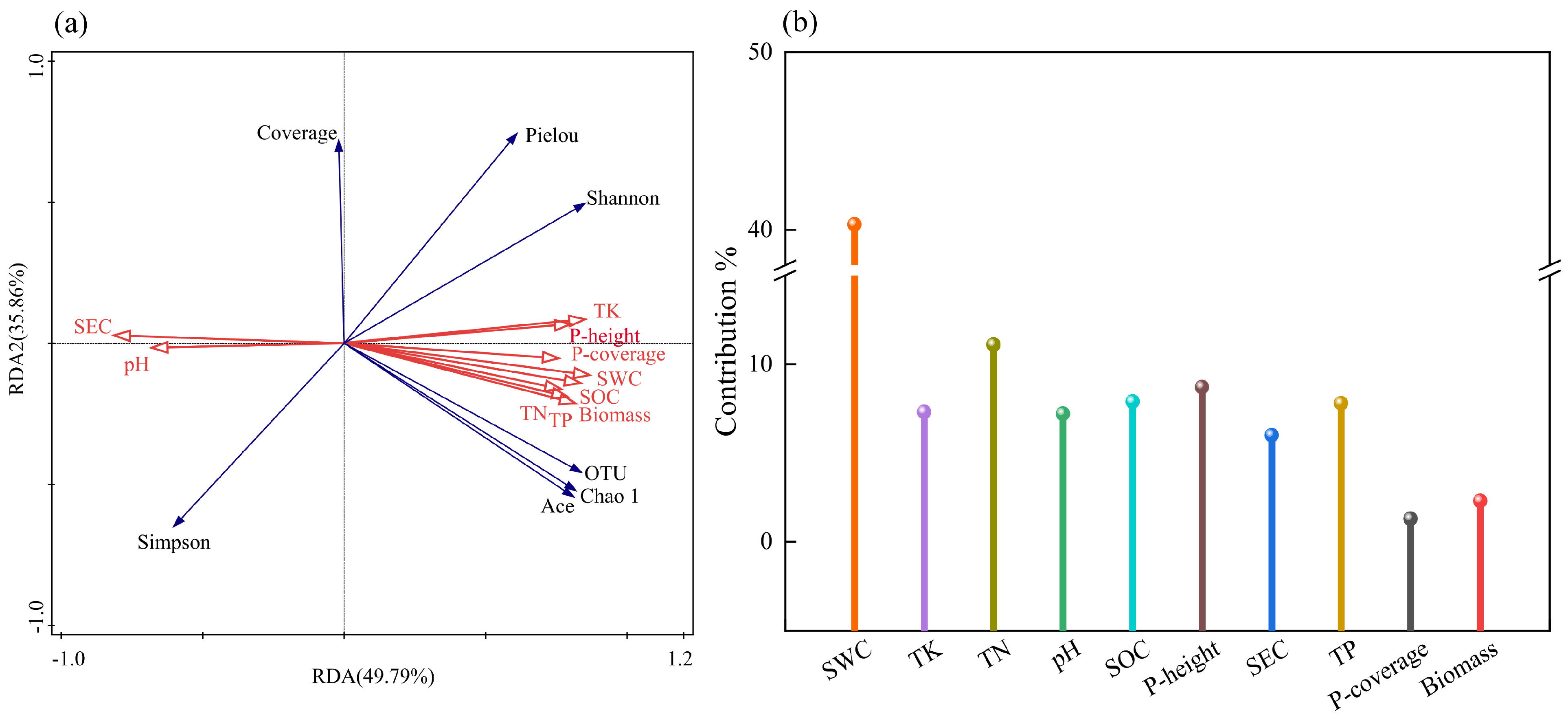
| Treatment | Plant Coverage/(%) | Plant Height/ (cm) | Biomass/(g·m−2) | SWC/(%) | SEC/ (μs·cm−1) | pH | SOC/ (g·kg−1) | TN/ (g·kg−1) | TP/ (g·kg−1) | TK/ (g·kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 35.11± 3.9c | 18.63± 2.34d | 346.21± 20.97d | 23.13± 3.34c | 1098.36± 63.67a | 8.66± 0.05a | 5.60± 0.46d | 0.77± 0.16d | 0.32± 0.02b | 19.08± 2.12c |
| H2 | 70.81± 6.82a | 34.87± 1.65a | 652.49± 20.65a | 40.38± 4.16a | 599.41± 38.50c | 7.97± 0.12c | 15.30± 1.53a | 1.55± 0.07a | 0.58± 0.06a | 25.28± 0.82a |
| H3 | 44.05± 2.69c | 24.18± 1.60c | 395.31± 30.94c | 27.34± 2.85c | 932.54± 120.11b | 8.35± 0.02b | 5.84± 0.25d | 1.06± 0.14b | 0.36± 0.05b | 20.00± 0.46bc |
| H4 | 56.12± 6.14b | 28.16± 2.07b | 468.17± 37.23b | 27.83± 1.27c | 832.02± 80.23b | 8.23± 0.03b | 8.43± 0.37c | 0.82± 0.02d | 0.39± 0.03b | 20.01± 1.21bc |
| H5 | 70.25± 4.29a | 31.69± 1.36a | 623.71± 10.64a | 35.43± 3.23b | 648.72± 62.92c | 8.08± 0.06c | 10.75± 0.60b | 1.20± 0.08b | 0.52± 0.01a | 22.05± 1.41b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Gao, P.; Cai, Z.; Li, F.; Shi, J. Topographic Habitat Drive the Change of Soil Fungal Community and Vegetation Soil Characteristics in the Rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region. J. Fungi 2025, 11, 531. https://doi.org/10.3390/jof11070531
Lyu L, Gao P, Cai Z, Li F, Shi J. Topographic Habitat Drive the Change of Soil Fungal Community and Vegetation Soil Characteristics in the Rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region. Journal of Fungi. 2025; 11(7):531. https://doi.org/10.3390/jof11070531
Chicago/Turabian StyleLyu, Liangyu, Pei Gao, Zongcheng Cai, Fayi Li, and Jianjun Shi. 2025. "Topographic Habitat Drive the Change of Soil Fungal Community and Vegetation Soil Characteristics in the Rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region" Journal of Fungi 11, no. 7: 531. https://doi.org/10.3390/jof11070531
APA StyleLyu, L., Gao, P., Cai, Z., Li, F., & Shi, J. (2025). Topographic Habitat Drive the Change of Soil Fungal Community and Vegetation Soil Characteristics in the Rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region. Journal of Fungi, 11(7), 531. https://doi.org/10.3390/jof11070531






