Antimicrobial Peptides Act-6 and Act 8-20 Derived from Scarabaeidae Cecropins Exhibit Differential Antifungal Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Design and Synthesis of Antimicrobial Peptides (AMPs)
2.3. Evaluation of Hemolytic Activity
2.4. Evaluation of Cytotoxic Activity
2.5. Antifungal Susceptibility Testing of AMPs Against Candida and Cryptococcus
2.6. Activity Against Biofilm Formation by C. albicans
2.7. Activity Against Morphogenesis of C. albicans
2.8. Effect of AMPs on C. Albicans Morphology
2.9. In Vivo Model of Disseminated Candidiasis
2.10. Statistical Analysis
3. Results
3.1. Act-6 and Act 8-20 Have Antifungal Activity Against Different Species of Candida and Cryptococcus
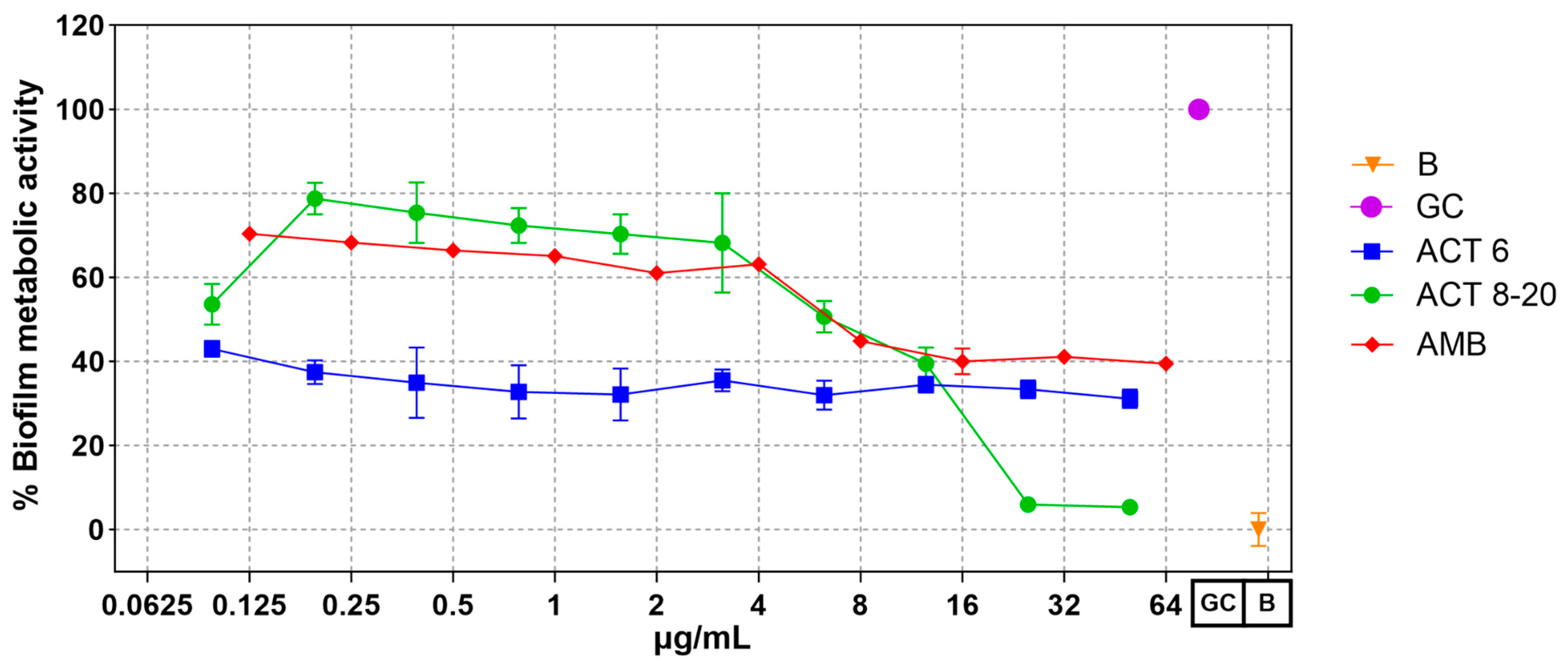
3.2. Act-6 and Act 8-20 Affect Biofilm Formation by C. albicans
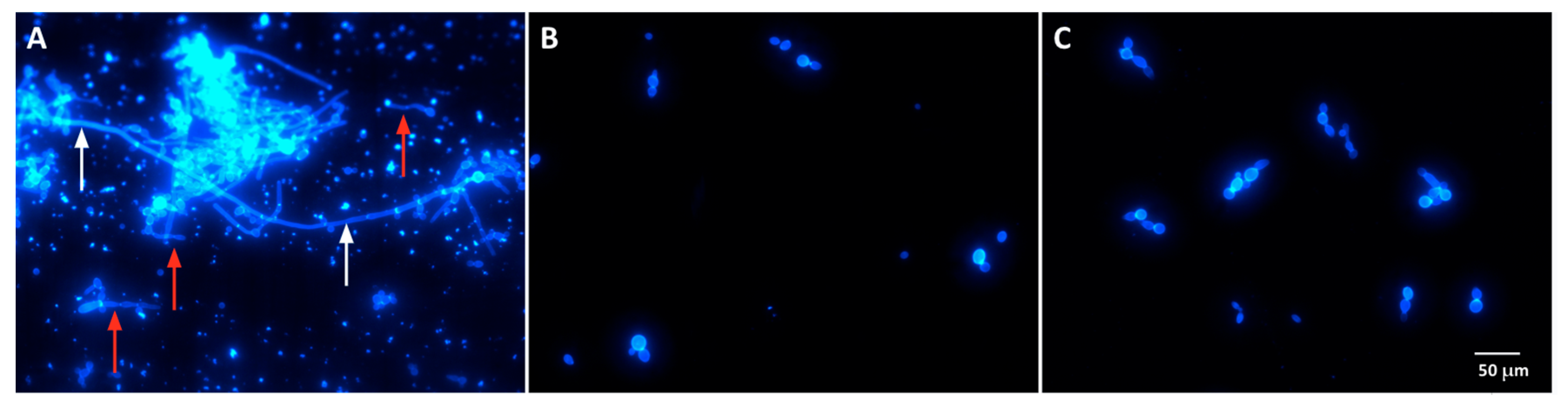
3.3. Act-6 and Act 8-20 Have the Ability to Inhibit Morphogenesis in C. albicans
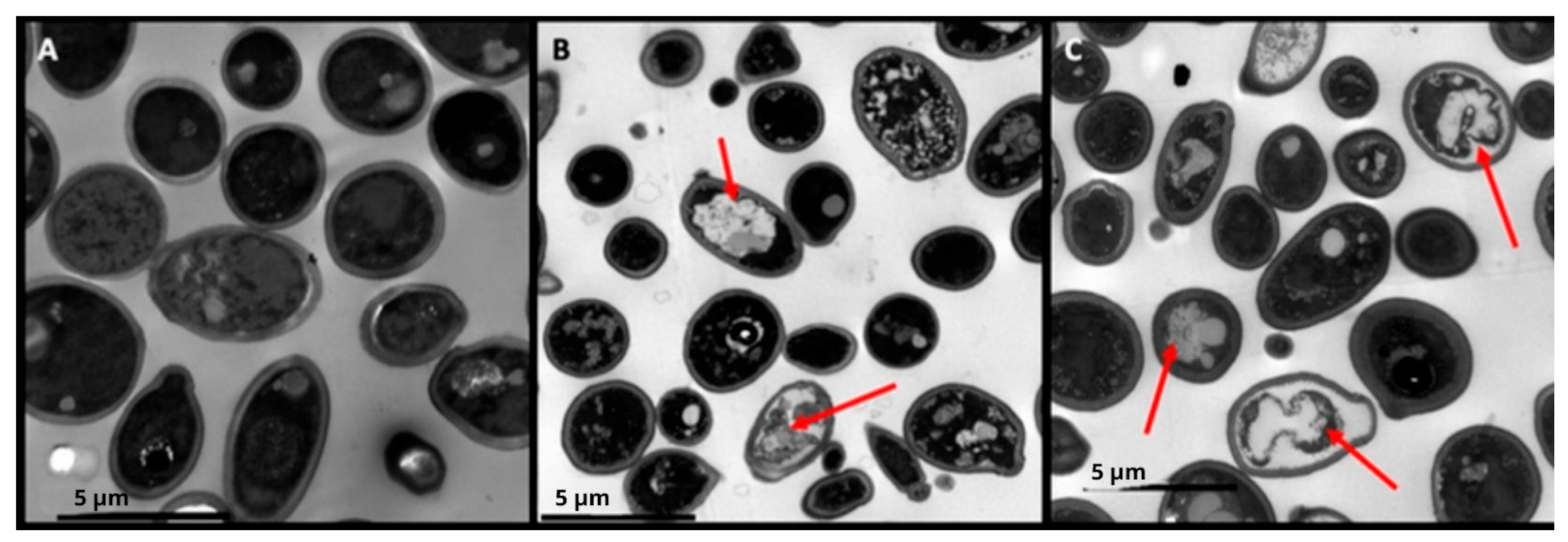
3.4. Act-6 and Act 8-20 Induce Morphological Alterations in C. albicans
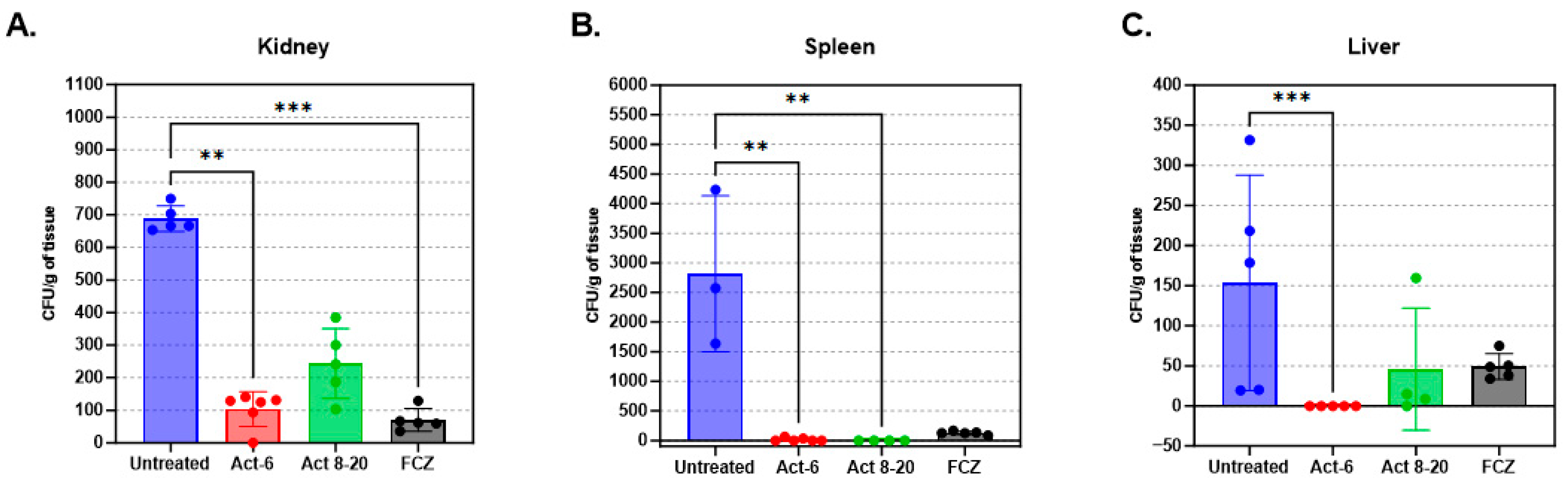
3.5. Fungal Load Decrease in Mice Organs Treated with AMPs
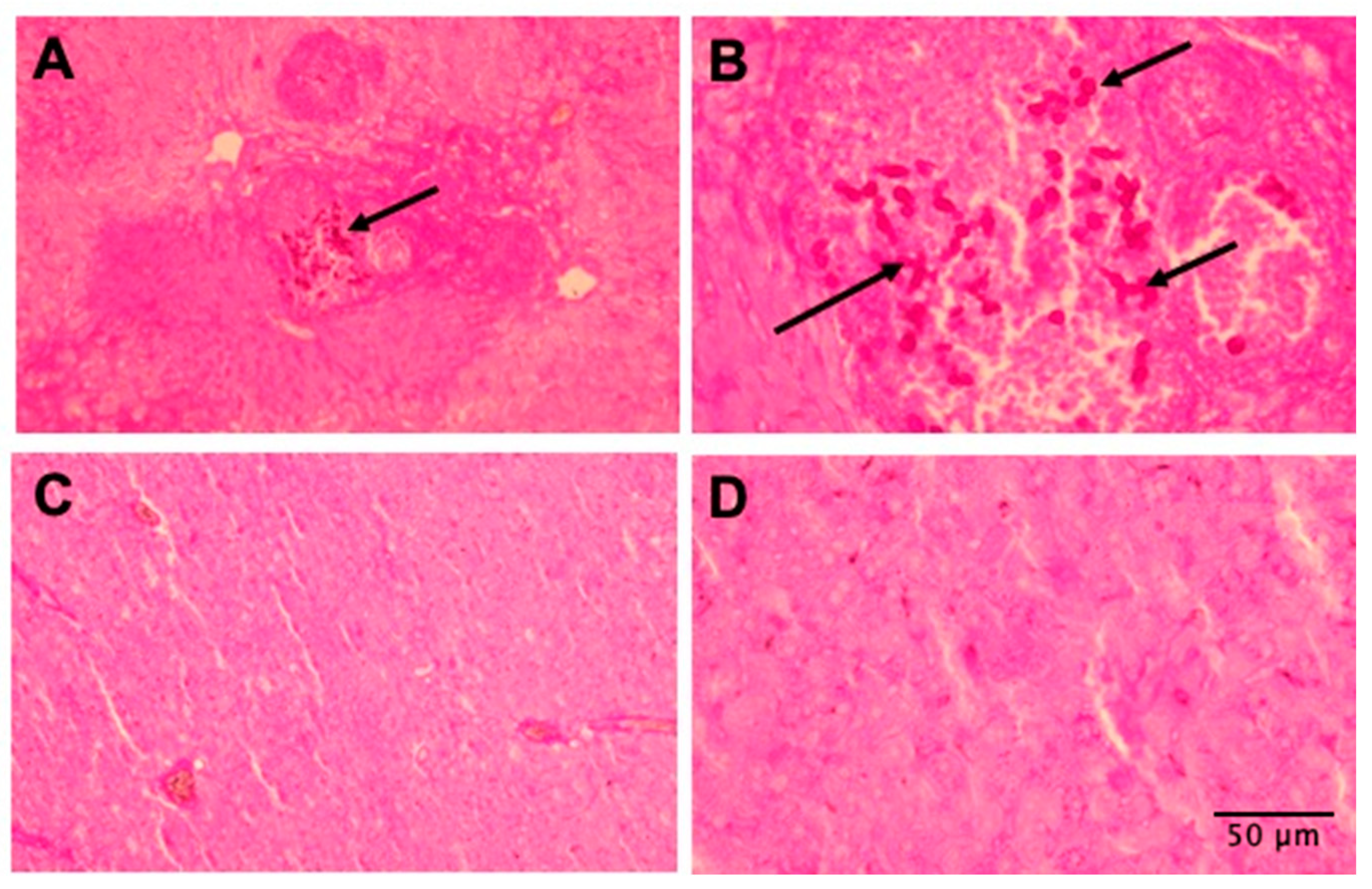
3.6. Tissues of Mice Treated with AMPs Are More Preserved
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Hani, U.; Shivakumar, H.; Vaghela, R.M.; Osmani, R.; Shrivastava, A. Candidiasis: A Fungal Infection- Current Challenges and Progress in Prevention and Treatment. Infect. Disord.-Drug Targets 2015, 15, 42–52. [Google Scholar] [CrossRef]
- Cortés, J.A.; Romero Moreno, L.F.; Aguirre León, C.A.; Pinzón Lozano, L.; Cuervo, S.I. Enfoque Clínico Del Síndrome Febril Agudo En Colombia. Infectio 2017, 21, 39–50. [Google Scholar] [CrossRef][Green Version]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive Candidiasis. Nat. Rev. Dis. Primer 2024, 10, 20. [Google Scholar] [CrossRef]
- Ben-Ami, R. Treatment of Invasive Candidiasis: A Narrative Review. J. Fungi 2018, 4, 97. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12, 884. [Google Scholar] [CrossRef]
- Sudbery, P.; Gow, N.; Berman, J. The Distinct Morphogenic States of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The Global Burden of HIV-Associated Cryptococcal Infection in Adults in 2020: A Modelling Analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005217-8.
- Mourad, A.; Perfect, J.R. The War on Cryptococcosis: A Review of the Antifungal Arsenal. Mem. Inst. Oswaldo Cruz 2018, 113, e170391. [Google Scholar] [CrossRef]
- Melhem, M.S.C.; Leite Júnior, D.P.; Takahashi, J.P.F.; Macioni, M.B.; Oliveira, L.D.; De Araújo, L.S.; Fava, W.S.; Bonfietti, L.X.; Paniago, A.M.M.; Venturini, J.; et al. Antifungal Resistance in Cryptococcal Infections. Pathogens 2024, 13, 128. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Ntwasa, M.; Goto, A.; Kurata, S. Coleopteran Antimicrobial Peptides: Prospects for Clinical Applications. Int. J. Microbiol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Henao Arias, D.C.; Toro, L.J.; Téllez Ramirez, G.A.; Osorio-Méndez, J.F.; Rodríguez-Carlos, A.; Valle, J.; Marín-Luevano, S.P.; Rivas-Santiago, B.; Andreu, D.; Castaño Osorio, J.C. Novel Antimicrobial Cecropins Derived from O. curvicornis and D. satanas Dung Beetles. Peptides 2021, 145, 170626. [Google Scholar] [CrossRef]
- Téllez Ramirez, G.A.; Osorio-Méndez, J.F.; Henao Arias, D.C.; Toro, S.L.J.; Franco Castrillón, J.; Rojas-Montoya, M.; Castaño Osorio, J.C. New Insect Host Defense Peptides (HDP) From Dung Beetle (Coleoptera: Scarabaeidae) Transcriptomes. J. Insect Sci. 2021, 21, 12. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jeong, J.-H.; Kim, Y. Design and Engineering of Antimicrobial Peptides Based on LPcin-YK3, an Antimicrobial Peptide Derivative from Bovine Milk. J. Microbiol. Biotechnol. 2018, 28, 381–390. [Google Scholar] [CrossRef]
- Cavaco, M.; Andreu, D.; ARB Castanho, M. The Challenge of Peptide Proteolytic Stability Studies: Scarce Data, Difficult Readability, and the Need for Harmonization. Angew. Chem. Int. Ed. Engl. 2021, 60, 1686–1688. [Google Scholar] [CrossRef]
- Cavaco, M.; Valle, J.; Flores, I.; Andreu, D.; ARB Castanho, M. Estimating Peptide Half-Life in Serum from Tunable, Sequence-Related Physicochemical Properties. Clin. Transl. Sci. 2021, 14, 1349–1358. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kang, J.H.; Hahm, K.-S. Structure-antibacterial, Antitumor and Hemolytic Activity Relationships of Cecropin A-magainin 2 and Cecropin A-melittin Hybrid Peptides. J. Pept. Res. 1999, 53, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alvarez, L.J.; Jiménez-Alcántar, P.; Ochoa-Zarzosa, A.; López-Meza, J.E. The Antimicrobial Peptide γ-Thionin from Habanero Chile (Capsicum Chinense) Induces Caspase-Independent Apoptosis on Human K562 Chronic Myeloid Leukemia Cells and Regulates Epigenetic Marks. Molecules 2023, 28, 3661. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts Guidelines for Diagnosing, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; Volume CLSI, ISBN 978-1-68440-163-5. [Google Scholar]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A Simple and Reproducible 96-Well Plate-Based Method for the Formation of Fungal Biofilms and Its Application to Antifungal Susceptibility Testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.E.; Rossi, D.C.P.; Ishida, K.; Spadari, C.C.; Melhem, M.S.C.; Garcia, D.M.; Caires, A.C.F.; Taborda, C.P.; Rodrigues, E.G. Antifungal Activity of the Biphosphinic Cyclopalladate C7a against Candida albicans Yeast Forms In Vitro and In Vivo. Front. Microbiol. 2017, 8, 771. [Google Scholar] [CrossRef]
- Torres, R.; Barreto-Santamaría, A.; Arévalo-Pinzón, G.; Firacative, C.; Gómez, B.L.; Escandón, P.; Patarroyo, M.A.; Muñoz, J.E. In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance. Antibiotics 2023, 12, 1234. [Google Scholar] [CrossRef]
- Rossi, D.C.; Muñoz, J.E.; Carvalho, D.D.; Belmonte, R.; Faintuch, B.; Borelli, P.; Miranda, A.; Taborda, C.P.; Daffre, S. Therapeutic Use of a Cationic Antimicrobial Peptide from the Spider Acanthoscurria gomesiana in the Control of Experimental Candidiasis. BMC Microbiol. 2012, 12, 28. [Google Scholar] [CrossRef]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial Role of Insect-Derived Bioactive Components against Inflammation and Its Associated Complications (Colitis and Arthritis) and Cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional Host Defense Peptides: Antimicrobial Peptides, the Small yet Big Players in Innate and Adaptive Immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.; Davidson, D.; Hancock, R. A Re-Evaluation of the Role of Host Defence Peptides in Mammalian Immunity. Curr. Protein Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef]
- Toro Segovia, L.J.; Téllez Ramírez, G.A.; Henao Arias, D.C.; Rivera Duran, J.D.; Bedoya, J.P.; Castaño Osorio, J.C. Identification and Characterization of Novel Cecropins from the Oxysternon conspicillatum Neotropic Dung Beetle. PLoS ONE 2017, 12, e0187914. [Google Scholar] [CrossRef]
- Kotey, F.C.; Dayie, N.T.; Tetteh-Uarcoo, P.B.; Donkor, E.S. Candida Bloodstream Infections: Changes in Epidemiology and Increase in Drug Resistance. Infect. Dis. Res. Treat. 2021, 14, 11786337211026927. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Joeng, J.-H.; Kim, Y. Design, Characterization, and Antimicrobial Activity of a Novel Antimicrobial Peptide Derived from Bovine Lactophoricin. J. Microbiol. Biotechnol. 2017, 27, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with Host Cells: Virulence Factors, Host Defense, Escape Strategies, and the Microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Muñoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Muñoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida Spp. J. Fungi 2022, 8, 1173. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, X.; Jiao, Z.; Peng, J.; Zhou, L.; Yang, L.; Huang, M.; Tian, C.; Guo, G. The Antimicrobial Peptide AMP-17 Derived from Musca Domestica Inhibits Biofilm Formation and Eradicates Mature Biofilm in Candida albicans. Antibiotics 2022, 11, 1474. [Google Scholar] [CrossRef]
- Lim, C.S.-Y.; Rosli, R.; Seow, H.F.; Chong, P.P. Candida and Invasive Candidiasis: Back to Basics. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 21–31. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, X.; Yang, L.; Su, P.; Fu, P.; Peng, J.; Yang, N.; Guo, G. Antimicrobial Peptide AMP-17 Affects Candida albicans by Disrupting Its Cell Wall and Cell Membrane Integrity. Infect. Drug Resist. 2020, 13, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Ciociola, T.; Elviri, L.; Zanello, P.P.; Giovati, L.; Arruda, D.C.; Muñoz, J.E.; Mortara, R.A.; Morace, G.; Borghi, E.; et al. A Naturally Occurring Antibody Fragment Neutralizes Infectivity of Diverse Infectious Agents. Sci. Rep. 2016, 6, 35018. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zou, C.; Wen, N.; Liu, X.; Meng, Z.; Feng, S.; Zheng, Z.; Meng, Q.; Wang, C. The Effect of Structural Modification of Antimicrobial Peptides on Their Antimicrobial Activity, Hemolytic Activity, and Plasma Stability. J. Pept. Sci. 2021, 27, e3306. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, C.; Li, G.; Lai, Z.; Tan, P.; Jian, Q.; Cheng, B.; Shan, A. Rational Avoidance of Protease Cleavage Sites and Symmetrical End-Tagging Significantly Enhances the Stability and Therapeutic Potential of Antimicrobial Peptides. J. Med. Chem. 2020, 63, 9421–9435. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, F.; Li, J.; Chen, F. Antifungal Activity of ToAP2D Peptide Against Sporothrix globosa. Front. Bioeng. Biotechnol. 2021, 9, 761518. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and In Vivo Evaluation of a Novel Histatin-5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef]

| AMP | Sequence | Length | Molecular Weight (Da) | pI | GRAVY | A*kT/e (Polar Angle) |
|---|---|---|---|---|---|---|
| Ox3-22 | GSKRWRKFEKRVKKVFEHTKEA | 22 | 2775.26 | 10.64 | −1.709 | 18.951 (113.2) |
| Act-6 | GSKRWRKFEKRVKKIFEKTKEAK | 23 | 2908.49 | 10.77 | −1.822 | 16.645 (115.99) |
| Act 8-20 | GSKRWRKFLKRVKKIFLHTKLAK | 23 | 2869.59 | 12.32 | −0.839 | 19.194 (107.57) |
| AMP | Values in μg/mL (μM) | IC50 | C. albicans ATCC 10231 | C. albicans SC5314 | C. glabrata ATCC 2001 | C. parapsilosis ATCC 22019 | C. krusei ATCC 6258 | C. tropicalis ATCC 750 | C. neoformans H99 | C. gattii H0058-I-2029 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ox3-22 | MIC | >50 (18.01) | >50 (18.01) | 1.56 (0.56) | 0.39 (0.14) | 0.78 (0.28) | 25 (9) | 25 (9) | 1.56 (0.53) | |
| TI (hemolysis) | >400 (144.13) | <8 | <8 | >257.38 | >1029.51 | >514.75 | >16.01 | >16.01 | >257.38 | |
| TI (PBMC cytotoxicity) | ||||||||||
| Act-6 | MIC | >50 (>17.19) | 25–50 (8.59–17.19) | 3.12 (1.07) | 3.12 (1.07) | 1.56 (0.53) | >50 (>17.19) | 25 (8.59) | 1.56 (0.53) | |
| TI (hemolysis) | >400 (137.53) | <8 | >16.01 | 128.53 | >128.53 | >259.49 | <8 | >16.01 | >259.49 | |
| TI (PBMC cytotoxicity | ||||||||||
| Act 8-20 | MIC | 12.5 (4.35) | 6.25–12.5 (2.17–4.35) | 12.5–25 (4.35–8.71) | 25 (8.71) | 3.1 (1.08) | 6.25 (2.17) | 3.1–6.25 (1.08–2.17) | 0.39 (0.108) | |
| TI (hemolysis) | >500 (174.24) | >40.06 | 40.06–80.29 | 20–40.06 | >20 | >161.33 | >80.29 | 80.29–161.33 | >1613.33 | |
| TI (PBMC cytotoxicity) | 159.2 (55.47) | 12.75 | 12.75–25.56 | 6.37–12.75 | 6.37 | 51.36 | 25.56 | 25.56–51.36 | 513.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, M.; Toro, L.J.; Firacative, C.; Gómez, B.L.; Rivas-Santiago, B.; Andreu, D.; Castaño, J.C.; Téllez, G.A.; Muñoz, J.E. Antimicrobial Peptides Act-6 and Act 8-20 Derived from Scarabaeidae Cecropins Exhibit Differential Antifungal Activity. J. Fungi 2025, 11, 519. https://doi.org/10.3390/jof11070519
Rodríguez M, Toro LJ, Firacative C, Gómez BL, Rivas-Santiago B, Andreu D, Castaño JC, Téllez GA, Muñoz JE. Antimicrobial Peptides Act-6 and Act 8-20 Derived from Scarabaeidae Cecropins Exhibit Differential Antifungal Activity. Journal of Fungi. 2025; 11(7):519. https://doi.org/10.3390/jof11070519
Chicago/Turabian StyleRodríguez, Melissa, Lily J. Toro, Carolina Firacative, Beatriz L. Gómez, Bruno Rivas-Santiago, David Andreu, Jhon C. Castaño, German A. Téllez, and Julián E. Muñoz. 2025. "Antimicrobial Peptides Act-6 and Act 8-20 Derived from Scarabaeidae Cecropins Exhibit Differential Antifungal Activity" Journal of Fungi 11, no. 7: 519. https://doi.org/10.3390/jof11070519
APA StyleRodríguez, M., Toro, L. J., Firacative, C., Gómez, B. L., Rivas-Santiago, B., Andreu, D., Castaño, J. C., Téllez, G. A., & Muñoz, J. E. (2025). Antimicrobial Peptides Act-6 and Act 8-20 Derived from Scarabaeidae Cecropins Exhibit Differential Antifungal Activity. Journal of Fungi, 11(7), 519. https://doi.org/10.3390/jof11070519









