Antifungal Minimal Inhibitory Concentrations of Mold Isolates from Patients with Cancer; Single-Center Experience, 2018–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Mold Isolates and Analysis of Minimal Inhibitory Concentrations
2.2. Determining ECVs and Wild-Type Upper Limit (wt-UL)
3. Results
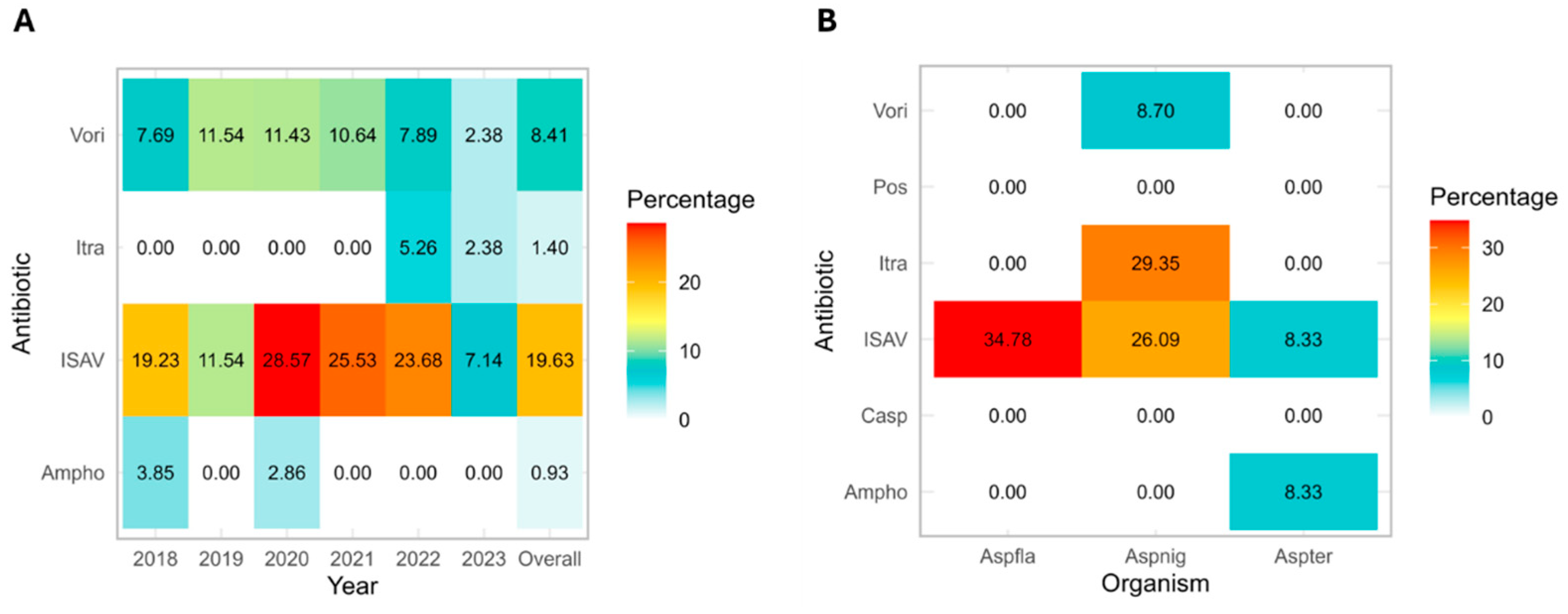
3.1. Distribution of Isolated Mold Pathogens
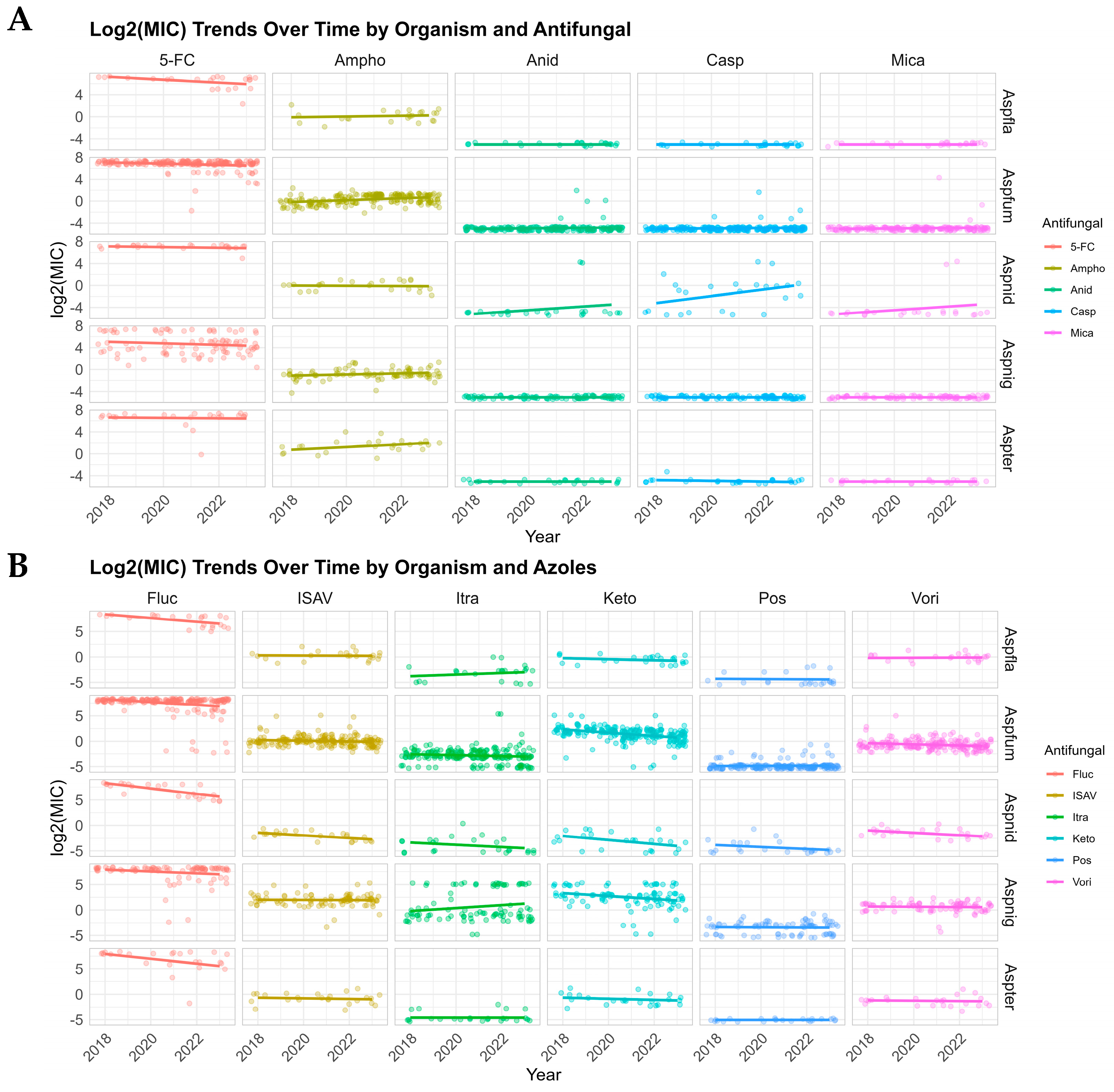
3.2. Distribution and Trends in MICs
3.3. Emergence of Non-Wild-Type Mold Isolates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FC | Flucytosine |
| Ampho | Amphotericin B |
| Anid | Anidulafungin |
| Aspfla | A. flavus |
| Aspfum | A. fumigatus |
| Aspnid | A. nidulans |
| Aspnig | A. niger |
| Aspter | A. terreus |
| AST | Antimicrobial susceptibility testing |
| Casp | Caspofungin |
| ECOFFs/ECVs | Epidemiological cutoff values |
| Fluc | Fluconazole |
| IA | Invasive aspergillosis |
| IMI | Invasive mold infections |
| ISAV | Isavuconazole |
| Itra | Itraconazole |
| Keto | Ketoconazole |
| MEC | Minimal effective concentrations |
| MIC | Minimal inhibitory concentrations |
| Mica | Micafungin |
| NWT | Non-wild-type |
| Pos | Posaconazole |
| Vori | Voriconazole |
| wt-UL | Upper limit of the wild-type population |
References
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- CDC. Data and Statistics on Fungal Diseases. Available online: https://www.cdc.gov/fungal/data-research/facts-stats/index.html (accessed on 24 June 2025).
- Cho, S.-Y.; Lee, D.-G.; Kim, W.-B.; Chun, H.-S.; Park, C.; Myong, J.-P.; Park, Y.-J.; Choi, J.-K.; Lee, H.-J.; Kim, S.-H.; et al. Epidemiology and Antifungal Susceptibility Profile of Aspergillus Species: Comparison between Environmental and Clinical Isolates from Patients with Hematologic Malignancies. J. Clin. Microbiol. 2019, 57, e02023-18. [Google Scholar] [CrossRef] [PubMed]
- Sipsas, N.V.; Kontoyiannis, D.P. Invasive fungal infections in patients with cancer in the Intensive Care Unit. Int. J. Antimicrob. Agents 2012, 39, 464–471. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.4, E.Def 9.4 and E.Def 11.0 Procedures. Available online: http://www.eucast.org (accessed on 14 May 2025).
- CLSI M57S; Epidemiological Cutoff Values for Antifungal Susceptibility Testing. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020.
- Espinel-Ingroff, A.; Sasso, M.; Turnidge, J.; Arendrup, M.; Botterel, F.; Bourgeois, N.; Bouteille, B.; Canton, E.; Cassaing, S.; Dannaoui, E.; et al. Etest ECVs/ECOFFs for detection of resistance in prevalent and three nonprevalent Candida spp. to triazoles and amphotericin B and Aspergillus spp. to caspofungin: Further assessment of modal variability. Antimicrob. Agents Chemother. 2021, 65, e01093-21. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.W. Breakpoints for antifungal agents: An update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist. Updates 2013, 16, 81–95. [Google Scholar] [CrossRef]
- Beredaki, M.-I.; Georgiou, P.-C.; Siopi, M.; Kanioura, L.; Andes, D.; Arendrup, M.C.; Mouton, J.W.; Meletiadis, J. Toward harmonization of voriconazole CLSI and EUCAST breakpoints for Candida albicans using a validated in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2020, 64, e00170-20. [Google Scholar] [CrossRef]
- Astvad, K.M.T.; Hare, R.K.; Arendrup, M.C. Evaluation of the in vitro activity of isavuconazole and comparator voriconazole against 2635 contemporary clinical Candida and Aspergillus isolates. Clin. Microbiol. Infect. 2017, 23, 882–887. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J. Clin. Microbiol. 2013, 51, 2608–2616. [Google Scholar] [CrossRef]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Brüggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K.; et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updates 2015, 21–22, 30–40. [Google Scholar] [CrossRef]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi. 2018, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Lane, S. Just as good but better tolerated: Posaconazole as first-line therapy for invasive aspergillosis. Hematologist 2021, 18. [Google Scholar]
- Aruanno, M.; Glampedakis, E.; Lamoth, F. Echinocandins for the Treatment of Invasive Aspergillosis: From Laboratory to Bedside. Antimicrob. Agents Chemother. 2019, 63, e00399-19. [Google Scholar] [CrossRef] [PubMed]
- CLSI M38; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017.
- Turnidge, J.; Kahlmeter, G.; Kronvall, G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006, 12, 418–425. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Monzon, A.; Cuenca-Estrella, M. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2008, 52, 2468–2472. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Rhomberg, P.R.; Wiederhold, N.P.; Gibas, C.; Sanders, C.; Fan, H.; Mele, J.; Kovanda, L.L.; Castanheira, M. In Vitro Activity of Isavuconazole against Opportunistic Fungal Pathogens from Two Mycology Reference Laboratories. Antimicrob. Agents Chemother. 2018, 62, e01230-18. [Google Scholar] [CrossRef] [PubMed]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef]
- Saral, R. Candida and Aspergillus Infections in Immunocompromised Patients: An Overview. Rev. Infect. Dis. 1991, 13, 487–492. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Franconi, I.; Rizzato, C.; Ghelardi, E.; Lupetti, A. Hospital distribution, seasonality, time trends and antifungal susceptibility profiles of all Aspergillus species isolated from clinical samples from 2015 to 2022 in a tertiary care hospital. BMC Microbiol. 2024, 24, 111. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Rhomberg, P.R.; Desphande, L.M.; Castanheira, M. Trends in the activity of mold-active azole agents against Aspergillus fumigatus clinical isolates with and without cyp51 alterations from Europe and North America (2017–2021). J. Clin. Microbiol. 2024, 62, e01141-23. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.; van der Velden, W.; Bouwman, F.; Stoop, F.J.; Blijlevens, N.M.A.; Melchers, W.J.G.; Verweij, P.E.; Donnelly, J.P. Epidemiology of invasive aspergillosis and triazole-resistant Aspergillus fumigatus in patients with haematological malignancies: A single-centre retrospective cohort study. J. Antimicrob. Chemother. 2018, 73, 1389–1394. [Google Scholar] [CrossRef]
- Heo, S.T.; Tatara, A.M.; Jiménez-Ortigosa, C.; Jiang, Y.; Lewis, R.E.; Tarrand, J.; Tverdek, F.; Albert, N.D.; Verweij, P.E.; Meis, J.F.; et al. Changes in In Vitro Susceptibility Patterns of Aspergillus to Triazoles and Correlation with Aspergillosis Outcome in a Tertiary Care Cancer Center, 1999–2015. Clin. Infect. Dis. 2017, 65, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Hamprecht, A.; Vehreschild, M.J.; Cornely, O.A.; Buchheidt, D.; Spiess, B.; Koldehoff, M.; Buer, J.; Meis, J.F.; Rath, P.M. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J. Antimicrob. Chemother. 2015, 70, 1522–1526. [Google Scholar] [CrossRef]
- Alanio, A.; Sitterle, E.; Liance, M.; Farrugia, C.; Foulet, F.; Botterel, F.; Hicheri, Y.; Cordonnier, C.; Costa, J.M.; Bretagne, S. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J. Antimicrob. Chemother. 2011, 66, 371–374. [Google Scholar] [CrossRef]
- Gregson, L.; Goodwin, J.; Johnson, A.; McEntee, L.; Moore, C.B.; Richardson, M.; Hope, W.W.; Howard, S.J. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: Correlation with itraconazole, voriconazole, and posaconazole. Antimicrob. Agents Chemother. 2013, 57, 5778–5780. [Google Scholar] [CrossRef] [PubMed]
- Buil, J.B.; Bruggemann, R.J.M.; Wasmann, R.E.; Zoll, J.; Meis, J.F.; Melchers, W.J.G.; Mouton, J.W.; Verweij, P.E. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J. Antimicrob. Chemother. 2018, 73, 134–142. [Google Scholar] [CrossRef]
- Howard, S.J.; Harrison, E.; Bowyer, P.; Varga, J.; Denning, D.W. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob. Agents Chemother. 2011, 55, 4802–4809. [Google Scholar] [CrossRef]
- Perez-Cantero, A.; Lopez-Fernandez, L.; Guarro, J.; Capilla, J. New Insights into the Cyp51 Contribution to Azole Resistance in Aspergillus Section Nigri. Antimicrob. Agents Chemother. 2019, 63, e00543-19. [Google Scholar] [CrossRef]
- Araujo, R.; Oliveira, M.; Amorim, A.; Sampaio-Maia, B. Unpredictable susceptibility of emerging clinical moulds to tri-azoles: Review of the literature and upcoming challenges for mould identification. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1289–1301. [Google Scholar] [CrossRef]
- Duong, T.N.; Le, T.V.; Tran, K.H.; Nguyen, P.T.; Nguyen, B.T.; Nguyen, T.A.; Nguyen, H.P.; Nguyen, B.T.; Fisher, M.C.; Rhodes, J.; et al. Azole-resistant Aspergillus fumigatus is highly prevalent in the environment of Vietnam, with marked variability by land use type. Environ. Microbiol. 2021, 23, 7632–7642. [Google Scholar] [CrossRef] [PubMed]
- Potruch, A.; Elinav, H.; Cohen, M.J.; Rouvinski, A.; Polacheck, I.; Korem, M. Comparative evaluation of Sensititre YeastOne and CLSI M38-Ed3 reference method for determining echinocandin minimum effective concentrations against Aspergillus isolates. Microbiol. Spectr. 2024, 12, e00280-24. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Zimbeck, A.J.; Baddley, J.W.; Marr, K.A.; Andes, D.R.; Walsh, T.J.; Kauffman, C.A.; Kontoyiannis, D.P.; Ito, J.I.; Pappas, P.G.; et al. In Vitro Echinocandin Susceptibility of Aspergillus Isolates from Patients Enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob. Agents Chemother. 2011, 55, 3944–3946. [Google Scholar] [CrossRef]
- Tavakoli, M.; Rivero-Menendez, O.; Abastabar, M.; Hedayati, M.T.; Sabino, R.; Siopi, M.; Zarrinfar, H.; Nouripour-Sisakht, S.; van der Lee, H.; Valadan, R.; et al. Genetic diversity and antifungal susceptibility patterns of Aspergillus nidulans complex obtained from clinical and environmental sources. Mycoses 2020, 63, 78–88. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Fothergill, A.; Fuller, J.; Johnson, E.; Pelaez, T.; Turnidge, J. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 2011, 55, 2855–2859. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Colombo, A.L.; Cordoba, S.; Dufresne, P.J.; Fuller, J.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; Kidd, S.E.; Meis, J.F.; et al. International Evaluation of MIC Distributions and Epidemiological Cutoff Value (ECV) Definitions for Fusarium Species Identified by Molecular Methods for the CLSI Broth Microdilution Method. Antimicrob. Agents Chemother. 2016, 60, 1079–1084. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lewis, R.E. Antifungal activity against Scedosporium species and novel assays to assess antifungal pharmacodynamics against filamentous fungi. Med. Mycol. 2009, 47, 422–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 27–46. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Castanheira, M. Susceptibility patterns of amphotericin B, itraconazole, posaconazole, voriconazole and caspofungin for isolates causing invasive mould infections from the SENTRY Antifungal Surveillance Program (2018–2021) and application of single-site epidemiological cutoff values to evaluate amphotericin B activity. Mycoses 2023, 66, 854–868. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Kontoyiannis, D.P. Role and Interpretation of Antifungal Susceptibility Testing for the Management of Invasive Fungal Infections. J. Fungi 2020, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Feuss, A.; Bougnoux, M.-E.; Dannaoui, E. The role of Olorofim in the treatment of filamentous fungal infections: A review of in Vitro and in vivo studies. J. Fungi 2024, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Almajid, A.; Bazroon, A.; Al-Awami, H.M.; Albarbari, H.; Alqahtani, I.; Almutairi, R.; Alsuwayj, A.; Alahmadi, F.; Aljawad, J.; Alnimer, R.; et al. Fosmanogepix: The novel anti-fungal agent’s comprehensive review of in vitro, in vivo, and current insights from advancing clinical trials. Cureus 2024, 16, e59210. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
| Organisms | Agent | MIC/MEC50 | MIC/MEC90 | Mode | Range | ECV (97.5%) | wt-UL; (Mode + 2) | CLSI ECV |
|---|---|---|---|---|---|---|---|---|
| Aspergillus flavus/oryzae (n = 23) | 5-FC | ≥64 | ≥64 | ≥64 | 4–≥64 | 256 | ||
| Ampho | 1 | 2 | 1 | 0.25–4 | 4 | 4 | 4 | |
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Casp | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Fluc | ≥128 | ≥128 | ≥128 | 32–≥128 | 512 | |||
| ISAV | 1 | 2 | 1 | 0.5–4 | 4 | 4 | 1 | |
| Itra | 0.12 | 0.25 | 0.12 | ≤0.06–1 | 0.5 | 0.5 | 1 | |
| Keto | 1 | 1 | 1 | 0.25–2 | 2 | 4 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Pos | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–0.25 | 0.12 | 0.5 | ||
| Vori | 1 | 2 | 1 | 0.5–2 | 2 | 4 | 2 | |
| Aspergillus fumigatus (n = 214) | 5-FC | ≥64 | ≥64 | ≥64 | 0.25–≥64 | 256 | ||
| Ampho | 1 | 2 | 2 | 0.25–4 | 4 | 8 | 2 | |
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–4 | 0.25 | |||
| Casp | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–4 | 0.25 | 0.5 | ||
| Fluc | ≥128 | ≥128 | ≥128 | ≤0.5–≥128 | 512 | |||
| ISAV | 1 | 2 | 1 | 0.25–≥16 | 2 | 4 | 1 | |
| Itra | 0.12 | 0.25 | 0.12 | ≤0.06–≥16 | 0.5 | 0.5 | 1 | |
| Keto | 4 | 8 | 4 | ≤0.06–≥16 | 8 | 16 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–≥8 | 0.25 | |||
| Pos | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–0.5 | 0.25 | |||
| Vori | 0.5 | 1 | 0.5 | 0.12–≥16 | 2 | 2 | 1 | |
| Aspergillus nidulans (n = 24) | 5-FC | ≥64 | ≥64 | ≥64 | 32–≥64 | 256 | ||
| Ampho | 1 | 2 | 1 | 0.25–2 | 4 | 4 | ||
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–≥8 | 0.25 | |||
| Casp | 0.5 | 4 | ≤0.06 | ≤0.06–≥8 | 0.25 | |||
| Fluc | ≥128 | ≥128 | ≥128 | 32–≥128 | 512 | |||
| ISAV | 0.25 | 0.5 | 0.25 | 0.12–0.5 | 0.5 | 1 | ||
| Itra | ≤0.06 | 0.25 | ≤0.06 | ≤0.06–1 | 0.25 | 0.25 | ||
| Keto | 0.12 | 0.5 | 0.12 | ≤0.06–0.5 | 2 | 0.5 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–≥8 | 0.25 | |||
| Pos | ≤0.06 | 0.12 | ≤0.06 | ≤0.06–0.5 | 0.25 | |||
| Vori | 0.25 | 0.5 | 0.25 | 0.12–1 | 1 | 1 | ||
| Aspergillus niger complex (n = 92) | 5-FC | 32 | ≥64 | ≥64 | 1–≥64 | 256 | ||
| Ampho | 0.5 | 1 | 0.5 | ≤0.12–2 | 2 | 2 | ||
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Casp | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | 0.25 | ||
| Fluc | ≥128 | ≥128 | ≥128 | ≤0.5–≥128 | 512 | |||
| ISAV | 4 | 8 | 4 | 0.12–≥16 | 8 | 16 | 4 | |
| Itra | 0.5 | ≥16 | 0.5 | ≤0.06–≥16 | 1 | 2 | 4 | |
| Keto | 8 | ≥16 | 8 | ≤0.06–≥16 | 64 | 32 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Pos | 0.12 | 0.25 | 0.12 | ≤0.06–0.5 | 0.5 | 0.5 | 2 | |
| Vori | 2 | 2 | 2 | 0.06–4 | 4 | 8 | 2 | |
| Aspergillus terreus complex (n = 24) | 5-FC | ≥64 | ≥64 | ≥64 | 1–≥64 | 256 | ||
| Ampho | 2 | 4 | 4 | 0.5–≥8 | 8 | 16 | 4 | |
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Casp | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06–0.12 | 0.25 | 0.12 | ||
| Fluc | ≥128 | ≥128 | ≥128 | ≤0.5–≥128 | 512 | |||
| ISAV | 0.5 | 1 | 0.5 | 0.12–2 | 2 | 2 | 1 | |
| Itra | ≤0.06 | 0.12 | ≤0.06 | ≤0.06–0.25 | 0.25 | 2 | ||
| Keto | 0.5 | 1 | 0.5 | 0.12–2 | 2 | 2 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Pos | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | 1 | ||
| Vori | 0.5 | 1 | 0.5 | 0.12–2 | 1 | 2 | 2 | |
| 5-FC | ≥64 | ≥64 | ≥64 | ≥64 | 256 | |||
| Aspergillus lentulus (n = 9) | Ampho | 4 | ≥8 | 4 | 1–≥8 | 8 | 16 | |
| Anid | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Casp | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Fluc | ≥128 | ≥128 | ≥128 | ≤0.5–≥128 | 512 | |||
| ISAV | 4 | 8 | 4 | 2–8 | 8 | 16 | ||
| Itra | 0.25 | 2 | 0.25 | 0.25–2 | 1 | |||
| Keto | 8 | ≥16 | 8 | 2–≥16 | 8 | 32 | ||
| Mica | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 | 0.25 | |||
| Pos | ≤0.06 | 0.5 | ≤0.06 | ≤0.06–0.5 | 0.25 | |||
| Vori | 2 | 4 | 2 | 0.25–4 | 8 | 8 |
| Organisms. | Agent | MIC/MEC50 | MIC/MEC90 | Mode | Range | ECV (97.5%) | wt-UL; (Mode + 2) |
|---|---|---|---|---|---|---|---|
| Fusarium spp. (n = 26) | 5-FC | ≥64 | ≥64 | ≥64 | ≥64 | 256 | |
| Ampho | 4 | ≥8 | 4 | 1–≥8 | 8 | 16 | |
| Anid | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Casp | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Fluc | ≥128 | ≥128 | ≥128 | 4–≥128 | 512 | ||
| ISAV | ≥16 | ≥16 | ≥16 | 8–≥16 | 64 | ||
| Itra | ≥16 | ≥16 | ≥16 | ≥16 | 64 | ||
| Keto | ≥16 | ≥16 | ≥16 | 2–≥16 | 64 | ||
| Mica | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Pos | ≥16 | ≥16 | ≥16 | 1–≥16 | 64 | ||
| Vori | ≥16 | ≥16 | ≥16 | 0.5–≥16 | 64 | ||
| Scedosporium spp. (n = 22) | 5-FC | ≥64 | ≥64 | ≥64 | 32–≥64 | 256 | |
| Ampho | ≥8 | ≥8 | ≥8 | 1–≥8 | 32 | ||
| Anid | 4 | ≥8 | 4 | ≤0.06–≥8 | 4 | 16 | |
| Casp | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Fluc | 16 | 64 | 16 | 8–≥128 | 32 | 64 | |
| ISAV | 8 | ≥16 | 8 | 0.5–≥16 | 32 | 32 | |
| Itra | 4 | ≥16 | ≥16 | 0.5–≥16 | 64 | ||
| Keto | 0.5 | 1 | 0.5 | ≤0.06–4 | 2 | 2 | |
| Mica | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Pos | 1 | 2 | 1 | 0.5–≥16 | 2 | 4 | |
| Vori | 1 | 4 | 1 | 0.12–≥16 | 4 | 4 | |
| Lomentospora prolificans (n = 17) | 5-FC | ≥64 | ≥64 | ≥64 | ≥64 | 256 | |
| Ampho | ≥8 | ≥8 | ≥8 | 4–≥8 | 32 | ||
| Anid | 4 | ≥8 | 4 | 2–≥8 | 4 | 16 | |
| Casp | ≥8 | ≥8 | ≥8 | 2–≥8 | 32 | ||
| Fluc | ≥128 | ≥128 | ≥128 | 16–≥128 | 512 | ||
| ISAV | ≥16 | ≥16 | ≥16 | 4–≥16 | 64 | ||
| Itra | ≥16 | ≥16 | ≥16 | 1–≥16 | 64 | ||
| Keto | ≥16 | ≥16 | ≥16 | 0.5–≥16 | 64 | ||
| Mica | ≥8 | ≥8 | ≥8 | 0.25–≥8 | 32 | ||
| Pos | ≥16 | ≥16 | ≥16 | 0.5–≥16 | 64 | ||
| Vori | ≥16 | ≥16 | ≥16 | 0.5–≥16 | 64 | ||
| Zygomycetes (n = 49) | 5-FC | ≥64 | ≥64 | ≥64 | 32–≥64 | 256 | |
| Ampho | 1 | 2 | 1 | ≤0.12–≥8 | 2 | 4 | |
| Anid | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Casp | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Fluc | ≥128 | ≥128 | ≥128 | 16–≥128 | 512 | ||
| ISAV | 4 | ≥16 | 2 | 0.5–≥16 | 8 | 8 | |
| Itra | 1 | ≥16 | 1 | 0.12–≥16 | 4 | 4 | |
| Keto | 1 | 4 | 1 | 0.25–≥16 | 8 | 4 | |
| Mica | ≥8 | ≥8 | ≥8 | ≤0.06–≥8 | 32 | ||
| Pos | 0.5 | 32 | 0.5 | ≤0.06–≥16 | 2 | 2 | |
| Vori | ≥16 | ≥16 | ≥16 | 2–≥16 | 64 |
| 5-FC (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |
| Aspergillus flavus/oryzae | 1 | 5 | 17 | |||||||||
| Aspergillus fumigatus | 1 | 1 | 3 | 14 | 194 | |||||||
| Aspergillus nidulans | 1 | 23 | ||||||||||
| Aspergillus niger complex | 1 | 1 | 8 | 17 | 18 | 20 | 27 | |||||
| Aspergillus terreus complex | 1 | 1 | 1 | 21 | ||||||||
| Aspergillus lentulus | 9 | |||||||||||
| Fusarium spp. | 26 | |||||||||||
| Scedosporium spp. | 1 | 19 | ||||||||||
| Lomentospora prolificans | 17 | |||||||||||
| Zygomycetes | 1 | 48 | ||||||||||
| Ampho (MIC; µg/mL) | ≤0.12 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | |||||
| Aspergillus flavus/oryzae | 1 | 5 | 8 | 8 | 1 | |||||||
| Aspergillus fumigatus | 2 | 32 | 80 | 98 | 2 | |||||||
| Aspergillus nidulans | 1 | 7 | 9 | 7 | ||||||||
| Aspergillus niger complex | 2 | 10 | 55 | 18 | 7 | |||||||
| Aspergillus terreus complex | 1 | 4 | 7 | 10 | 2 | |||||||
| Aspergillus lentulus | 1 | 3 | 4 | 1 | ||||||||
| Fusarium spp. | 4 | 6 | 10 | 6 | ||||||||
| Scedosporium spp. | 1 | 6 | 13 | |||||||||
| Lomentospora prolificans | 2 | 15 | ||||||||||
| Zygomycetes | 3 | 3 | 9 | 25 | 6 | 3 | ||||||
| Anid (MIC/MEC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | ||||
| Aspergillus flavus/oryzae | 23 | |||||||||||
| Aspergillus fumigatus | 209 | 2 | 2 | 1 | ||||||||
| Aspergillus nidulans | 22 | 2 | ||||||||||
| Aspergillus niger complex | 92 | |||||||||||
| Aspergillus terreus complex | 24 | |||||||||||
| Aspergillus lentulus | 9 | |||||||||||
| Fusarium spp. | 2 | 1 | 23 | |||||||||
| Scedosporium spp. | 1 | 3 | 13 | 3 | ||||||||
| Lomentospora prolificans | 3 | 11 | 3 | |||||||||
| Zygomycetes | 1 | 12 | 36 | |||||||||
| Casp (MIC/MEC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | ||||
| Aspergillus flavus/oryzae | 23 | |||||||||||
| Aspergillus fumigatus | 209 | 3 | 1 | 1 | ||||||||
| Aspergillus nidulans | 8 | 2 | 3 | 8 | 1 | 2 | ||||||
| Aspergillus niger complex | 92 | |||||||||||
| Aspergillus terreus complex | 23 | 1 | ||||||||||
| Aspergillus lentulus | 9 | |||||||||||
| Fusarium spp. | 2 | 1 | 23 | |||||||||
| Scedosporium spp. | 1 | 1 | 1 | 2 | 3 | 12 | ||||||
| Lomentospora prolificans | 1 | 2 | 14 | |||||||||
| Zygomycetes | 1 | 1 | 47 | |||||||||
| Fluc (MIC; µg/mL) | ≤0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | ≥128 | |||
| Aspergillus flavus/oryzae | 2 | 7 | 14 | |||||||||
| Aspergillus fumigatus | 5 | 2 | 1 | 3 | 15 | 188 | ||||||
| Aspergillus nidulans | 4 | 7 | 13 | |||||||||
| Aspergillus niger complex | 2 | 1 | 1 | 5 | 7 | 76 | ||||||
| Aspergillus terreus complex | 1 | 1 | 2 | 6 | 14 | |||||||
| Aspergillus lentulus | 1 | 1 | 7 | |||||||||
| Fusarium spp. | 1 | 1 | 24 | |||||||||
| Scedosporium spp. | 6 | 7 | 3 | 3 | 1 | |||||||
| Lomentospora prolificans | 1 | 16 | ||||||||||
| Zygomycetes | 2 | 2 | 6 | 38 | ||||||||
| ISAV (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |||
| Aspergillus flavus/oryzae | 4 | 11 | 6 | 2 | ||||||||
| Aspergillus fumigatus | 3 | 45 | 124 | 32 | 5 | 2 | 3 | |||||
| Aspergillus nidulans | 7 | 12 | 5 | |||||||||
| Aspergillus niger complex | 1 | 1 | 1 | 22 | 43 | 20 | 4 | |||||
| Aspergillus terreus complex | 2 | 3 | 10 | 7 | 2 | |||||||
| Aspergillus lentulus | 3 | 4 | 2 | |||||||||
| Fusarium spp. | 1 | 25 | ||||||||||
| Scedosporium spp. | 1 | 3 | 1 | 9 | 6 | |||||||
| Lomentospora prolificans | 1 | 2 | 14 | |||||||||
| Zygomycetes | 2 | 8 | 14 | 14 | 1 | 10 | ||||||
| Itra (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |||
| Aspergillus flavus/oryzae | 7 | 11 | 3 | 2 | ||||||||
| Aspergillus fumigatus | 34 | 104 | 57 | 14 | 2 | 1 | 2 | |||||
| Aspergillus nidulans | 13 | 8 | 2 | 1 | ||||||||
| Aspergillus niger complex | 2 | 18 | 31 | 11 | 2 | 1 | 1 | 26 | ||||
| Aspergillus terreus complex | 19 | 4 | 1 | |||||||||
| Aspergillus lentulus | 7 | 1 | 1 | |||||||||
| Fusarium spp. | 26 | |||||||||||
| Scedosporium spp. | 1 | 5 | 3 | 2 | 2 | 7 | ||||||
| Lomentospora prolificans | 1 | 16 | ||||||||||
| Zygomycetes | 1 | 8 | 18 | 12 | 2 | 2 | 6 | |||||
| Keto (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |||
| Aspergillus flavus/oryzae | 4 | 7 | 10 | 2 | ||||||||
| Aspergillus fumigatus | 3 | 2 | 1 | 8 | 20 | 57 | 96 | 23 | 4 | |||
| Aspergillus nidulans | 7 | 9 | 3 | 5 | ||||||||
| Aspergillus niger complex | 2 | 2 | 1 | 5 | 9 | 22 | 31 | 20 | ||||
| Aspergillus terreus complex | 1 | 6 | 10 | 5 | 2 | |||||||
| Aspergillus lentulus | 1 | 1 | 5 | 2 | ||||||||
| Fusarium spp. | 2 | 3 | 21 | |||||||||
| Scedosporium spp. | 2 | 2 | 9 | 5 | 1 | 1 | ||||||
| Lomentospora prolificans | 1 | 1 | 2 | 4 | 9 | |||||||
| Zygomycetes | 5 | 7 | 18 | 8 | 8 | 2 | 1 | |||||
| Mica (MIC/MEC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | ||||
| Aspergillus flavus/oryzae | 23 | |||||||||||
| Aspergillus fumigatus | 211 | 1 | 1 | 1 | ||||||||
| Aspergillus nidulans | 22 | 2 | ||||||||||
| Aspergillus niger complex | 92 | |||||||||||
| Aspergillus terreus complex | 24 | |||||||||||
| Aspergillus lentulus | 9 | |||||||||||
| Fusarium spp. | 2 | 2 | 22 | |||||||||
| Scedosporium spp. | 1 | 2 | 1 | 4 | 12 | |||||||
| Lomentospora prolificans | 1 | 2 | 3 | 11 | ||||||||
| Zygomycetes | 1 | 1 | 47 | |||||||||
| Pos (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |||
| Aspergillus flavus/oryzae | 17 | 3 | 3 | |||||||||
| Aspergillus fumigatus | 197 | 11 | 4 | 2 | ||||||||
| Aspergillus nidulans | 17 | 5 | 1 | 1 | ||||||||
| Aspergillus niger complex | 28 | 46 | 15 | 3 | ||||||||
| Aspergillus terreus complex | 24 | |||||||||||
| Aspergillus lentulus | 6 | 2 | 1 | |||||||||
| Fusarium spp. | 1 | 3 | 22 | |||||||||
| Scedosporium spp. | 2 | 11 | 5 | 2 | ||||||||
| Lomentospora prolificans | 1 | 16 | ||||||||||
| Zygomycetes | 1 | 8 | 16 | 11 | 7 | 1 | 5 | |||||
| Vori (MIC; µg/mL) | ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |||
| Aspergillus flavus/oryzae | 6 | 14 | 3 | |||||||||
| Aspergillus fumigatus | 1 | 30 | 109 | 56 | 13 | 3 | 1 | 1 | ||||
| Aspergillus nidulans | 3 | 10 | 9 | 2 | ||||||||
| Aspergillus niger complex | 1 | 1 | 3 | 28 | 51 | 8 | ||||||
| Aspergillus terreus complex | 1 | 9 | 11 | 2 | 1 | |||||||
| Aspergillus lentulus | 1 | 5 | 3 | |||||||||
| Fusarium spp. | 1 | 1 | 2 | 7 | 15 | |||||||
| Scedosporium spp. | 1 | 1 | 5 | 6 | 4 | 2 | 1 | |||||
| Lomentospora prolificans | 1 | 1 | 14 | |||||||||
| Zygomycetes | 1 | 5 | 14 | 29 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, H.A.; Dadwal, S.S.; She, R.C. Antifungal Minimal Inhibitory Concentrations of Mold Isolates from Patients with Cancer; Single-Center Experience, 2018–2023. J. Fungi 2025, 11, 518. https://doi.org/10.3390/jof11070518
Mahmud HA, Dadwal SS, She RC. Antifungal Minimal Inhibitory Concentrations of Mold Isolates from Patients with Cancer; Single-Center Experience, 2018–2023. Journal of Fungi. 2025; 11(7):518. https://doi.org/10.3390/jof11070518
Chicago/Turabian StyleMahmud, Hafij Al, Sanjeet Singh Dadwal, and Rosemary C. She. 2025. "Antifungal Minimal Inhibitory Concentrations of Mold Isolates from Patients with Cancer; Single-Center Experience, 2018–2023" Journal of Fungi 11, no. 7: 518. https://doi.org/10.3390/jof11070518
APA StyleMahmud, H. A., Dadwal, S. S., & She, R. C. (2025). Antifungal Minimal Inhibitory Concentrations of Mold Isolates from Patients with Cancer; Single-Center Experience, 2018–2023. Journal of Fungi, 11(7), 518. https://doi.org/10.3390/jof11070518






