Functional Characterization of Deubiquitinase UBP Family and Proteomic Analysis of Aaubp14-Mediated Pathogenicity Mechanism in Alternaria alternata
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. DNA and RNA Extraction, Gene Cloning, and Sequence Analysis
2.3. Generate Gene Deletion Mutants and Complementation Strains
2.4. Morphological Features Analysis
2.5. Pathogenicity Assay
2.6. Toxicity Test and UHPLC-MS/MS Analysis of ACT-Toxin
2.7. Quantitative Real-Time PCR
2.8. Carbon Sources Utilization Test
2.9. Protein Extraction and Western Blotting
2.10. LC–MS/MS Analysis of Ubiquitylated Peptides
2.11. Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
3.1. Characterization of UBPs in the Tangerine Pathotype of A. alternata
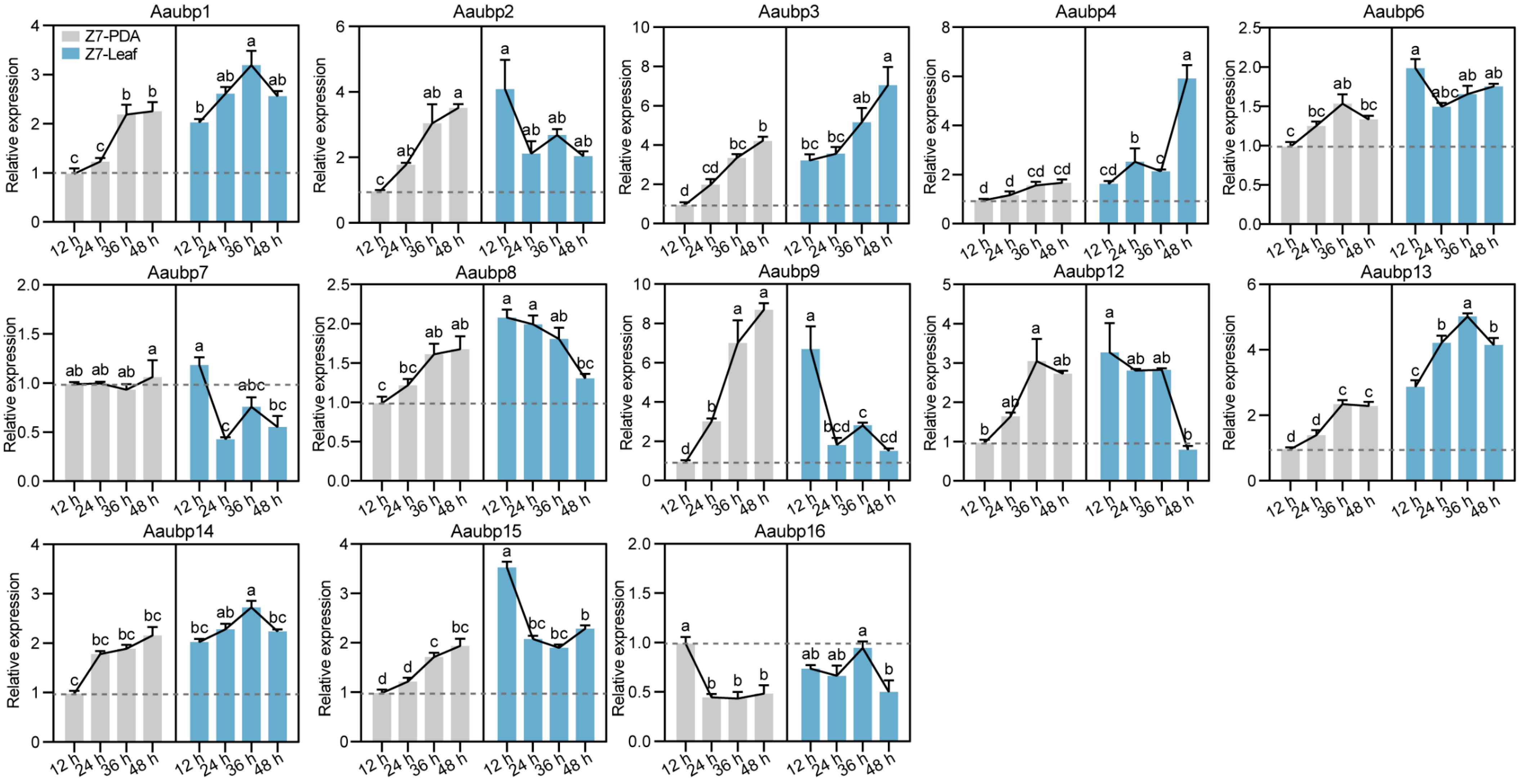
3.2. Expression Dynamics of Aaubp Genes During Vegetative Growth and Infection
3.3. Characterization of Vegetative Growth of the ΔAaubp Mutants
3.4. Characterization of Conidiation and Conidial Morphology of the ΔAaubp Mutants
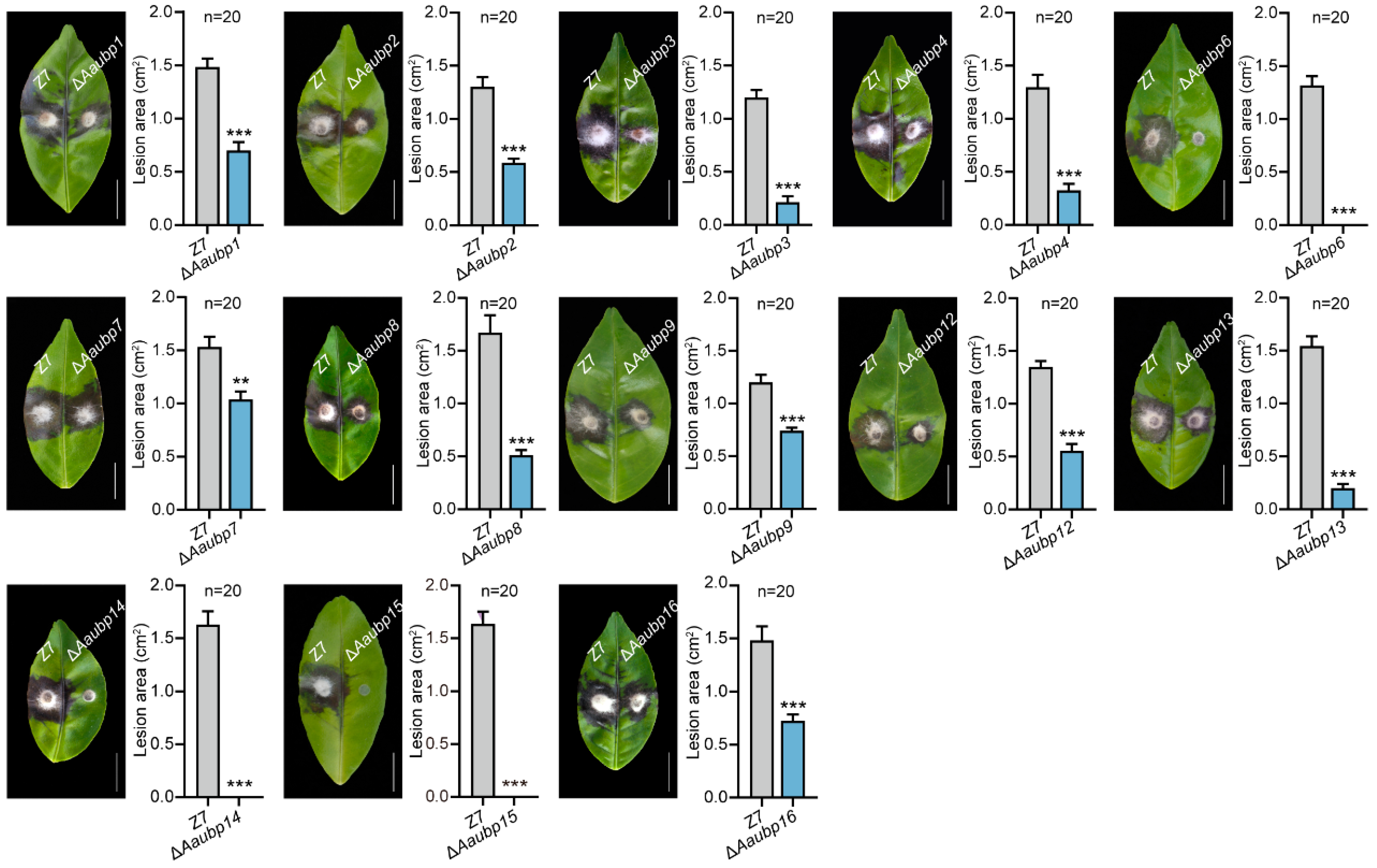
3.5. The Aaubp Genes Are Involved in Pathogenicity
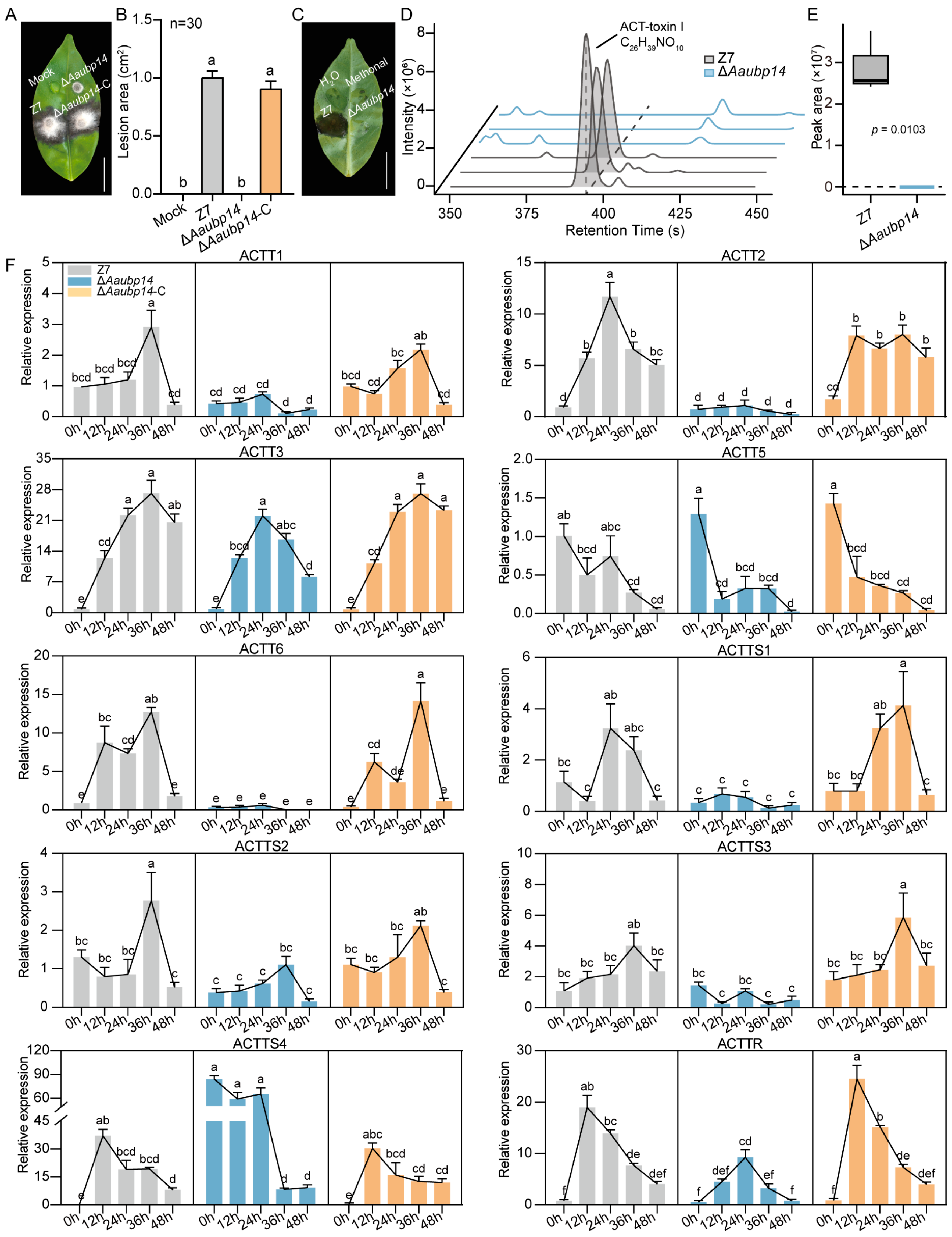
3.6. Deletion of Aaubp14 Disrupts the Biosynthesis of ACT-Toxin
3.7. Quantitative Proteomics Analysis of Hyper-Ubiquitinated Proteins in ΔAaubp14
3.8. Functional Insights into Aaubp14-Mediated Ubiquitinated Proteins
3.9. Aaubp14 Regulates the Expression of Virulence-Related Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peever, T.L.; Ibañez, A.; Akimitsu, K.; Timmer, L.W. Worldwide Phylogeography of the Citrus Brown Spot Pathogen. Alternaria alternata. Phytopathology 2002, 92, 794–802. [Google Scholar] [CrossRef]
- Akimitsu, K.; Peever, T.L.; Timmer, L.W. Molecular, Ecological and Evolutionary Approaches to Understanding Alternaria Diseases of Citrus. Mol. Plant Pathol. 2003, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fu, Y.; Nie, D.; Stewart, J.E.; Peever, T.L.; Li, H. Identification of a Novel Phylogenetic Lineage of Alternaria alternata Causing Citrus Brown Spot in China. Fungal Biol. 2015, 119, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Timmer, L.W.; Solel, Z.; Orozco-Santos, M. Alternaria Brown Spot of Mandarins. In Compendium of Citrus Diseases; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2000; pp. 19–21. [Google Scholar]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-Selective Toxins Produced by the Plant Pathogenic Fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Yu, D.; Xu, J.; Chung, K.R.; Li, H. Genomic and Transcriptomic Analyses of the Tangerine Pathotype of Alternaria alternata in Response to Oxidative Stress. Sci. Rep. 2016, 6, 32437. [Google Scholar] [CrossRef]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and Biological Activities of Two Host-Specific Toxins from the Tangerine Pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Gai, Y.; Ma, H.; Chen, Y.; Li, L.; Cao, Y.; Wang, M.; Sun, X.; Jiao, C.; Riely, B.K.; Li, H. Chromosome-Scale Genome Sequence of Alternaria alternata Causing Alternaria Brown Spot of Citrus. Mol. Plant-Microbe Interact. 2021, 34, 726–732. [Google Scholar] [CrossRef]
- Johnson, L.J.; Johnson, R.D.; Akamatsu, H.; Salamiah, A.; Otani, H.; Kohmoto, K.; Kodama, M. Spontaneous Loss of a Conditionally Dispensable Chromosome from the Alternaria alternata Apple Pathotype Leads to Loss of Toxin Production and Pathogenicity. Curr. Genet. 2001, 40, 65–72. [Google Scholar] [CrossRef]
- Hatta, R.; Ito, K.; Hosaki, Y.; Tanaka, T.; Tanaka, A.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. A Conditionally Dispensable Chromosome Controls Host-Specific Pathogenicity in the Fungal Plant Pathogen Alternaria alternata. Genetics 2002, 161, 59–70. [Google Scholar] [CrossRef]
- Masunaka, A.; Tanaka, A.; Tsuge, T.; Peever, T.L.; Timmer, L.W.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. Distribution and Characterization of AKT Homologs in the Tangerine Pathotype of Alternaria alternata. Phytopathology 2000, 90, 762–768. [Google Scholar] [CrossRef]
- Ajiro, N.; Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Izumi, Y.; et al. Role of the Host-Selective ACT-Toxin Synthesis Gene ACTTS2 Encoding an Enoyl-Reductase in Pathogenicity of the Tangerine Pathotype of Alternaria alternata. Phytopathology 2010, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Ishii, Y.; Honda, A.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; et al. Function of Genes Encoding Acyl-coA Synthetase and Enoyl-coA Hydratase for Host-Selective ACT-Toxin Biosynthesis in the Tangerine Pathotype of Alternaria alternata. Phytopathology 2009, 99, 369–377. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Tada, Y.; Ichimura, K.; et al. ACTTS3 Encoding a Polyketide Synthase Is Essential for the Biosynthesis of ACT-Toxin and Pathogenicity in the Tangerine Pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2010, 23, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, H.; Zheng, F.; Chen, Y.; Wang, M.; Jiao, C.; Li, H.; Gai, Y. The Transcription Regulator ACTR Controls ACT-Toxin Biosynthesis and Pathogenicity in the Tangerine Pathotype of Alternaria alternata. Microbiol. Res. 2021, 248, 126747. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, H.; Shen, X.; Ruan, R.; Rokas, A.; Li, H. Genomic Features and Evolution of the Conditionally Dispensable Chromosome in the Tangerine Pathotype of Alternaria alternata. Mol. Plant Pathol. 2019, 20, 1425–1438. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Wang, N.Y.; Chung, K.R. The FUS3 MAPK Signaling Pathway of the Citrus Pathogen Alternaria alternata Functions Independently or Cooperatively with the Fungal Redox-Responsive AP1 Regulator for Diverse Developmental, Physiological and Pathogenic Processes. Fungal Genet. Biol. 2010, 47, 381–391. [Google Scholar] [CrossRef]
- Lin, C.H.; Chung, K.R. Specialized and Shared Functions of the Histidine Kinase- and HOG1 MAP Kinase-Mediated Signaling Pathways in Alternaria alternata, a Filamentous Fungal Pathogen of Citrus. Fungal Genet. Biol. 2010, 47, 818–827. [Google Scholar] [CrossRef]
- Yago, J.I.; Lin, C.H.; Chung, K.R. The SLT2 Mitogen-activated Protein Kinase-mediated Signalling Pathway Governs Conidiation, Morphogenesis, Fungal Virulence and Production of Toxin and Melanin in the Tangerine Pathotype of Alternaria alternata. Mol. Plant Pathol. 2011, 12, 653–665. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.H.; Chung, K.R. Roles for SKN7 Response Regulator in Stress Resistance, Conidiation and Virulence in the Citrus Pathogen Alternaria alternata. Fungal Genet. Biol. 2012, 49, 802–813. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chung, K.R. Calcineurin Phosphatase and Phospholipase C Are Required for Developmental and Pathological Functions in the Citrus Fungal Pathogen Alternaria alternata. Microbiology 2014, 160, 1453–1465. [Google Scholar] [CrossRef][Green Version]
- Yu, P.L.; Chen, L.H.; Chung, K.R. How the Pathogenic Fungus Alternaria alternata Copes with Stress via the Response Regulators SSK1 and SHO1. PLoS ONE 2016, 11, e0149153. [Google Scholar] [CrossRef]
- Fu, H.; Chung, K.R.; Gai, Y.; Mao, L.; Li, H. The Basal Transcription Factor II H Subunit Tfb5 Is Required for Stress Response and Pathogenicity in the Tangerine Pathotype of Alternaria alternata. Mol. Plant Pathol. 2020, 21, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, Y.; Gai, Y.; Ma, H.; Zhu, Z.; Chung, K.R.; Li, H. Genome-Wide Identification and Functional Characterization of GATA Transcription Factor Gene Family in Alternaria alternata. J. Fungi 2021, 7, 1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, Y.; Jiao, C.; Sun, X.; Gai, Y.; Zhu, Z.; Li, H. The Alternaria alternata StuA Transcription Factor Interacting with the pH-Responsive Regulator PacC for the Biosynthesis of Host-Selective Toxin and Virulence in Citrus. Microbiol. Spectr. 2023, 11, e0233523. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.H.; Chung, K.R. A Nonribosomal Peptide Synthetase Mediates Siderophore Production and Virulence in the Citrus Fungal Pathogen Alternaria alternata. Mol. Plant Pathol. 2013, 14, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Chen, C.W.; Choo, C.Y.L.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Proper Functions of Peroxisomes Are Vital for Pathogenesis of Citrus Brown Spot Disease Caused by Alternaria alternata. J. Fungi 2020, 6, 248. [Google Scholar] [CrossRef]
- Wu, P.C.; Choo, C.Y.L.; Lu, H.Y.; Wei, X.Y.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Pexophagy Is Critical for Fungal Development, Stress Response, and Virulence in Alternaria alternata. Mol. Plant Pathol. 2022, 23, 1538–1554. [Google Scholar] [CrossRef]
- Choo, C.Y.L.; Wu, P.C.; Yago, J.I.; Chung, K.R. The Pex3-Mediated Peroxisome Biogenesis Plays a Critical Role in Metabolic Biosynthesis, Stress Response, and Pathogenicity in Alternaria alternata. Microbiol. Res. 2023, 266, 127236. [Google Scholar] [CrossRef]
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The Ubiquitin System. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Grabbe, C.; Husnjak, K.; Dikic, I. The Spatial and Temporal Organization of Ubiquitin Networks. Nat. Rev. Mol. Cell Biol. 2011, 12, 295–307. [Google Scholar] [CrossRef]
- Pickart, C.M. Mechanisms Underlying Ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and Function of Deubiquitinating Enzymes. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1695, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Turcu, F.E.; Ventii, K.H.; Wilkinson, K.D. Regulation and Cellular Roles of Ubiquitin-Specific Deubiquitinating Enzymes. Annu. Rev. Biochem. 2009, 78, 363–397. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A Genomic and Functional Inventory of Deubiquitinating Enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef]
- Wang, W.; Cai, X.; Chen, X. Recent Progress of Deubiquitinating Enzymes in Human and Plant Pathogenic Fungi. Biomolecules 2022, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, Z.; Hou, Y.; Liu, C.; Hendy, A.; Xing, J.; Chen, X. Systematic Characterization of the Ubiquitin-Specific Proteases in Magnaporthe oryzae. Phytopathol. Res. 2020, 2, 8. [Google Scholar] [CrossRef]
- Cai, X.; Xiang, S.; He, W.; Tang, M.; Zhang, S.; Chen, D.; Zhang, X.; Liu, C.; Li, G.; Xing, J.; et al. Deubiquitinase Ubp3 Regulates Ribophagy and Deubiquitinates Smo1 for Appressorium-mediated Infection by Magnaporthe oryzae. Mol. Plant Pathol. 2022, 23, 832–844. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.; Matar, K.A.O.; Zheng, T.; Zhao, Q.; Xie, Y.; Gao, X.; Li, M.; Wang, B.; Lu, G. The Deubiquitinating Enzyme MoUbp8 Is Required for Infection-Related Development, Pathogenicity, and Carbon Catabolite Repression in Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2020, 104, 5081–5094. [Google Scholar] [CrossRef]
- Que, Y.; Xu, Z.; Wan, C.; Lv, W.; Yue, X.; Xu, L.; Tang, S.; Dai, H.; Wang, Z. The Putative Deubiquitinating Enzyme MoUbp4 Is Required for Infection-Related Morphogenesis and Pathogenicity in the Rice Blast Fungus Magnaporthe oryzae. Curr. Genet. 2020, 66, 561–576. [Google Scholar] [CrossRef]
- Chen, A.; Han, X.; Liu, C.; Zhou, Y.; Ren, Y.; Shen, X.; Shim, W.B.; Chai, Y.; Ma, Z.; Chen, Y. Profiling of Deubiquitinases That Control Virulence in the Pathogenic Plant Fungus Fusarium graminearum. New Phytol. 2024, 242, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, X.; Ruan, R.; Fu, H.; Li, H. Csn5 Is Required for the Conidiogenesis and Pathogenesis of the Alternaria alternata Tangerine Pathotype. Front. Microbiol. 2018, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Lv, W.; Zhang, Q.; Zhou, C. Coding the α-Subunit of SNF1 Kinase, Snf1 Is Required for the Conidiogenesis and Pathogenicity of the Alternaria alternata Tangerine Pathotype. Fungal Biol. 2020, 124, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Han, Y.; Yang, L.; Wu, M.; Zhang, J.; Cheng, J.; Wang, M.; Jiang, D.; Chen, W.; Li, G. CmpacC Regulates Mycoparasitism, Oxalate Degradation and Antifungal Activity in the Mycoparasitic Fungus Coniothyrium minitans. Environ. Microbiol. 2015, 17, 4711–4729. [Google Scholar] [CrossRef]
- Goswami, R.S. Targeted Gene Replacement in Fungi Using a Split-Marker Approach. In Plant Fungal Pathogens; Bolton, M., Thomma, B., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 835, ISBN 978-1-61779-500-8. [Google Scholar]
- Zhou, Y.J.; Zhang, J.; Wang, X.D.; Yang, L.; Jiang, D.H.; Li, G.Q.; Hsiang, T.; Zhuang, W.Y. Morphological and Phylogenetic Identification of Botrytis sinoviticola, a Novel Cryptic Species Causing Gray Mold Disease of Table Grapes in China. Mycologia 2014, 106, 43–56. [Google Scholar] [CrossRef]
- Kong, L.; Yang, J.; Li, G.; Qi, L.; Zhang, Y.; Wang, C.; Zhao, W.; Xu, J.; Peng, Y. Different Chitin Synthase Genes Are Required for Various Developmental and Plant Infection Processes in the Rice Blast Fungus Magnaporthe oryzae. PLoS Pathog. 2012, 8, e1002526. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, C.; Xing, J.; Chen, X. A Deubiquitinating Enzyme Ubp14 Is Required for Development, Stress Response, Nutrient Utilization, and Pathogenesis of Magnaporthe oryzae. Front. Microbiol. 2018, 9, 769. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Yao, Y.; Xu, L.; Xiang, Y.; Zhao, H.; Wang, L.; Zuo, Z.; Huang, X.; Zhao, C. Flubendazole Demonstrates Valid Antitumor Effects by Inhibiting STAT3 and Activating Autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 293. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Consortium The Gene Ontology Resource: 20 Years and Still GOing Strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]
- Gong, W.; Liu, X.; Lv, X.; Zhang, Y.; Niu, Y.; Jin, K.; Li, B.; Zuo, Q. Ubiquitination Plays an Important Role during the Formation of Chicken Primordial Germ Cells. J. Anim. Sci. 2024, 102, skae251. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape Stringapp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Finley, D.; Ulrich, H.D.; Sommer, T.; Kaiser, P. The Ubiquitin–Proteasome System of Saccharomyces cerevisiae. Genetics 2012, 192, 319–360. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Akimitsu, K. Functional Analysis of a Multicopy Host-Selective ACT-Toxin Biosynthesis Gene in the Tangerine Pathotype of Alternaria alternata Using RNA Silencing. Mol. Plant-Microbe Interact. 2008, 21, 1591–1599. [Google Scholar] [CrossRef]
- Meng, S.; Huang, S.; Liu, J.; Gai, Y.; Li, M.; Duan, S.; Zhang, S.; Sun, X.; Yang, Q.; Wang, Y.; et al. Histone Methylation Is Required for Virulence, Conidiation, and Multi-Stress Resistance of Alternaria alternata. Front. Microbiol. 2022, 13, 924476. [Google Scholar] [CrossRef]
- Ma, H.; Li, L.; Gai, Y.; Zhang, X.; Chen, Y.; Zhuo, X.; Cao, Y.; Jiao, C.; Gmitter, F.G.; Li, H. Histone Acetyltransferases and Deacetylases Are Required for Virulence, Conidiation, DNA Damage Repair, and Multiple Stresses Resistance of Alternaria alternata. Front. Microbiol. 2021, 12, 783633. [Google Scholar] [CrossRef]
- Tsai, H.C.; Yang, S.L.; Chung, K.R. Cyclic AMP-Dependent Protein Kinase A Negatively Regulates Conidia Formation by the Tangerine Pathotype of Alternaria Alternata. World J. Microbiol. Biotechnol. 2013, 29, 289–300. [Google Scholar] [CrossRef]
- Wang, P.H.; Wu, P.C.; Huang, R.; Chung, K.R. The Role of a Nascent Polypeptide-Associated Complex Subunit Alpha in Siderophore Biosynthesis, Oxidative Stress Response, and Virulence in Alternaria alternata. Mol. Plant-Microbe Interact. 2020, 33, 668–679. [Google Scholar] [CrossRef]
- Ma, H.; Wang, M.; Gai, Y.; Fu, H.; Zhang, B.; Ruan, R.; Chung, K.-R.; Li, H. Thioredoxin and Glutaredoxin Systems Required for Oxidative Stress Resistance, Fungicide Sensitivity, and Virulence of Alternaria alternata. Appl. Environ. Microbiol. 2018, 84, e0008618. [Google Scholar] [CrossRef]
- Oh, E.; Akopian, D.; Rape, M. Principles of Ubiquitin-Dependent Signaling. Annu. Rev. Cell Dev. Biol. 2018, 34, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Mevissen, T.E.T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, M.; Papa, F.R.; Chen, P.; Swaminathan, S.; Johnson, P.; Stillman, L.; Amerik, A.Y.; Li, S.-J. The DOA Pathway: Studies on the Functions and Mechanisms of Ubiquitin-Dependent Protein Degradation in the Yeast Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 1995, 60, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhou, Y.; Ren, Y.; Liu, C.; Han, X.; Wang, J.; Ma, Z.; Chen, Y. Ubiquitination of Acetyltransferase Gcn5 Contributes to Fungal Virulence in Fusarium graminearum. mBio 2023, 14, e0149923. [Google Scholar] [CrossRef]
- Hämmerle, M.; Bauer, J.; Rose, M.; Szallies, A.; Thumm, M.; Düsterhus, S.; Mecke, D.; Entian, K.-D.; Wolf, D.H. Proteins of Newly Isolated Mutants and the Amino-Terminal Proline Are Essential for Ubiquitin-Proteasome-Catalyzed Catabolite Degradation of Fructose-1,6-Bisphosphatase of Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 25000–25005. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Li, H.; Chen, H.; Huang, B. The Deubiquitinating Enzyme MrUbp14 Is Involved in Conidiation, Stress Response, and Pathogenicity in Metarhizium robertsii. Front. Fungal Biol. 2022, 3, 896466. [Google Scholar] [CrossRef]
- Eisele, F.; Braun, B.; Pfirrmann, T.; Wolf, D.H. Mutants of the Deubiquitinating Enzyme Ubp14 Decipher Pathway Diversity of Ubiquitin–Proteasome Linked Protein Degradation. Biochem. Biophys. Res. Commun. 2006, 350, 329–333. [Google Scholar] [CrossRef]
- Li, Y.; Lan, Q.; Gao, Y.; Xu, C.; Xu, Z.; Wang, Y.; Chang, L.; Wu, J.; Deng, Z.; He, F.; et al. Ubiquitin Linkage Specificity of Deubiquitinases Determines Cyclophilin Nuclear Localization and Degradation. iScience 2020, 23, 100984. [Google Scholar] [CrossRef]
- Amerik, A.Y.; Swaminathan, S.; Krantz, B.A.; Wilkinson, K.D.; Hochstrasser, M. In Vivo Disassembly of Free Polyubiquitin Chains by Yeast Ubp14 Modulates Rates of Protein Degradation by the Proteasome. EMBO J. 1997, 16, 4826–4838. [Google Scholar] [CrossRef]
- Masunaka, A.; Ohtani, K.; Peever, T.L.; Timmer, L.W.; Tsuge, T.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. An Isolate of Alternaria alternata That Is Pathogenic to Both Tangerines and Rough Lemon and Produces Two Host-Selective Toxins, ACT- and ACR-Toxins. Phytopathology 2005, 95, 241–247. [Google Scholar] [CrossRef]
- van Roermund, C.W.T.; Waterham, H.R.; Ijlst, L.; Wanders, R.J.A. Fatty Acid Metabolism in Saccharomyces cerevisiae. Cell. Mol. Life Sci. 2003, 60, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, Y.; Tang, J.; Jiao, C.; Li, H. Biological Functions of the Peroxisomal Docking Proteins, AaPex13 and AaPex14, in the Tangerine Pathotype of Alternaria alternata. Mycosystema 2022, 41, 1199–1216. [Google Scholar] [CrossRef]
- Henry, K.W.; Wyce, A.; Lo, W.S.; Duggan, L.J.; Emre, N.C.T.; Kao, C.-F.; Pillus, L.; Shilatifard, A.; Osley, M.A.; Berger, S.L. Transcriptional Activation via Sequential Histone H2B Ubiquitylation and Deubiquitylation, Mediated by SAGA-Associated Ubp8. Genes Dev. 2003, 17, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.G.; Nelson, Z.W.; Gottschling, D.E. Ubp10/Dot4p Regulates the Persistence of Ubiquitinated Histone H2B: Distinct Roles in Telomeric Silencing and General Chromatin. Mol. Cell. Biol. 2005, 25, 6123–6139. [Google Scholar] [CrossRef]
- Fang, K.; Yao, X.; Tian, Y.; He, Y.; Lin, Y.; Lei, W.; Peng, S.; Pan, G.; Shi, H.; Zhang, D.; et al. Ubiquitin-Specific Protease UBP14 Stabilizes HY5 by Deubiquitination to Promote Photomorphogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2024, 121, e2404883121. [Google Scholar] [CrossRef]
- Mirabito, P.M.; Adams, T.H.; Timberlake, W.E. Interactions of Three Sequentially Expressed Genes Control Temporal and Spatial Specificity in Aspergillus Development. Cell 1989, 57, 859–868. [Google Scholar] [CrossRef]
- Wu, P.C.; Choo, Y.L.; Wei, S.Y.; Yago, J.I.; Chung, K.R. Contribution of Autophagy to Cellular Iron Homeostasis and Stress Adaptation in Alternaria alternata. Int. J. Mol. Sci. 2024, 25, 1123. [Google Scholar] [CrossRef]
- Hanna, J.; Hathaway, N.A.; Tone, Y.; Crosas, B.; Elsasser, S.; Kirkpatrick, D.S.; Leggett, D.S.; Gygi, S.P.; King, R.W.; Finley, D. Deubiquitinating Enzyme Ubp6 Functions Noncatalytically to Delay Proteasomal Degradation. Cell 2006, 127, 99–111. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Zhou, H.; Jiao, C.; Li, H. Functional Characterization of Deubiquitinase UBP Family and Proteomic Analysis of Aaubp14-Mediated Pathogenicity Mechanism in Alternaria alternata. J. Fungi 2025, 11, 495. https://doi.org/10.3390/jof11070495
Tang J, Zhou H, Jiao C, Li H. Functional Characterization of Deubiquitinase UBP Family and Proteomic Analysis of Aaubp14-Mediated Pathogenicity Mechanism in Alternaria alternata. Journal of Fungi. 2025; 11(7):495. https://doi.org/10.3390/jof11070495
Chicago/Turabian StyleTang, Jiejing, Hang Zhou, Chen Jiao, and Hongye Li. 2025. "Functional Characterization of Deubiquitinase UBP Family and Proteomic Analysis of Aaubp14-Mediated Pathogenicity Mechanism in Alternaria alternata" Journal of Fungi 11, no. 7: 495. https://doi.org/10.3390/jof11070495
APA StyleTang, J., Zhou, H., Jiao, C., & Li, H. (2025). Functional Characterization of Deubiquitinase UBP Family and Proteomic Analysis of Aaubp14-Mediated Pathogenicity Mechanism in Alternaria alternata. Journal of Fungi, 11(7), 495. https://doi.org/10.3390/jof11070495







