Synergistic Effects of Rhizophagus irregularis and Trichoderma harzianum Co-Inoculation on Enhancing Drought Tolerance and Secondary Metabolite Production in Licorice (Glycyrrhiza uralensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Soil Characteristics
2.2. Microbial Inoculation
2.3. Experimental Design and Growth Conditions
2.4. Parameter Measurements
2.5. Statistical Analysis
3. Results
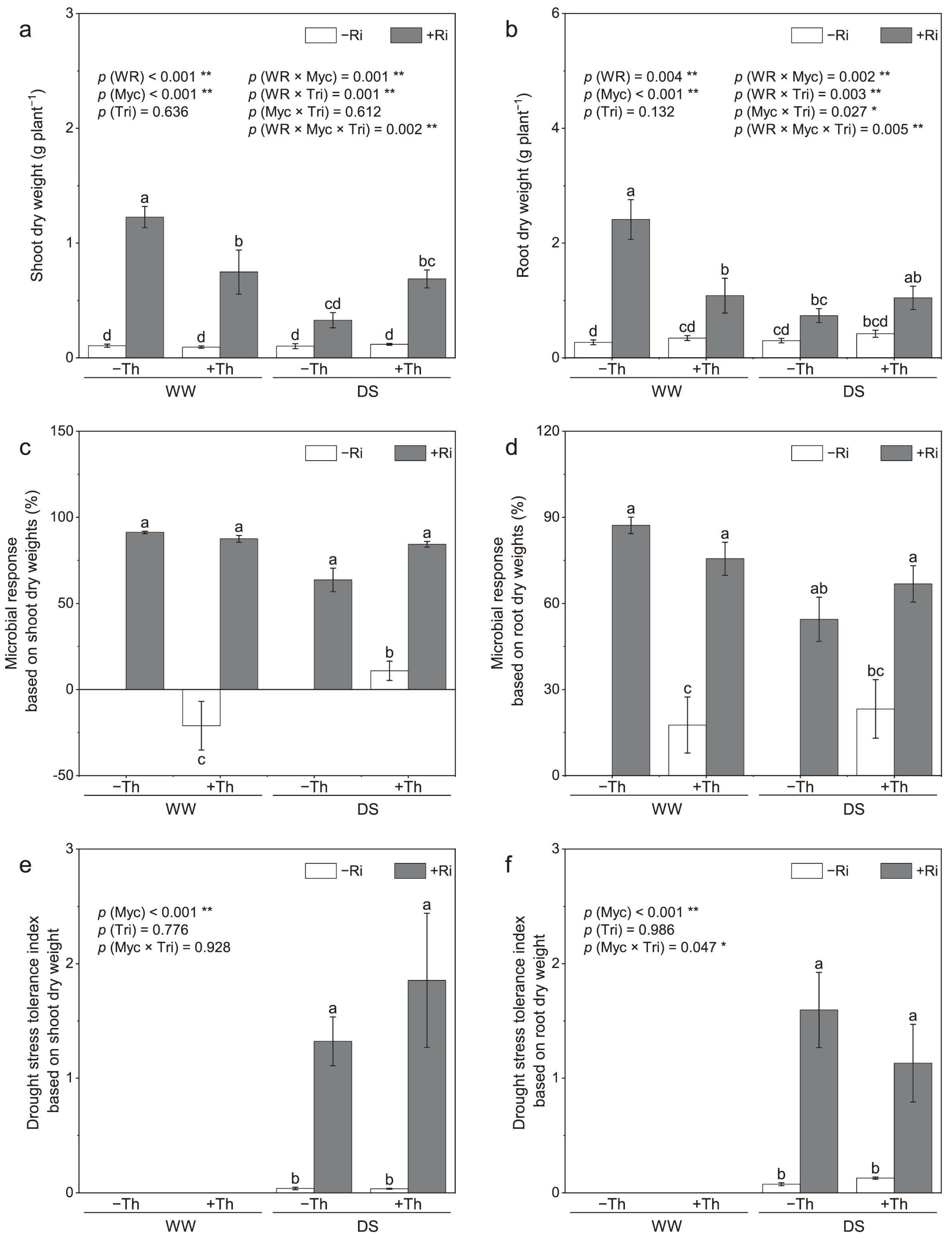
3.1. Plant Biomass and Microbial Response
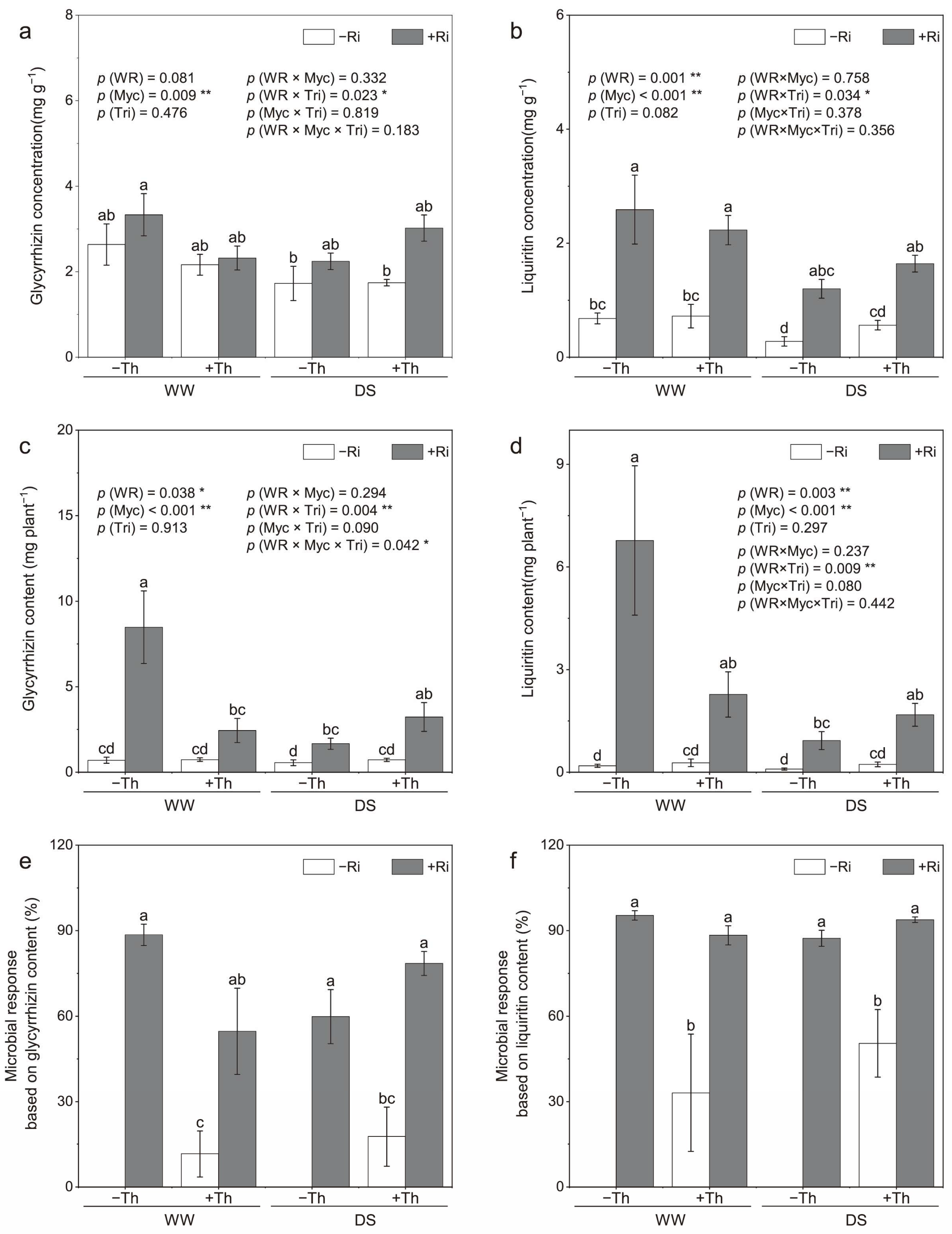
3.2. Root Glycyrrhizin, Liquiritin Concentrations, and Yields
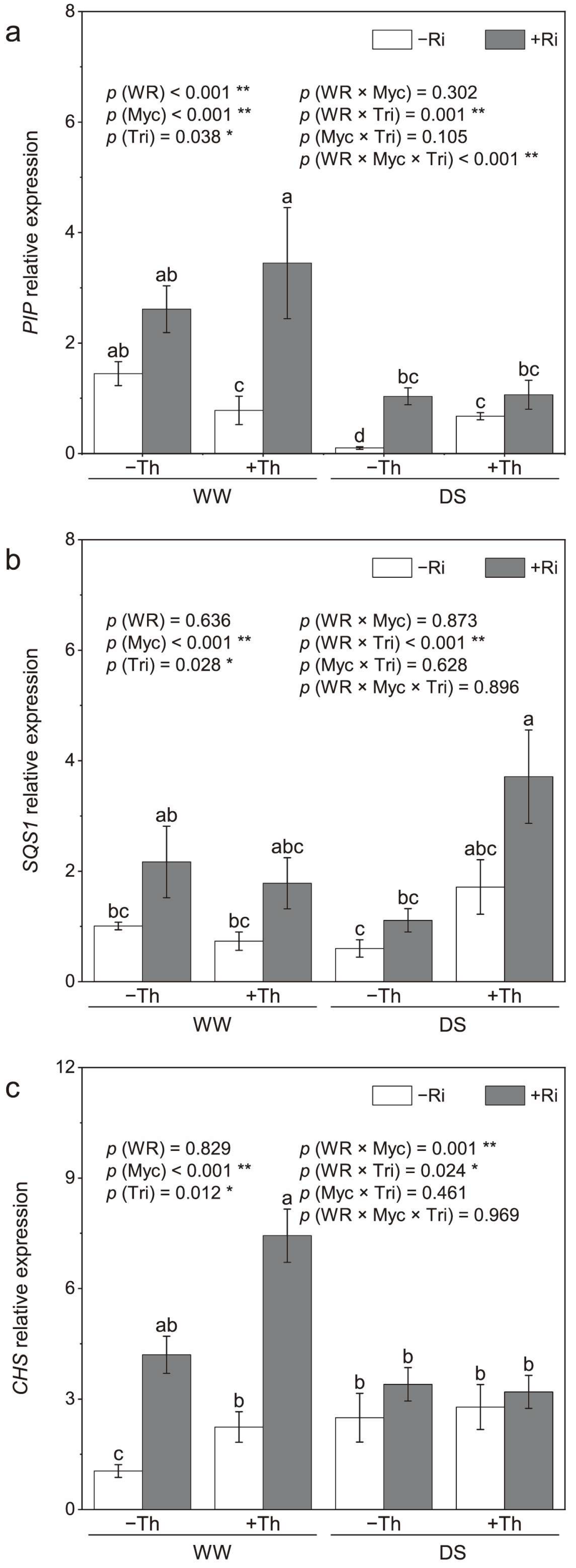
3.3. Root PIP, SQS1, and CHS Relative Expression
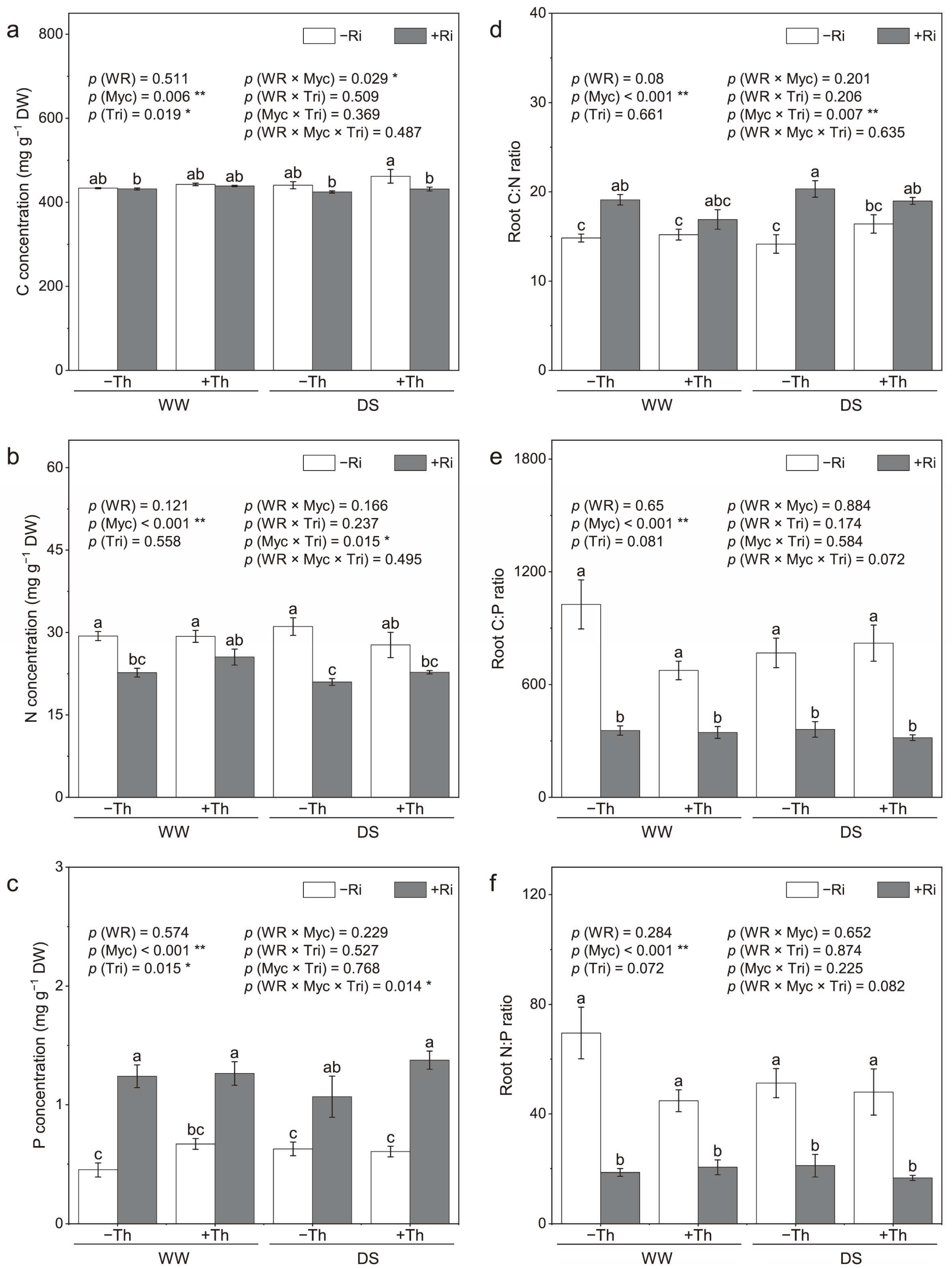
3.4. Root Nutrient Concentrations and Stoichiometry
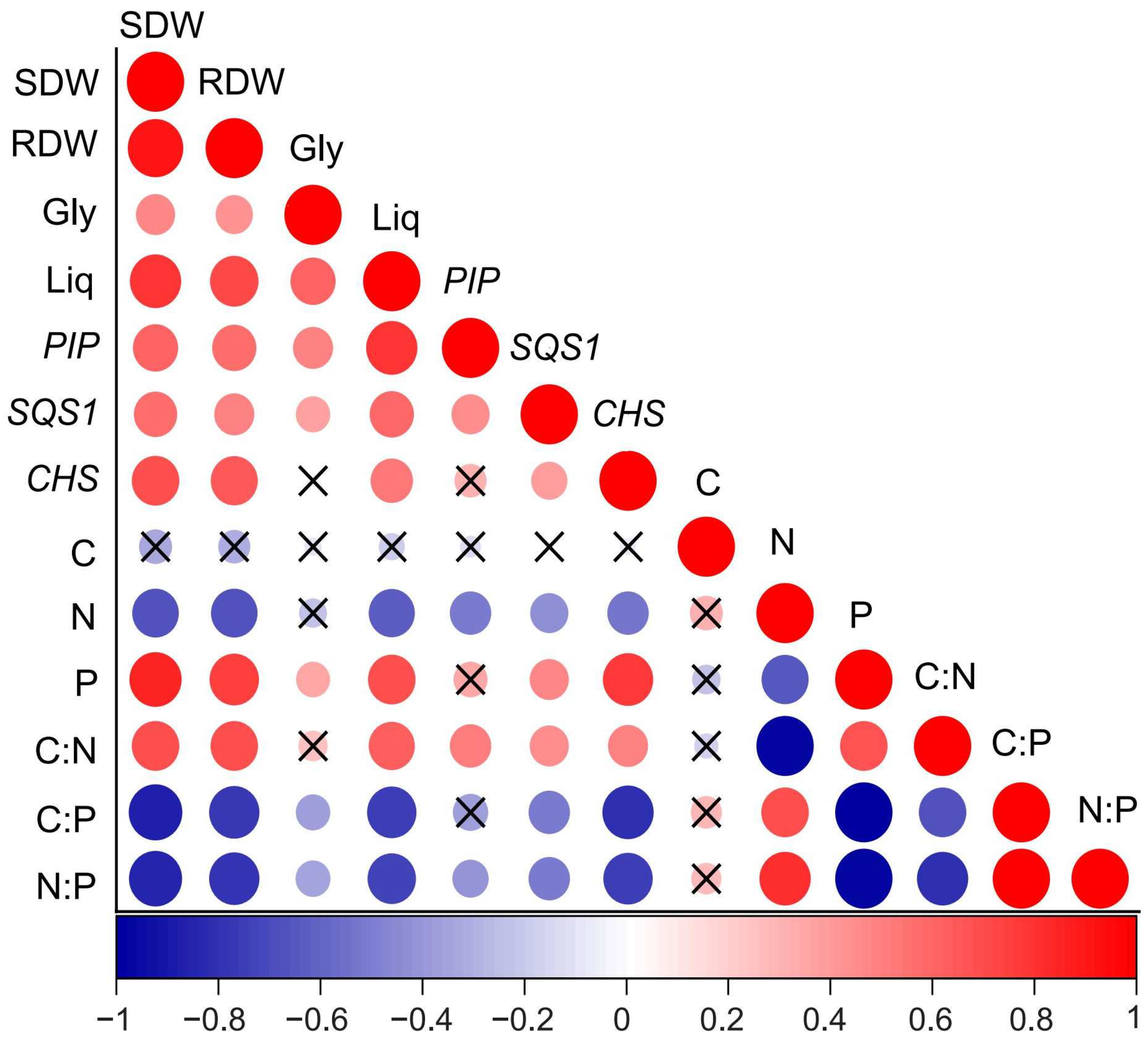
3.5. Correlations Between Plant Traits
4. Discussion
4.1. Enhanced Biomass and Stress Tolerance
4.2. Accumulation of Secondary Metabolites
4.3. Gene Expression and Stress Response
4.4. Nutrient Utilization and Stoichiometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Yang, G.; Sheng, Y.; Li, P.; Qiu, H.; Zhou, X.; Huang, L.; Chao, Z. Glomus mosseae inoculation improves the root system architecture, photosynthetic efficiency and flavonoids accumulation of liquorice under nutrient stress. Front. Plant Sci. 2017, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Brand, E.; Wang, W.; Zhao, Z. Licorice: Resources, applications in ancient and modern times. J. Ethnopharmacol. 2022, 298, 115594. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [PubMed]
- Vasisht, K.; Sharma, N.; Karan, M. Current perspective in the international trade of medicinal plants material: An update. Curr. Pharm. Des. 2016, 22, 4288–4336. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Cheng, B.F.; Lian, J.J.; Guo, D.D.; Qin, J.W.; Zhang, Y.B.; Yang, H.J.; Wang, M.; Wang, L.; Feng, Z.W. Liquiritin, a flavone compound from licorice, inhibits IL-1β-induced inflammatory responses in SW982 human synovial cells. J. Funct. Foods 2017, 33, 142–148. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: Update and review. Phytother. Res, 2015; 29, 1868–1886. [Google Scholar] [CrossRef]
- Hayashi, H.; Sudo, H. Economic importance of licorice. Plant Biotechnol. 2009, 26, 101–104. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Zhou, J.; Fu, W.; Guo, L.; Zhang, X.; Chen, B. Integrated transcriptomics and metabolomics reveal specific phenolic and flavonoid accumulation in licorice (Glycyrrhiza uralensis Fisch.) induced by arbuscular mycorrhiza symbiosis under drought stress. Plant Physiol. Biochem. 2023, 205, 108173. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Zhou, X.; Jiang, X.; Xu, L.; Wu, S.; Zhao, A.; Zhang, X.; Chen, B. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, B.; Zheng, S.; Zhang, X.; Wang, X.; Dong, W.; Xie, Q.; Wang, G.; Xiao, Y.; Chen, F. A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 2021, 184, 5527–5540. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Mbarki, S.; Cerdà, A.; Brestic, M.; Mahendra, R.; Abdelly, C.; Pascual, J.A. Vineyard compost supplemented with Trichoderma harzianum T78 improve saline soil quality. Land Degrad. Dev. 2017, 28, 1028–1037. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: A meta-analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Kapoor, R.; Chaudhary, V.; Bhatnagar, A.K. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 2007, 17, 581–587. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Yu, M.; Wu, Z.; Zhao, A.; Li, J.; Zhang, X.; Chen, B. Improved phosphorus nutrition by arbuscular mycorrhizal symbiosis as a key factor facilitating glycyrrhizin and liquiritin accumulation in Glycyrrhiza uralensis. Plant Soil 2019, 439, 243–257. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.; He, C.; Yao, J.; Wei, M.; He, X. Complementary effects of dark septate endophytes and Trichoderma strains on growth and active ingredient accumulation of Astragalus mongholicus under drought stress. J. Fungi 2022, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanzar, E.; Aliasgharzad, N.; Neyshabouri, M.R.; Khoshru, B.; Arzanlou, M.; Asgari Lajayer, B. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int. J. Environ. Sci. Technol. 2020, 17, 869–878. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Rashad, Y.M.; Ibrahim, S.A. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp. Physiol. Mol. Plant Pathol. 2018, 103, 84–91. [Google Scholar] [CrossRef]
- Clarke, J.M.; DePauw, R.M.; Townley-Smith, T.F. Evaluation of methods for quantification of drought tolerance in wheat. Crop Sci. 1992, 32, 723–728. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, W.; Jiang, X.; Wu, Z.; Zhang, X.; Chen, B. Arbuscular mycorrhizal fungus improves Rhizobium–Glycyrrhiza seedling symbiosis under drought stress. Agronomy 2019, 9, 572. [Google Scholar] [CrossRef]
- Bouskout, M.; Bourhia, M.; Al Feddy, M.N.; Dounas, H.; Salamatullah, A.M.; Soufan, W.; Nafidi, H.A.; Ouahmane, L. Mycorrhizal fungi inoculation improves Capparis spinosas yield, nutrient uptake and photosynthetic efficiency under water deficit. Agronomy 2022, 12, 149. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, X.; Yan, Q.; Xie, Y.; Chen, J.; Liang, G.; Chen, M.; Xiao, S.; Chen, Y.; Liu, J. Arbuscular mycorrhizal fungi improve the growth, water status, and nutrient uptake of Cinnamomum migao and the soil nutrient stoichiometry under drought stress and recovery. J. Fungi 2023, 9, 321. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Sheikh, I.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Amelioration of drought stress in Foxtail millet (Setaria italica L.) by P-solubilizing drought-tolerant microbes with multifarious plant growth promoting attributes. Environ. Sustain. 2020, 3, 23–34. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Sadashivaiah Sunil, L.; Chandrakanth, R. Gene expression in medicinal plants in stress conditions. In Stress-Responsive Factors and Molecular Farming in Medicinal Plants; Singh, D., Mishra, A.K., Srivastava, A.K., Eds.; Springer: Singapore, 2023; pp. 89–105. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial regulation of plant secondary metabolites: Impact, mechanisms and prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.T.; Hussain, A.; Aimen, A.; Jamshaid, M.U.; Ditta, A.; Asghar, H.N.; Zahir, Z.A. Improving resilience against drought stress among crop plants through inoculation of plant growth-promoting rhizobacteria. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Hashem, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 387–408. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol. Biochem. 2021, 157, 108243. [Google Scholar] [CrossRef]
- Kalamulla, R.; Yapa, N. Co-inoculation of AMF and other microbial biofertilizers for better nutrient acquisition from the soil system. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer: Singapore, 2024; pp. 99–111. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Chen, Z.; Kowalchuk, G.A.; Fu, X.; Kuramae, E.E. Microbial inoculants modulate growth traits, nutrients acquisition and bioactive compounds accumulation of Cyclocarya paliurus (Batal.) Iljinskaja under degraded field condition. For. Ecol. Manag. 2021, 482, 118897. [Google Scholar] [CrossRef]
- Thangavel, P.; Anjum, N.A.; Muthukumar, T.; Sridevi, G.; Vasudhevan, P.; Maruthupandian, A. Arbuscular mycorrhizae: Natural modulators of plant–nutrient relation and growth in stressful environments. Arch. Microbiol. 2022, 204, 264. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Yao, Y.; Li, H.; Wang, Q.; Niu, B. Microbial interactions within beneficial consortia promote soil health. Sci. Total Environ. 2023, 900, 165801. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Sun, M.; Feng, H.; Wei, X.; Xie, W.; Fu, W.; Guo, L.; Zhang, X.; Hao, Z.; Chen, B. Synergistic Effects of Rhizophagus irregularis and Trichoderma harzianum Co-Inoculation on Enhancing Drought Tolerance and Secondary Metabolite Production in Licorice (Glycyrrhiza uralensis). J. Fungi 2025, 11, 488. https://doi.org/10.3390/jof11070488
Zhang K, Sun M, Feng H, Wei X, Xie W, Fu W, Guo L, Zhang X, Hao Z, Chen B. Synergistic Effects of Rhizophagus irregularis and Trichoderma harzianum Co-Inoculation on Enhancing Drought Tolerance and Secondary Metabolite Production in Licorice (Glycyrrhiza uralensis). Journal of Fungi. 2025; 11(7):488. https://doi.org/10.3390/jof11070488
Chicago/Turabian StyleZhang, Kangxu, Mengyao Sun, Haiyan Feng, Xia Wei, Wei Xie, Wei Fu, Lanping Guo, Xin Zhang, Zhipeng Hao, and Baodong Chen. 2025. "Synergistic Effects of Rhizophagus irregularis and Trichoderma harzianum Co-Inoculation on Enhancing Drought Tolerance and Secondary Metabolite Production in Licorice (Glycyrrhiza uralensis)" Journal of Fungi 11, no. 7: 488. https://doi.org/10.3390/jof11070488
APA StyleZhang, K., Sun, M., Feng, H., Wei, X., Xie, W., Fu, W., Guo, L., Zhang, X., Hao, Z., & Chen, B. (2025). Synergistic Effects of Rhizophagus irregularis and Trichoderma harzianum Co-Inoculation on Enhancing Drought Tolerance and Secondary Metabolite Production in Licorice (Glycyrrhiza uralensis). Journal of Fungi, 11(7), 488. https://doi.org/10.3390/jof11070488






