Interactions Between Morel Cultivation, Soil Microbes, and Mineral Nutrients: Impacts and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Physicochemical Properties
2.3. Soil Enzymatic Activity
2.4. High-Throughput Sequencing of Microbial Communities
2.5. Statistical Analysis
3. Results
3.1. Yields of Different Morchella Species
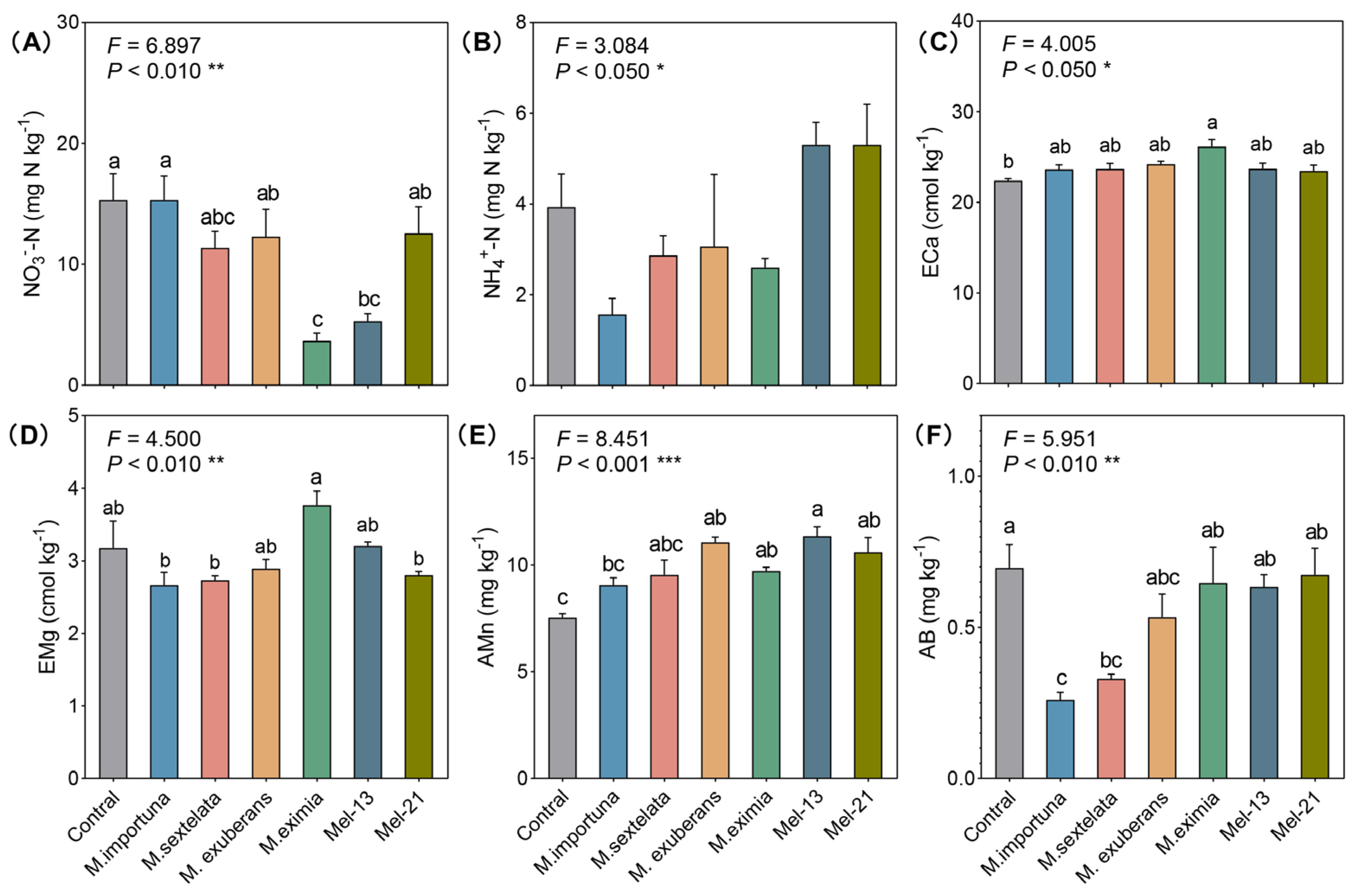
3.2. Soil Physiochemical Characteristics
3.3. Enzyme Activity of Microorganisms
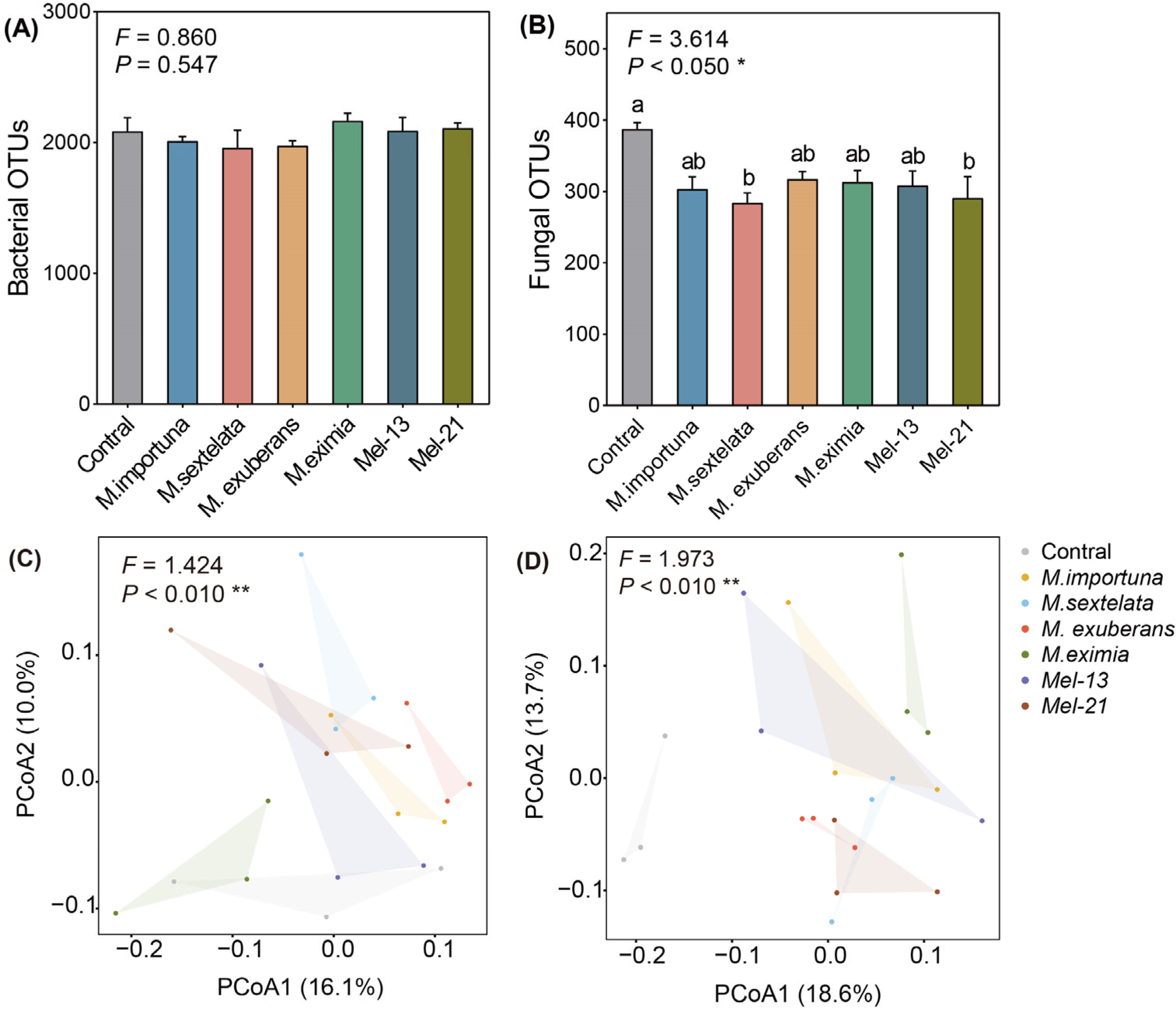
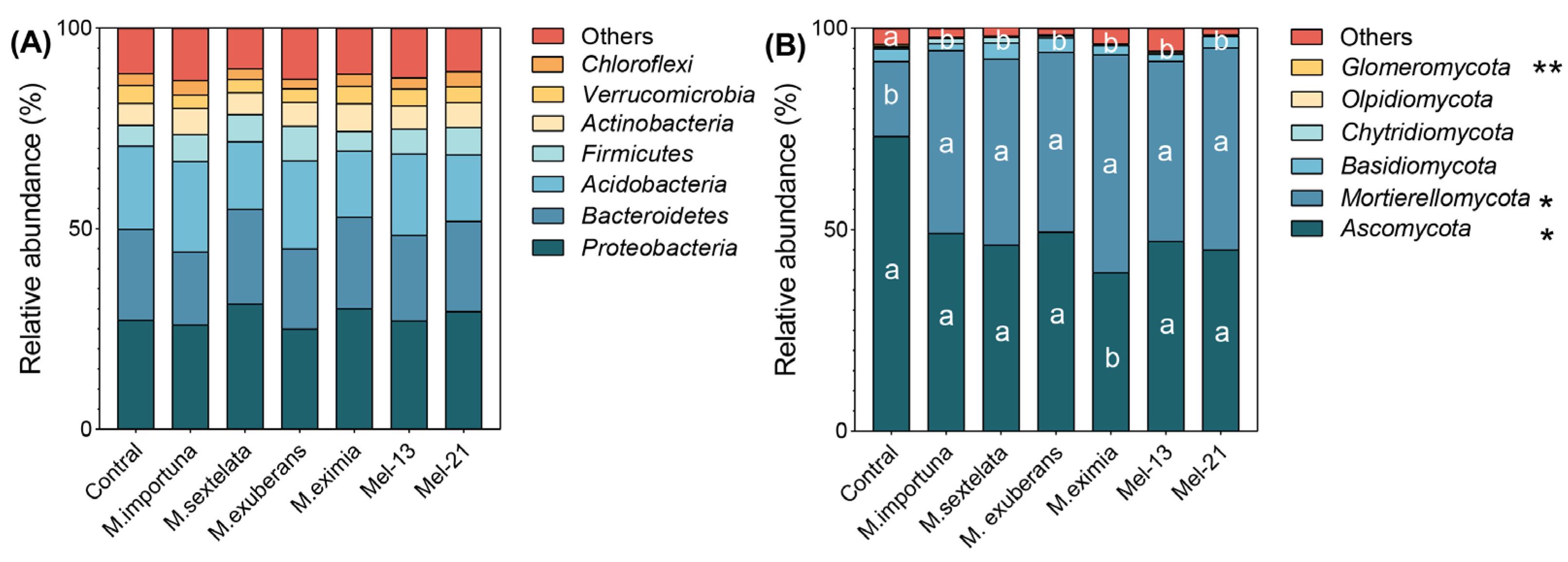
3.4. Soil Bacterial and Fungal Community Structure
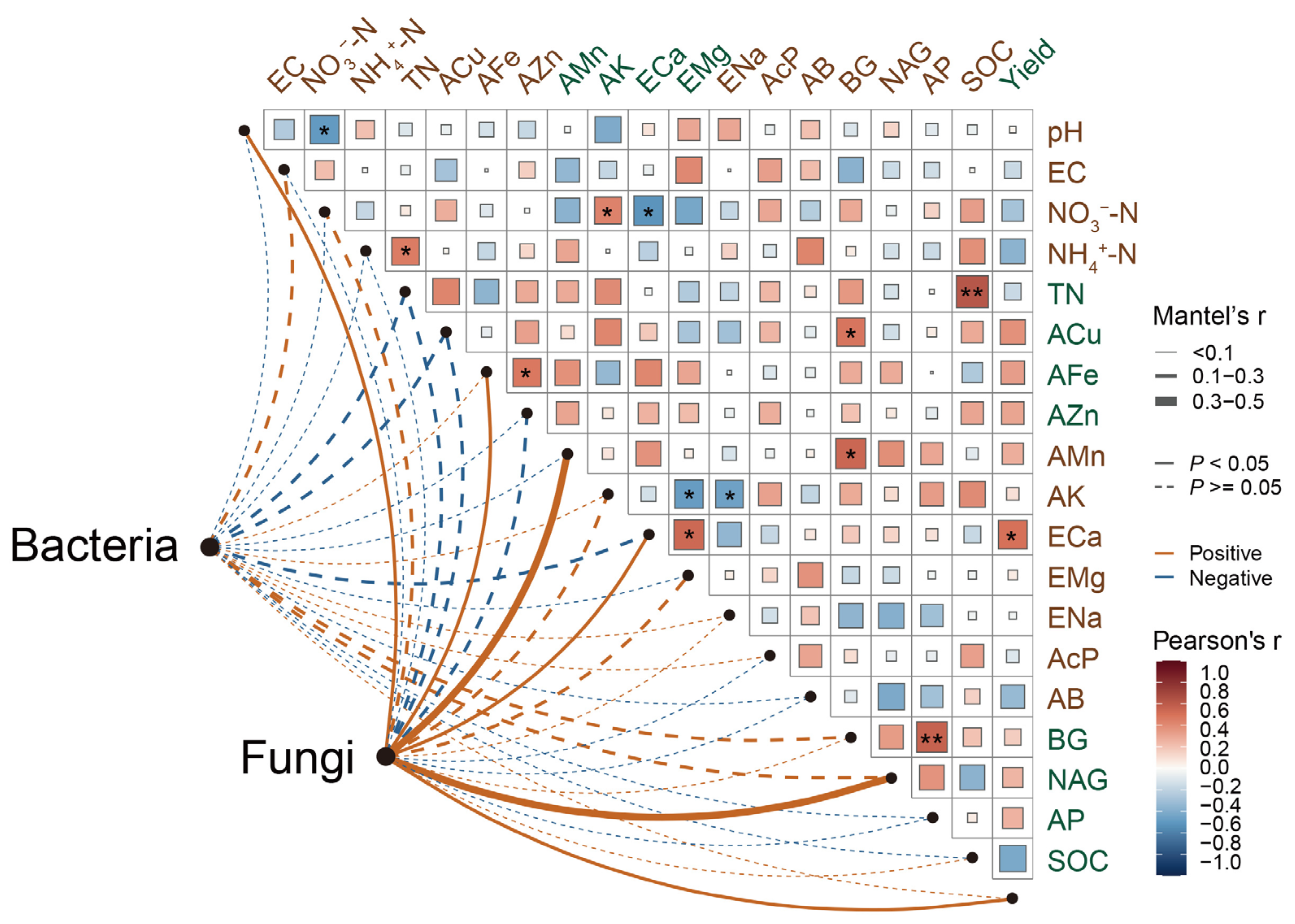
3.5. Correlations Among Soil Microbial Communities, Mineral Element Availability, and Morel Productivity
4. Discussion
4.1. Effects of Morel Cultivation on Soil Microbial Communities
4.2. Effects of Morel Cultivation on Soil Mineral Elements
4.3. Practical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.H.; Zhao, Q.; Yang, Z.L. A review on research advances, issues, and perspectives of morels. Mycology 2015, 6, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.J.; Yang, L. Recent advances on bioactive ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, T.; Liu, L.; Chen, Y.; Tang, J.; Peng, W.; Tan, H. Application of the mushroom volatile 1–octen–3–ol to suppress a morel disease caused by Paecilomyces penicillatus. Appl. Microbiol. Biot. 2022, 106, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Du, X.H.; Yang, Z.L. Mating systems in true morels (Morchella). Microbiol. Mol. Biol. Rev. 2021, 85, e0022020. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True morels (Morchella)–nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Guo, H.-B.; Bi, K.-X.; Sibirina, L.A.; Qi, X.-J.; Yu, X.-D. Determining why continuous cropping reduces the production of the morel Morchella sextelata. Front. Microbiol. 2022, 13, 903983. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White mold on cultivated morels caused by Paecilomyces penicillatus. FEMS Microbiol. Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef]
- Lohberger, A.; Spangenberg, J.E.; Ventura, Y.; Bindschedler, S.; Verrecchia, E.P.; Bshary, R.; Junier, P. Effect of organic carbon and nitrogen on the interactions of Morchella spp. and bacteria dispersing on their mycelium. Front. Microbiol. 2019, 10, 124. [Google Scholar] [CrossRef]
- Longley, R.; Benucci, G.N.M.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Loizides, M. Morels: The story so far. Field Mycol. 2017, 18, 42–53. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Chen, C.; Wang, J.; Han, Y.; Long, Z. Effects of element complexes containing Fe, Zn and Mn on artificial morel’s biological characteristics and soil bacterial community structures. PLoS ONE 2017, 12, e0174618. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Chen, Z.; He, P.X.; Liu, W.; Zhang, W.Y.; Cao, X.M. Allelopathic effects of phenolic acid extracts on Morchella mushrooms, pathogenic fungus, and soil–dominant fungus uncover the mechanism of morel continuous cropping obstacle. Arch. Microbiol. 2024, 206, 55. [Google Scholar] [CrossRef]
- Kalyoncu, F.; Oskay, M.; Kalyoncu, M. The effects of some environmental parameters on mycelial growth of six Morchella species. J. Pure Appl. Microbiol. 2009, 3, 467–472. [Google Scholar]
- Tan, H.; Liu, T.H.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W.H. Morel production related to soil microbial diversity and evenness. Microbiol. Spectr. 2021, 9, e0022921. [Google Scholar] [CrossRef]
- Orlofsky, E.; Zabari, L.; Bonito, G.; Masaphy, S. Changes in soil bacteria functional ecology associated with Morchella rufobrunnea fruiting in a natural habitat. Environ. Microbiol. 2021, 23, 6651–6662. [Google Scholar] [CrossRef]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi–omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Fang, J.Y.; Wang, Z.H.; Guo, D.L.; Flynn, D.F.B.; Geng, Z. Stoichiometry and large–scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U S Dept. Agric. Circ. 1954, 939, 1–19. [Google Scholar]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Han, Y.T.; Yue, Z.L.; Zhang, G.Y.; Gao, J.; Li, P.; Wang, L.A.; Li, S.M.; Zhao, Z.Z.; Liu, X.W.; Wang, L. Fungal diversity and metabolic pathways in rhizosphere soil of Morchella esculenta with different planting years. Jiangsu Agric. Sci. 2023, 51, 212–218. [Google Scholar] [CrossRef]
- Kang, C.; Zeng, W.J.; Zheng, X.; Wang, W.K.; Yang, L.; Liu, Z.X.; Wang, J. Impacts of Morchella on physicochemical properties, microbial diversity and community structure of overburden soil layer. Jiangsu Agric. Sci. 2023, 51, 221–228. Available online: https://link.cnki.net/doi/10.15889/j.issn.1002-1302.2023.14.030 (accessed on 27 January 2025).
- Zhang, Y.; Sun, S.; Luo, D.; Mao, P.; Rosazlina, R.; Martin, F.; Xu, L. Decline in morel production upon continuous cropping is related to changes in soil mycobiome. J. Fungi 2023, 9, 492. [Google Scholar] [CrossRef]
- Zhao, N.; Li, R.; Wang, X.; Duan, J.; Zheng, Q.; Li, M. Effects of exogenous Calcium Carbonate and temperature on active organic Carbon components and microbial community composition in yellow soil. J. Soil Water Conserv. 2023, 37, 238–245. Available online: https://link.cnki.net/doi/10.13870/j.cnki.stbcxb.2023.06.030 (accessed on 6 April 2025).
- Yue, Y.H.; Hao, H.B.; Wang, Q.; Xiao, T.T.; Zhang, Y.C.; Chen, Q.; Chen, H.; Zhang, J.J. Dynamics of the soil microbial community associated with Morchella cultivation: Diversity, assembly mechanism and yield prediction. Front. Microbiol. 2024, 15, 1345231. [Google Scholar] [CrossRef]
- Zhang, F.S.; Long, L.; Hu, Z.Y.; Yu, X.R.; Liu, Q.Y.; Bao, J.K.; Long, Z.F. Analyses of artificial morel soil bacterial community structure and mineral element contents in ascocarp and the cultivated soil. Can. J. Microbiol. 2019, 65, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, T.; Li, H.; Zhang, B.; Li, W.; Zhang, B.; Wang, X.; Wang, J.; Chen, Q.; He, X.; et al. Safe production strategies for soil-covered cultivation of morel in heavy metal-contaminated soils. J. Fungi 2023, 9, 765. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, C.; Huang, W.; Li, X. Controlled surface fire for improving yields of Morchella importuna. Mycol. Prog. 2017, 16, 1057–1063. [Google Scholar] [CrossRef]
- Phanpadith, P.; Yu, Z.; Yu, D.; Phongsavath, S.; Shen, K.; Zheng, W.; Phommakoun, B. Promotion of maize growth by a yellow morel, Morchella crassipes. Symbiosis 2020, 80, 33–41. [Google Scholar] [CrossRef]


| Control | M. importuna | M. sextelata | M. exuberans | M. eximia | Mel-13 | Mel-21 | |
|---|---|---|---|---|---|---|---|
| pH | 7.85 ± 0.04 | 7.76 ± 0.05 | 7.75 ± 0.04 | 7.84 ± 0.06 | 7.92 ± 0.08 | 7.88 ± 0.1 | 7.77 ± 0.05 |
| EC | 279.44 ± 26.61 | 239.11 ± 7.93 | 268.00 ± 22.33 | 218.00 ± 15.21 | 269.67 ± 13.24 | 246.11 ± 3.07 | 260.56 ± 6.28 |
| SOC | 13.57 ± 0.17 | 11.70 ± 0.34 | 12.18 ± 0.31 | 12.18 ± 0.50 | 11.00 ± 1.2 | 12.12 ± 0.48 | 12.95 ± 0.38 |
| TN | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.01 | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.14 ± 0.01 |
| AcP | 51.78 ± 6.96 | 40.17 ± 5.54 | 48.45 ± 5.84 | 45.12 ± 8.37 | 40.39 ± 2.41 | 43.13 ± 4.87 | 46.75 ± 8.79 |
| AK | 224.51 ± 11.39 | 230.49 ± 4.98 | 234.90 ± 1.71 | 232.21 ± 4.91 | 209.71 ± 3.15 | 222.51 ± 5.63 | 224.96 ± 5.04 |
| AFe | 8.023 ± 0.38 | 8.97 ± 0.14 | 8.75 ± 0.48 | 10.02 ± 0.69 | 10.69 ± 0.47 | 8.33 ± 0.48 | 10.26 ± 1.06 |
| AZn | 1.31 ± 0.23 | 1.12 ± 0.26 | 1.70 ± 0.25 | 1.70 ± 0.45 | 1.66 ± 0.28 | 1.23 ± 0.41 | 1.66 ± 0.26 |
| ACu | 1.50 ± 0.07 | 1.59 ± 0.06 | 1.72 ± 0.05 | 1.88 ± 0.26 | 1.48 ± 0.07 | 1.34 ± 0.01 | 1.66 ± 0.14 |
| ENa | 1.81 ± 0.02 | 1.75 ± 0.00 | 1.78 ± 0.01 | 1.80 ± 0.02 | 1.78 ± 0.01 | 1.79 ± 0.01 | 1.78 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Fan, M.; Qin, H.; Zhang, Z.; Liu, S.; Wu, S.; Wang, Y.; Yuan, X. Interactions Between Morel Cultivation, Soil Microbes, and Mineral Nutrients: Impacts and Mechanisms. J. Fungi 2025, 11, 405. https://doi.org/10.3390/jof11060405
Fu Y, Fan M, Qin H, Zhang Z, Liu S, Wu S, Wang Y, Yuan X. Interactions Between Morel Cultivation, Soil Microbes, and Mineral Nutrients: Impacts and Mechanisms. Journal of Fungi. 2025; 11(6):405. https://doi.org/10.3390/jof11060405
Chicago/Turabian StyleFu, Yiwen, Muxin Fan, Haiyan Qin, Zeyu Zhang, Shijun Liu, Shuwen Wu, Yun Wang, and Xia Yuan. 2025. "Interactions Between Morel Cultivation, Soil Microbes, and Mineral Nutrients: Impacts and Mechanisms" Journal of Fungi 11, no. 6: 405. https://doi.org/10.3390/jof11060405
APA StyleFu, Y., Fan, M., Qin, H., Zhang, Z., Liu, S., Wu, S., Wang, Y., & Yuan, X. (2025). Interactions Between Morel Cultivation, Soil Microbes, and Mineral Nutrients: Impacts and Mechanisms. Journal of Fungi, 11(6), 405. https://doi.org/10.3390/jof11060405





