Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Healthy and Infected Papayas
2.2. Isolation of Pathogenic Fungi from Papaya Fruit
2.3. Pathogenicity Test
2.4. Morphological Characterization of Fungi
2.5. Molecular Identification of Fungi
2.5.1. DNA Extraction and Purification
2.5.2. PCR Amplification
2.5.3. Amplification on Agarose Gel Electrophoresis
2.5.4. Sequencing
2.5.5. Phylogenetic Analysis
2.6. Influence of Environmental Parameters on Mycelial Growth
2.6.1. Effect of Temperature on Mycelial Growth
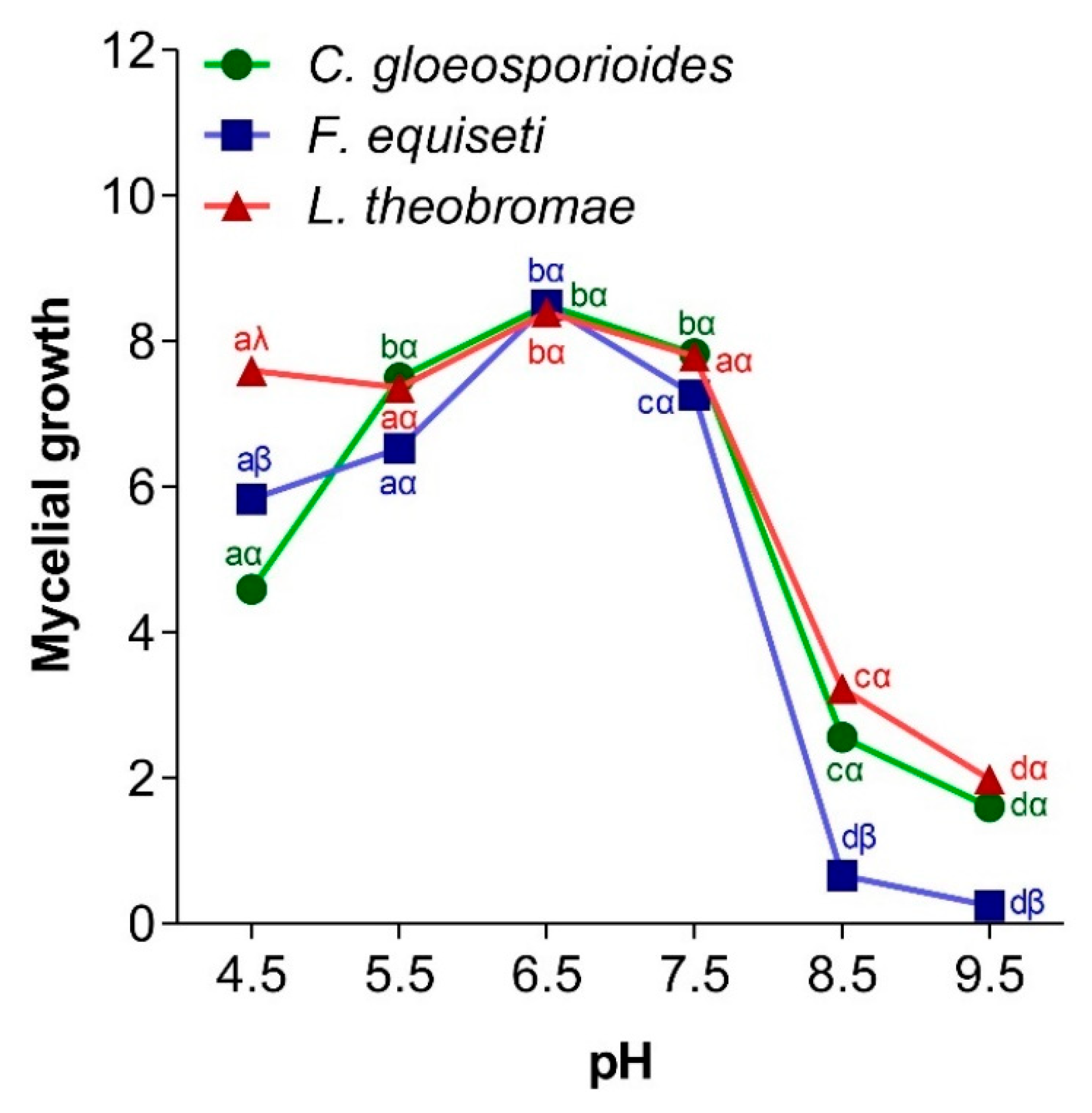
2.6.2. Effect of pH on Mycelial Growth
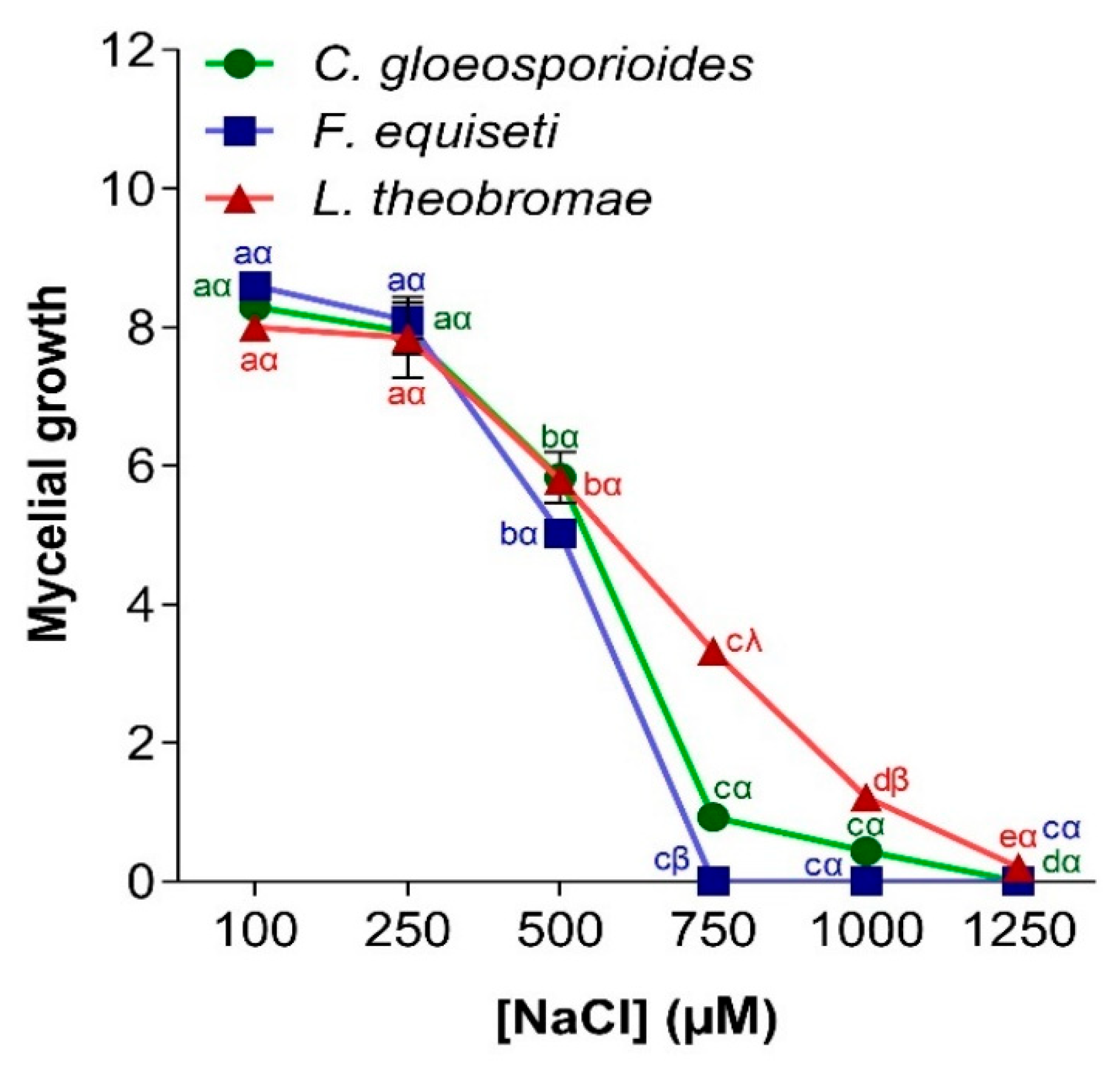
2.6.3. Effect of Salt Stress on Mycelial Growth
2.6.4. Effect of Water Potential on Mycelial Growth
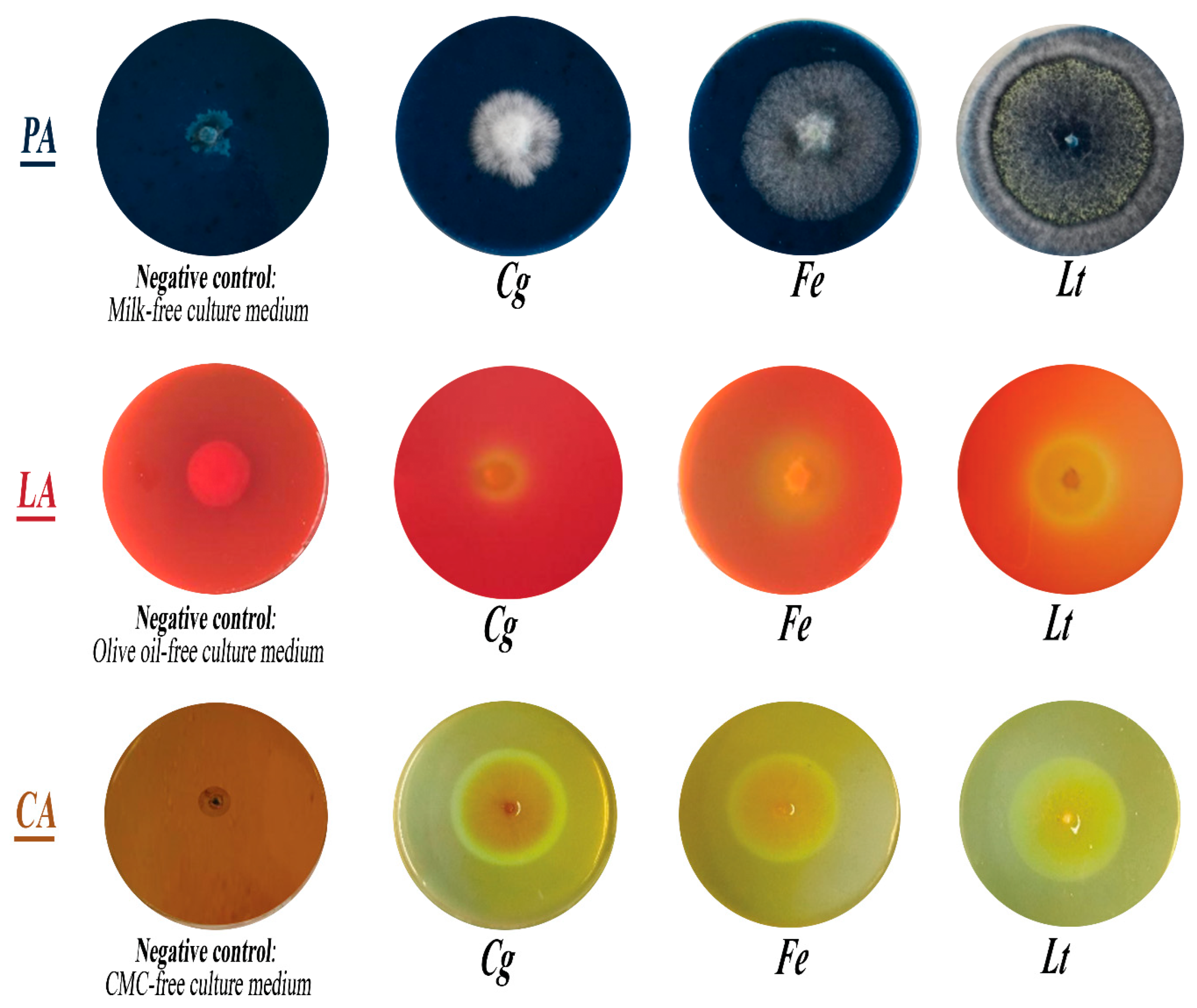
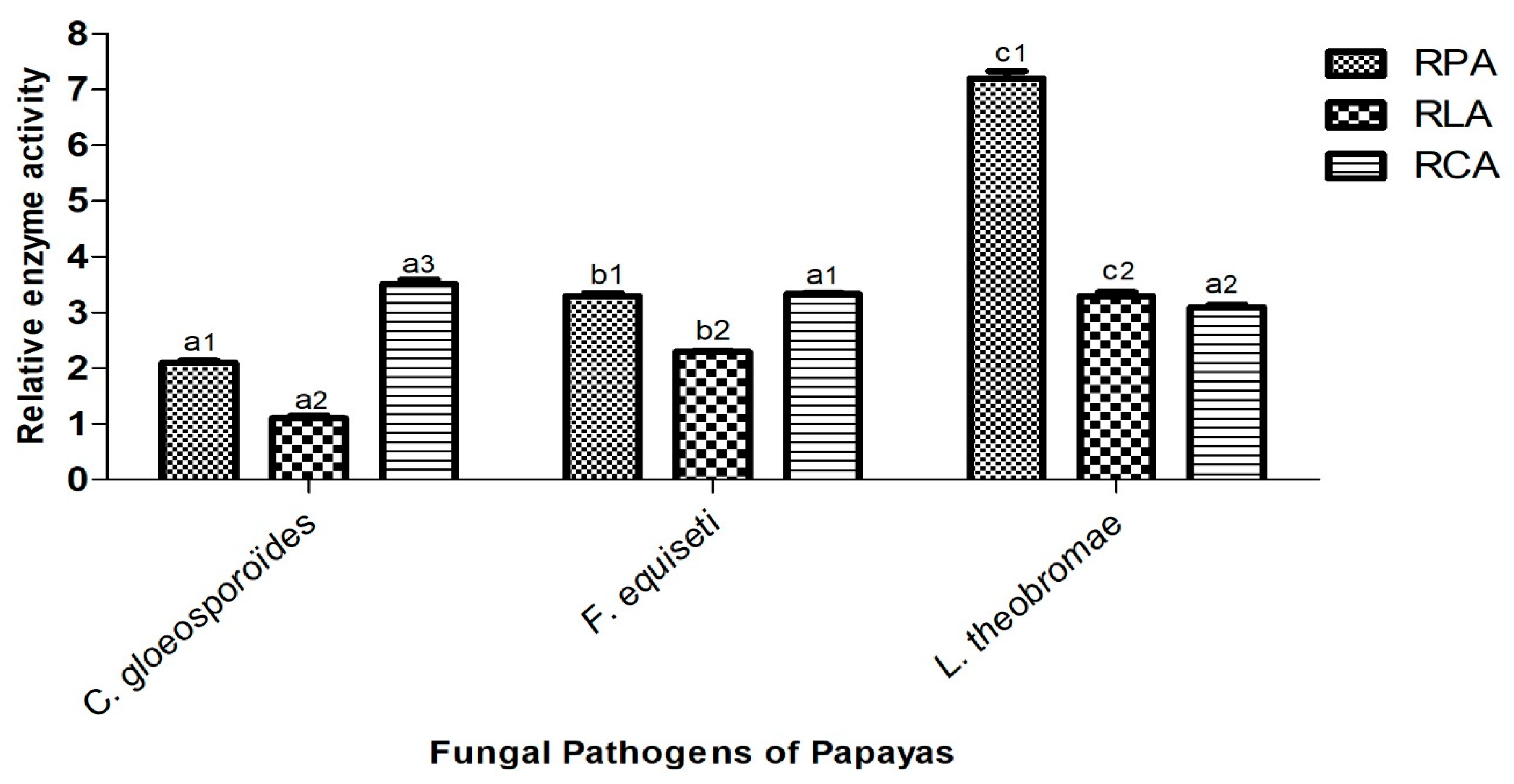
2.7. Biochemical Characterization: Highlighting of Hydrolytic Enzymes Produced by Fungi
- Production of protease
- Production of lipase
- Cellulase production
2.8. Statistical Analysis
3. Results
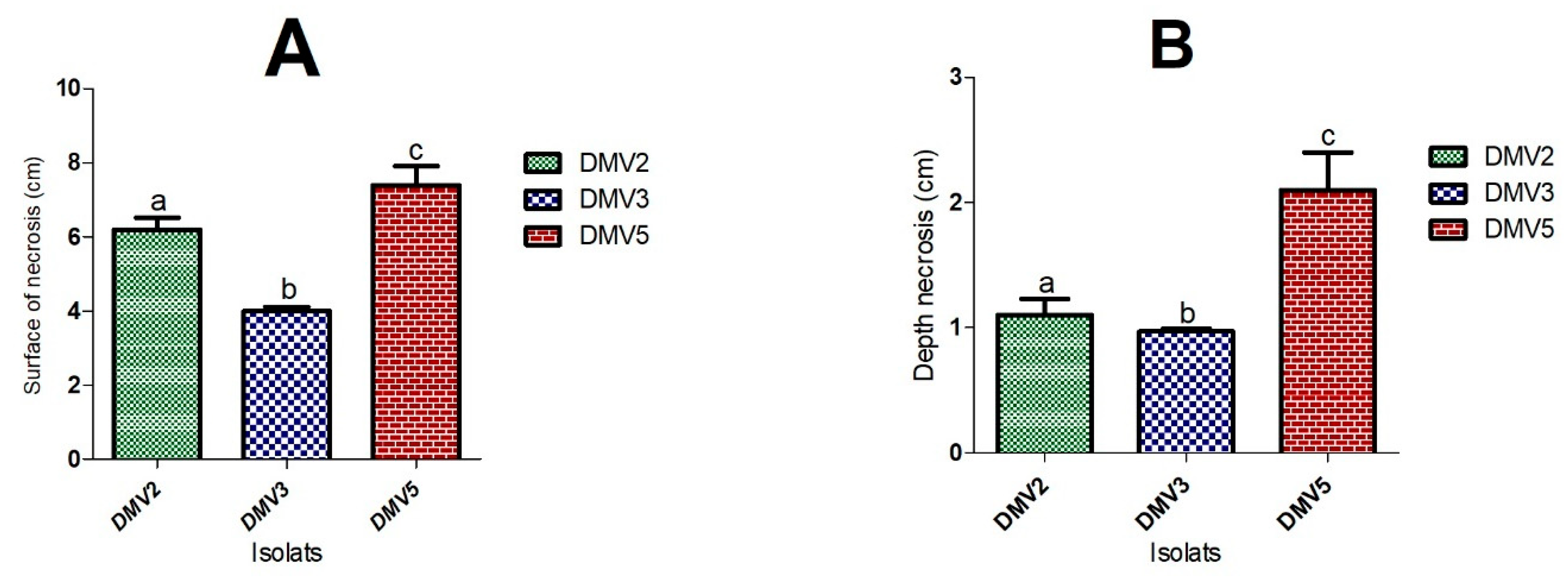
3.1. Fungal Isolates and Rot Symptoms on Papayas
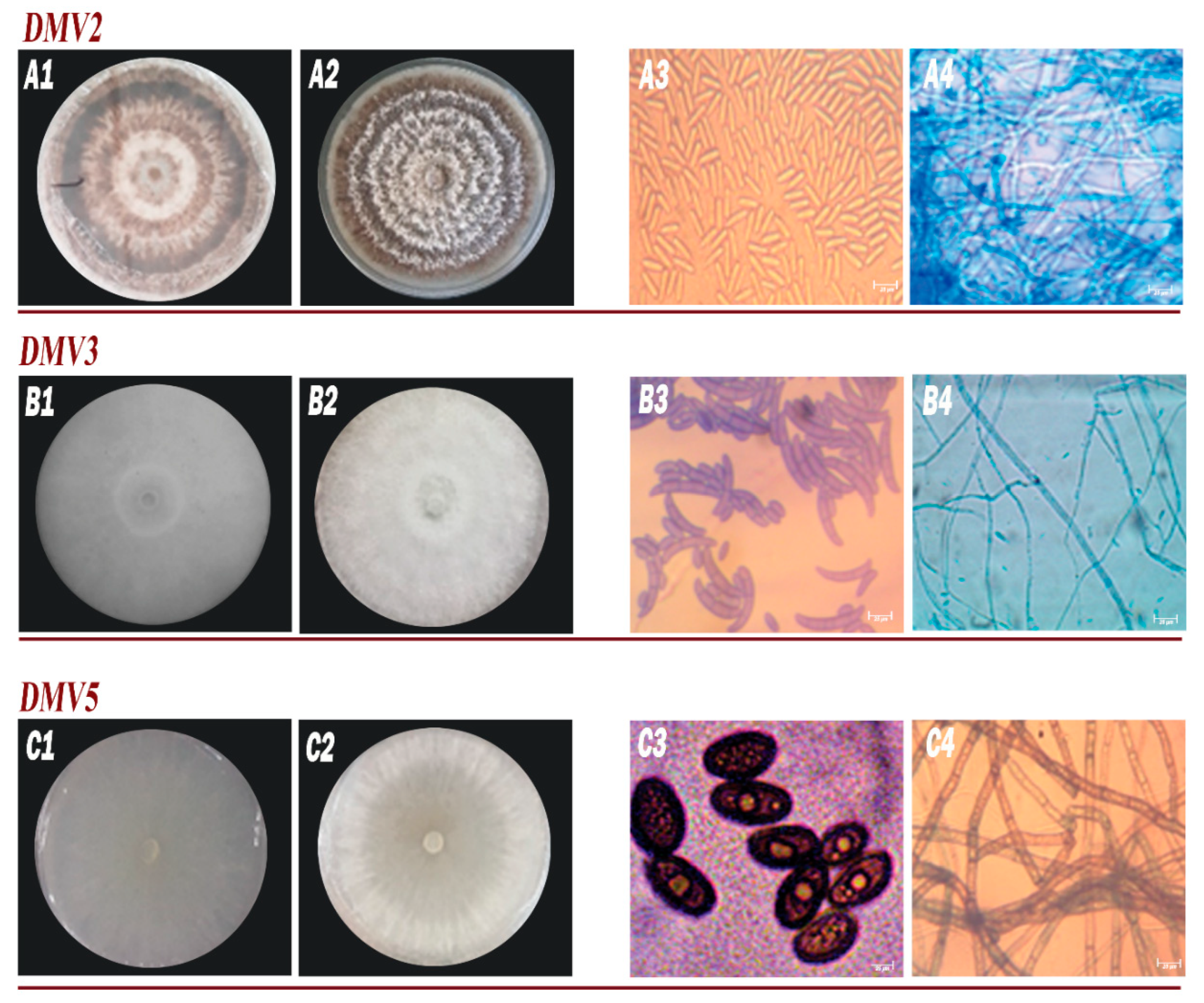
3.2. Phenotypic and Morphological Characteristics of Isolates
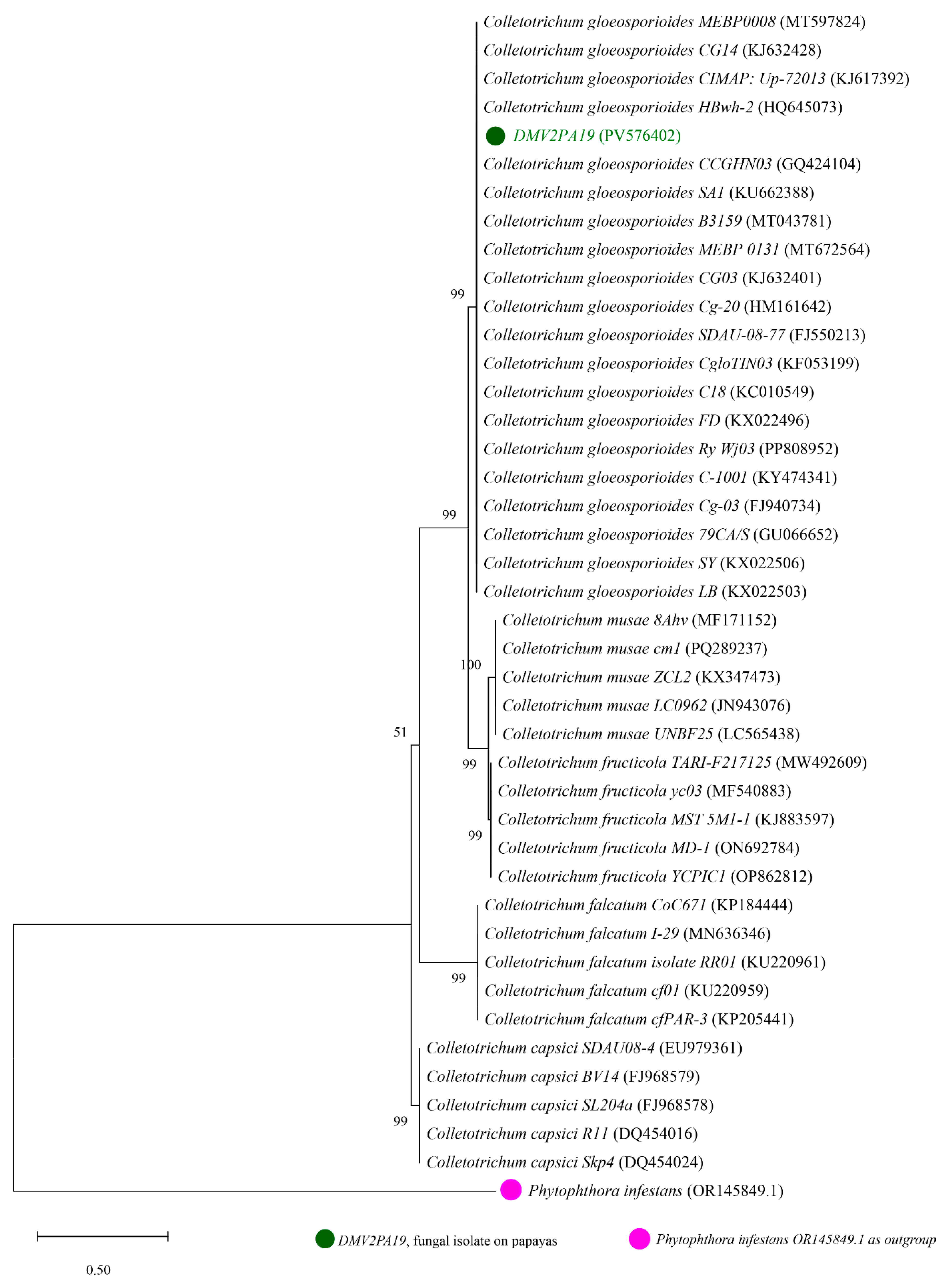
3.3. Molecular Identification and Phylogenetic Analysis
3.4. Influence of Environmental Factors on Mycelial Growth of Papaya Pathogenic Fungi
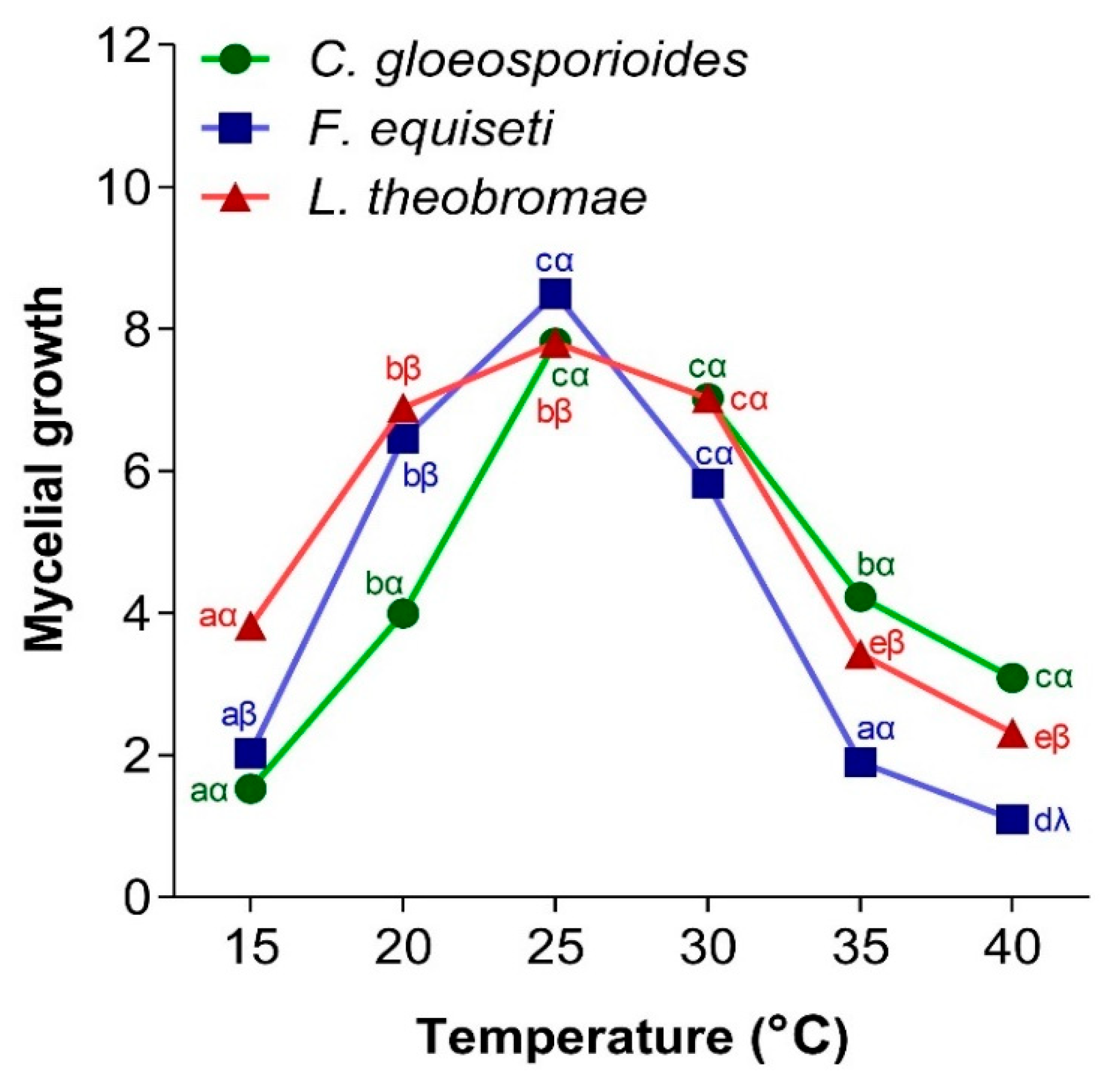
3.4.1. Effect of Temperature on Fungal Mycelial Growth
3.4.2. Effect of pH on Fungal Mycelial Growth
3.4.3. Effect of Salt Stress on Pathogen Growth
3.4.4. Effect of Water Potential on Pathogen Mycelial Growth
3.5. Capacity of Isolated Fungi to Secrete Hydrolytic Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, J.; Ke, S.; He, X.; Zhang, B.; Zheng, Z.; Chen, P. Research on the Isolation of Endophytic Fungi from Papaya and the Prevention of Colletotrichum gloeosporioides. J. Fungi 2024, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Pudhuvai, B.; Sharma, C.; Kumar, A.; Sharma, V.; Yadav, D.; Jin, J.O. Carica papaya, L.: A Tropical Fruit with Benefits beyond the Tropics. Diversity 2022, 14, 683. [Google Scholar] [CrossRef]
- Saurabh, P.; Peter, C.; Shaw, N.; Amitha, K.H. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunotoxicol. 2016, 13, 590–602, 2429. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Alara, J.A. Carica papaya: Comprehensive Overview of the Nutritional Values, Phytochemicals and Pharmacological Activities. Adv. Tradit. Med. 2022, 22, 17–47. [Google Scholar] [CrossRef]
- Balogun, A.A.; Ariahu, C.C. Quality Evaluation of Fresh Papaya Fruits Stored in Evaporative Coolers. Asian Food Sci. J. 2020, 15, 6–16. [Google Scholar] [CrossRef]
- FAO. Stratégie Nationale de Développement de l’Arboriculture Fruitière (2020–2025) Avec Un Accent Particulier Sur Les Filières Orange, Mangue et Papaye. Base Données FAOLEX, SNDAF. 2023. Available online: https://www.fao.org/faolex/results/details/fr/c/LEX-FAOC211661/ (accessed on 14 May 2025).
- Victor, D.M.; Priscille, E.; Steve, V.; Fabiola, D.; Moise, N.; Olivier, Y.; Patience, M.; Severin, T.; Modeste, S. In Vitro and in Situ Activity of Cymbopogon citratus Essential Oil Against Alternaria Alternata and Phomopsis Carica-papayae, Causal Agents of Papaya Leaf Diseases. J. Plant Sci. 2024, 12, 55–63. [Google Scholar] [CrossRef]
- Vieira, W.A.D.S.; Veloso, J.S.; da Silva, A.C.; dos Santos Nunes, A.; Doyle, V.P.; Castlebury, L.A.; Câmara, M.P.S. Elucidating the Colletotrichum spp. Diversity Responsible for Papaya Anthracnose in Brazil. Fungal Biol. 2022, 126, 623–630. [Google Scholar] [CrossRef]
- Ampères, M.; Boat, B.; Taïeb, N.; Agriopoulou, S.; Miche, L.; Sameza, M.L.; Dupuy, N.; Roussos, S.; Ampères, M.; Boat, B. Identification of Native Soil-Derived Trichoderma spp. Isolates and Analysis of Their Antagonist Traits against Lasiodiplodia theobromae Causing Stem-End Rot in Papaya to Cite This Version: HAL Id: Hal-03777688 Identification of Native Soil-Derived Tri. Arch. Phytopathol. Plant Prot. 2022, 55, 1766–1794. [Google Scholar] [CrossRef]
- Sekeli, R.; Hamid, M.H.; Razak, R.A.; Wee, C.Y.; Ong-Abdullah, J. Malaysian Carica papaya L. Var. Eksotika: Current Research Strategies Fronting Challenges. Front. Plant Sci. 2018, 9, 1380. [Google Scholar] [CrossRef]
- Martínez-Soto, D.; Yu, H.; Allen, K.S.; Ma, L.J. Differential Colonization of the Plant Vasculature between Endophytic Versus Pathogenic Fusarium oxysporum Strains. Mol. Plant-Microbe Interact. 2023, 36, 4–13. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal Activity of Five Plant-Extracted Essential Oils against Anthracnose in Papaya Fruit. Biol. Agric. Hortic. 2018, 34, 18–26. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Jarial, K.; Jarial, R.S.; Banyal, S.K. Prevalence of Different Post Harvest Rots of Papaya in Subtropical Zone of Himachal Pradesh. Indian J. Ecol. 2024, 51, 218–224. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit Stem-End Rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef]
- Hassan, H.; Mohamed, M.T.M.; Yusoff, S.F.; Hata, E.M.; Tajidin, N.E. Selecting Antagonistic Yeast for Postharvest Biocontrol of Colletotrichum gloeosporioides in Papaya Fruit and Possible Mechanisms Involved. Agronomy 2021, 11, 760. [Google Scholar] [CrossRef]
- Getnet, M.; Alemu, K.; Tsedaley, B. Status of Postharvest Papaya Anthracnose (Colletotrichum gloeosporioides) in Assosa Zone, Western Ethiopia. Discov. Food 2024, 4, 25. [Google Scholar] [CrossRef]
- Zakaria, L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae 2023, 9, 322. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Saad, N.; Hun, T.G.; Hata, E.M. First Report of Fusarium equiseti Causing Fruit Rot Disease of Watermelon in Malaysia. Plant Dis. 2022, 106, 326. [Google Scholar] [CrossRef]
- Jahun, B.M.; Ilu, K.J.; Yahaya, S.M.; Ahmed, B.; Salami, K.D. Fungi Causing Post-Harvest Spoilage Carica papaya Linn Fruits of Two Selected Markets in Kano State, Nigeria. J. Appl. Sci. Environ. Manag. 2021, 25, 727–731. [Google Scholar] [CrossRef]
- Chaudhary Scholar, M.M.; Patel, D.; Chaudhary, M.M.; Chaudhary, D.H.; Dighule, S.B. Isolation and Characterization of Fungi Associated with Deterioration of Papaya Fruits. J. Pharmacogn. Phytochem. 2020, 9, 3434–3437. Available online: https://www.phytojournal.com/search?q=Isolation+and+Characterization+of+Fungi+Associated+with+Deterioration+of+Papaya+Fruits (accessed on 14 May 2025).
- Tan, G.H.; Ali, A.; Siddiqui, Y. Major Fungal Postharvest Diseases of Papaya: Current and Prospective Diagnosis Methods. Crop Prot. 2023, 174, 106399. [Google Scholar] [CrossRef]
- Karunamoorthy, J.A.L.; Al-Taie, B.S.; Allawi, M.Y.; Jambari, N.N.; Rahmad, N.; Al-Obaidi, J.R. First Report of Fusarium equiseti and Lasiodiplodia theobromae Infection in Local Malaysian Papaya (Carica papaya L.). Trop. Agric. 2023, 100, 115–120. Available online: https://journals.sta.uwi.edu/ojs/index.php/ta/article/view/8498 (accessed on 14 May 2025).
- Hami, A.; Rasool, R.S.; Khan, N.A.; Mansoor, S.; Mir, M.A.; Ahmed, N.; Masoodi, K.Z. Morpho-Molecular Identification and First Report of Fusarium equiseti in Causing Chilli wilt from Kashmir (Northern Himalayas). Sci. Rep. 2021, 11, 3610. [Google Scholar] [CrossRef]
- Bencheikh, A.; Rouag, N.; Mamache, W.; Belabed, I. First Report of Fusarium equiseti Causing Crown Rot and Damping-off on Durum wheat in Algeria. Arch. Phytopathol. Plant Prot. 2020, 53, 915–931. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, X.; He, R.; Wang, M.; Zhang, Y.; Yang, Y. Biological Characteristics, Pathogenicity, and Sensitivity to Fungicides of Four Species of Lasiodiplodia on Avocado Fruits. Horticulturae 2024, 10, 1190. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Nhien, D.B.; Thu, L.T.M.; Trong, N.D.; Hiep, P.C.; Thuan, V.M.; Quang, L.T.; Thuc, L.V.; Xuan, D.T. Using Trichoderma asperellum to Antagonize Lasiodiplodia theobromae Causing Stem-End Rot Disease on Pomelo (Citrus maxima). J. Fungi 2023, 9, 981. [Google Scholar] [CrossRef]
- Ablormeti, F.K.; Coleman, S.R.; Honger, J.O.; Owusu, E.; Bedu, I.; Aidoo, O.F.; Cornelius, E.W.; Odamtten, G.T. Management of Lasiodiplodia theobromae, the Causal Agent of Mango Tree Decline Disease in Ghana. Afr. Crop Sci. J. 1970, 29, 193–207. [Google Scholar] [CrossRef]
- Davy, M.; Sameza, M.L.; Tchameni, S.N.; Ntah, M.; Ayong, A.; Ampère Boat Bedine, M.; Youassi, O.Y.; Kamsu, P.N.; Michel, P.; Jazet, D. Antifungal Effects of Clove (Syzygium aromaticum) Essential Oil against Colletotrichum gloeosporioides, the Fungus Associated with Papaya (Carica papaya L.) Fruit Anthracnose. Int. J. Appl. Microbiol. Biotechnol. Res. 2020, 8, 51–57. [Google Scholar] [CrossRef]
- Rovetto, E.I.; Luz, C.; La Spada, F.; Meca, G.; Riolo, M.; Cacciola, S.O. Diversity of Mycotoxins and Other Secondary Metabolites Recovered from Blood Oranges Infected by Colletotrichum, Alternaria, and Penicillium Species. Toxins 2023, 15, 407. [Google Scholar] [CrossRef]
- Félix, C.; Libório, S.; Nunes, M.; Félix, R.; Duarte, A.S.; Alves, A.; Esteves, A.C. Lasiodiplodia theobromae as a Producer of Biotechnologically Relevant Enzymes. Int. J. Mol. Sci. 2018, 19, 29. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Wang, C.; Han, M.; Min, Y.; Hu, J.; Pan, Y.; Huang, L.; Nie, J. Colletotrichum fructicola Co-Opts Cytotoxic Ribonucleases That Antagonize Host Competitive Microorganisms to Promote Infection. mBio 2024, 15, e0105324. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Meneses, R.; Gonçalves, M.F.M.; Tilleman, L.; Duarte, A.S.; Jorrín-Novo, J.V.; Van de Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Esteves, A.C.; et al. A Multi-Omics Analysis of the Grapevine Pathogen Lasiodiplodia theobromae Reveals That Temperature Affects the Expression of Virulence- and Pathogenicity-Related Genes. Sci. Rep. 2019, 9, 13144. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- María, A.I.-Z.; Silvia, B.-B.; José, L.A.-R.; Mónica, H.-L.; Laura, L.B.-N. Morphological, Pathogenic and Molecular Characterization of Fungal Species Associated with Mango Fruits in Mexico. Afr. J. Microbiol. Res. 2023, 17, 193–201. [Google Scholar] [CrossRef]
- Anthony, S.; Abeywickrama, K.; Dayananda, R.; Wijeratnam, S.; Arambewela, L. Fungal Pathogens Associated with Banana Fruit in Sri Lanka, and their Treatment with Essential Oils. Mycopathologia 2004, 157, 91–97. [Google Scholar] [CrossRef]
- Vu, T.X.; Tran, T.B.; Tran, M.B.; Do, T.T.K.; Do, L.M.; Dinh, M.T.; Thai, H.D.; Pham, D.N.; Tran, V.T. Efficient Control of the Fungal Pathogens Colletotrichum gloeosporioides and Penicillium digitatum Infecting Citrus Fruits by Native Soilborne Bacillus velezensis Strains. Heliyon 2023, 9, e13663. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi; CRC Press: Boca Raton, FL, USA, 2010; 1201. [Google Scholar] [CrossRef]
- Botton, B.; Breton, A.; Fevra, M.; Gauthier, S.; Guy, P.; Larpent, J.P.; Reymond, P.; Sanglier, J.J.; Vayssier, Y.; Veau, P. Moisissures Utiles et Nuisibles. Importance Industrielle, 2nd ed.; Masson: Paris, France, 1990; p. 512. [Google Scholar]
- White, J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protocols: A Guide to Methods and Applications; Academic Press Inc.: New York, NY, USA, 1990; Volume 21, pp. 99–104. Available online: https://www.researchgate.net/publication/223397588_White_T_J_T_D_Bruns_S_B_Lee_and_J_W_Taylor_Amplification_and_direct_sequencing_of_fungal_ribosomal_RNA_Genes_for_phylogenetics (accessed on 14 May 2025).
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Compaoré, H.; Sawadogo-lingani, H.; Samandoulougou, S. Aptitude de Trois Souches de Moisissures à Produire Des Enzymes Extracellulaires En Milieu Solide Au Burkina Faso. J. Appl. Biosci. 2017, 110, 10776–10782. [Google Scholar] [CrossRef][Green Version]
- Kannan, R.; Dhivya, V. In-Vitro Effect of Different Abiotic Stresses on the Growth and Sporulation of Colletotrichum gloeosporioides Causing Anthracnose of Mango. Indian J. Agric. Res. 2023, 57, 261–265. [Google Scholar] [CrossRef]
- Dossa, J.S.B.; Togbe, E.C.; Pernaci, M.; Agbossou, E.K.; Ahohuendo, B.C. Effet des facteurs de l’environnement sur les Fusarium pathogènes des plantes cultivées. Int. J. Biol. Chem. Sci. 2019, 13. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of High Temperature and Salinity Tolerant Trichoderma Isolates with Antagonistic Activity against Sclerotium rolfsii. Springerplus 2014, 3, 641. [Google Scholar] [CrossRef] [PubMed]
- Jabiri, S.; El Hamss, H.; Amraoui, M.B.; Lahlali, R. Influence of Culture Media and Environmental Factors (Water Potential and Temperature) on Mycelial Growth of Phytopythium vexans (de Bary), the Causal Agent of Dieback Disease in Apple Trees. Appl. Microbiol. 2022, 2, 861–872. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Ricardo-Díaz, J.; Quiñones-Muñoz, T. Fungal Strain Selection for Protease Production by Solid-State Fermentation Using Agro-Industrial Waste as Substrates. Chem. Pap. 2019, 73, 2603–2610. [Google Scholar] [CrossRef]
- Saran, S.; Isar, J.; Saxena, R.K. A Modified Method for the Detection of Microbial Proteases on Agar Plates Using Tannic Acid. J. Biochem. Biophys. Methods 2007, 70, 697–699. [Google Scholar] [CrossRef]
- Youassi, Y.; Ambata, A.; Benisa, J.; Ndondoni, D.; Ntah, A.; Bedine, A.; Nguemezi, T.; Sameza, M.L. The Potential of Trichoderma asperellum Organic Extract and Its Emulsion to Inhibit Cocoa (Theobroma cacao L.) Black Pod Disease and Induce Biochemical Defenses against Phytophthora megakarya. J. Plant Prot. Res. 2024, 64, 19. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karbalaei-Heidari, H.R. Isolation, Identification, Biochemical Properties and Potential Application of an Organic Solvent-Tolerant Lipase from Pseudomonas sp. Strain NEB-1. Iran. J. Sci. Technol. 2014, 38, 221–229. [Google Scholar] [CrossRef]
- Abada, E.A.; Elbaz, R.M.; Sonbol, H.; Korany, S.M. Optimization of Cellulase Production from Bacillus albus (Mn755587) and Its Involvement in Bioethanol Production. Polish J. Environ. Stud. 2021, 30, 2459–2466. [Google Scholar] [CrossRef]
- Nehad, E.A.; Yoness, M.F.; Reem, A.A. Optimization and Purification of Cellulase Produced by Penicillium decumbens and Its Application. Egypt. Pharm. J. 2019, 18, 391–402. [Google Scholar] [CrossRef]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand. Plants 2023, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Gnanesh, B.N.; Arunakumar, G.S.; Tejaswi, A.; Supriya, M.; Manojkumar, H.B.; Devi, S.S. Characterization and Pathogenicity of Lasiodiplodia theobromae Causing Black Root Rot and Identification of Novel Sources of Resistance in Mulberry Collections. Plant Pathol. J. 2022, 38, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Y.; Yu, H.; Zhou, J.; Hu, M.; Liu, A.; Wu, J.; Wang, H.; Zhang, C. Colletotrichum spp. Diversity Between Leaf Anthracnose and Crown Rot From the Same Strawberry Plant. Front. Microbiol. 2022, 13, 860694. [Google Scholar] [CrossRef]
- Helal, R.B.; Hosen, S.; Shamsi, S. Mycoflora Associated with Post-Harvest Disease of Papaya (Carica papaya L.) and Their Pathogenic Potentiality. Bangladesh J. Bot. 2018, 47, 389–395. [Google Scholar] [CrossRef]
- Gómez Godínez, L.J.; Rodríguez Escobar, J.G.; Rodríguez-Falconi, R.; Arteaga-Garibay, R.I.; Avendaño-Arrazate, C.H. Isolation and Characterization of Fungal Pathogens Associated with Carica papaya L. and Their Biocontrol with Trichoderma sp. Agro Prod. 2024, 185–197. [Google Scholar] [CrossRef]
- Pacheco-Esteva, M.C.; Soto-Castro, D.; Vásquez- López, A.; Tovar-Pedraza, J.M. Colletotrichum brevisporum Causing Anthracnose of Papaya (Carica papaya L.) in Mexico. Can. J. Plant Pathol. 2023, 45, 360–366. [Google Scholar] [CrossRef]
- Ghaljaei, F.; Hastings-tolsma, M.; Rezaee, N.; Ph, D. Reduced Sensitivity of Azoxystrobin and Thiophanate-Methyl Resistance in Lasiodiplodia theobromae from Papaya. Pesticide Biochemistry and Physiology. Pediatr. Neonatol. 2022, 162, 60–68. [Google Scholar] [CrossRef]
- de Medeiros Ferro, M.M.; Assunção, I.P.; de Andrade Lima, G.S.; de Farias, A.R.G.; de Neto, F.A.; de Melo, M.P. Lasiodiplodia theobromae and Lasiodiplodia brasiliense Causing Dieback and Rot Fruit of Jackfruit Tree in Brazil. Crop Prot. 2024, 184, 106763. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Assunção, I.P.; Lima, G.S.A.; Marques, M.W.; Lima, W.G.; Monteiro, J.H.A.; de Queiroz Balbino, V.; Michereff, S.J.; Phillips, A.J.L.; Câmara, M.P.S. Species of Lasiodiplodia Associated with Papaya Stem-End Rot in Brazil. Fungal Divers. 2014, 67, 127–141. [Google Scholar] [CrossRef]
- Rayón-Díaz, E.; Birke-Biewendt, A.B.; Velázquez-Estrada, R.M.; González-Estrada, R.R.; Ramírez-Vázquez, M.; Rosas-Saito, G.H.; Gutierrez-Martinez, P. Sodium Silicate and Chitosan: An Alternative for the in vitro Control of Colletotrichum gloeosporioides Isolated from Papaya (Carica papaya L.). Rev. Bio Ciencias 2021, 8, 15741. [Google Scholar] [CrossRef]
- Li, X.; Geng, C.; Huang, X.; Chen, S.; Yang, J.; Han, Y.; Lu, F.; Duan, K.; Gao, Q. Variations in Mycelial Growth and Virulence below 26 °C among Five Colletotrichum Strains from Strawberry. J. Gen. Plant Pathol. 2024, 90, 229–240. [Google Scholar] [CrossRef]
- Chutima, K.T. Biocontrol Ability of Marine Yeasts against Postharvest Diseases in Mangos Caused by Colletotrichum gloeosporioides and Lasiodiplodia theobromae. Eur. J. Plant Pathol. 2024, 168, 709–721. [Google Scholar] [CrossRef]
- Chen, X.Y.; Dai, D.J.; Zhao, S.F.; Shen, Y.; Wang, H.D.; Zhang, C.Q. Genetic Diversity of Colletotrichum spp. Causing Strawberry Anthracnose in Zhejiang, China. Plant Dis. 2020, 104, 1351–1357. [Google Scholar] [CrossRef]
- Gupta, A.; Choudhary, R.; Bashyal, M.; Rawat, K.; Singh, D.; Solanki, I.S. First Report of Root and Stem Rot Disease on Papaya Caused by Fusarium falciforme in India. Plant Dis. 2021, 103, 752–756. [Google Scholar] [CrossRef]
- Wita, A.; Białas, W.; Wilk, R.; Szychowska, K.; Czaczyk, K. The Influence of Temperature and Nitrogen Source on Cellulolytic Potential of Microbiota Isolated from Natural Environment. Polish J. Microbiol. 2019, 68, 105–114. [Google Scholar] [CrossRef]
- Sajili, M.H.; Wahida, W.N.; Badaluddin, A.; Khandaker, M.; Mohammed, S.; Kadir, J. Influence of Culture Media, Temperature and PH on Colletrotrichum gleosporioides, Isolated from Carica papaya in Besut, Terengganu, Malaysia. J. Agrobiotech 2017, 8, 49–55. Available online: https://journal.unisza.edu.my/agrobiotechnology/index.php/agrobiotechnology/article/view/114 (accessed on 14 May 2025).
- Estrada, A.B.; Dodd, J.C.; Jeffries, P. Effect of Humidity and Temperature on Conidial Germination and Appressorium Development of Two Philippine Isolates of the Mango Anthracnose Pathogen Colletotrichum gloeosporioides. Plant Pathol. 2000, 49, 608–618. [Google Scholar] [CrossRef]
- Palmero, D.; Cara, M.D.; Iglesias, C.; Gálvez, L.; Tello, J.C. Comparative Study of the Pathogenicity of Seabed Isolates of Fusarium equiseti and the effect of the composition of the mineral salt medium and temperature on mycelial growth. Braz. J. Microbiol. 2011, 42, 948–953. [Google Scholar] [CrossRef]
- Yan, H.; Nelson, B. Effect of Temperature on Fusarium solani and Fusarium tricinctum Growth and Disease Development in Soybean. Can. J. Plant Pathol. 2020, 42, 527–537. [Google Scholar] [CrossRef]
- Almiman, B. Identifying the Optimal Temperature and Water Activity Conditions of Phytopathogenic Fungi Recovered from Al-Baha Province. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 640–651. [Google Scholar] [CrossRef]
- Fovo, J.D.; Dostaler, D.; Bernier, L. Influence of Culture Media and Temperature on Growth and Sporulation of Lasiodiplodia theobromae, Pestalotiopsis microspora and Fusarium oxysporum Isolated from Ricinodendron heudelotii in Cameroon. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3098–3112. [Google Scholar] [CrossRef]
- Félix, C.; Duarte, A.S.; Vitorino, R.; Guerreiro, A.C.L.; Domingues, P.; Correia, A.C.M.; Alves, A.; Esteves, A.C. Temperature Modulates the Secretome of the Phytopathogenic Fungus Lasiodiplodia theobromae. Front. Plant Sci. 2016, 7, 1096. [Google Scholar] [CrossRef]
- Zhao, X.; Chai, J.; Wang, F.; Jia, Y. Optimization of Submerged Culture Parameters of the Aphid Pathogenic Fungus Fusarium equiseti Based on Sporulation and Mycelial Biomass. Microorganisms 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, K.; Chanu, W.T.; Sinha, B.; Thangjam, B.; Hasan, W.; Devi, K.S.; Chakma, T.; Phurailatpam, S.; Mishra, L.K.; Singh, G.M. Deciphering Growth Abilities of Fusarium oxysporum f. sp. pisi under Variable Temperature, PH and Nitrogen. Front. Microbiol. 2023, 14, 1228442. [Google Scholar] [CrossRef]
- Febbiyanti, T.R.; Widodo, W.; Wiyono, S.; Yahya, S. Effect of PH and Storage Period to the Growth of Lasiodiplodia theobromae Which Causes the Stem Canker on the Rubber Plant. J. Penelit. Karet 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Latha, P.; Prakasam, V.; Jonathan, E.I.; Samiyappan, R.; Natarajan, C. Effect of Culture Media and Environmental Factors on Mycelial Growth and Pycnidial Production of Lasiodiplodia theobromae in Physic Nut (Jatropha curcas). J. Environ. Biol. 2013, 34, 683–687. Available online: https://pubmed.ncbi.nlm.nih.gov/24640243/ (accessed on 14 May 2025).
- Akhtar, N.; Chandra, R.; Mazhar, Z. Influence of Media, Temperature and PH on Growth of Colletotrichum capsici (Syd.) Causing Anthracnose Disease of Chilli. J. Pharmacogn. Phytochem. 2018, 7, 3280–3284. Available online: https://www.phytojournal.com/archives/2018.v7.i4.5458/influence-of-media-temperature-and-ph-on-growth-of-ltemgtcolletotrichum-capsici-ltemgtsyd-causing-anthracnose-disease-of-chilli (accessed on 14 May 2025).
- Hasan, M.F.; Islam, M.A.; Sikdar, B. PCR and Sequencing Base Detection of Gummosis Disease on Citrus aurantifolia Caused by Lasiodiplodia theobromae and Evaluation of Its Antagonisms. J. Adv. Microbiol. 2020, 20, 77–90. [Google Scholar] [CrossRef]
- Feng, Q.; Cao, S.; Liao, S.; Wassie, M.; Sun, X.; Chen, L.; Xie, Y. Fusarium equiseti-Inoculation Altered Rhizosphere Soil Microbial Community, Potentially Driving Perennial Ryegrass Growth and Salt Tolerance. Sci. Total Environ. 2023, 871, 162153. [Google Scholar] [CrossRef]
- Li, F.; Lu, D.; Meng, F.; Tian, C. Transcription Factor CgSte12 Regulates Pathogenicity by Affecting Appressorium Structural Development in the Anthracnose-Causing Fungus Colletotrichum gloeosporioides. Phytopathology 2024, 114, 1832–1842. [Google Scholar] [CrossRef]
- González, N.; Ragazzo, J.; Barros, C.; Narváez, Z.; Montserrat, S. Yeasts with Potential Biocontrol of Colletotrichum gloeosporioides in Avocado (Persea americana Mill. Cv. Hass) and Characterization of Yamadazyma Mexicana Mechanisms. Eur. J. Plant Pathol. 2023, 165, 525–543. [Google Scholar] [CrossRef]
- Gunamalai, L.; Duanis-Assaf, D.; Sharir, T.; Maurer, D.; Feygenberg, O.; Sela, N.; Alkan, N. Comparative Characterization of Virulent and Less-Virulent Lasiodiplodia Theobromae Isolates. Mol. Plant-Microbe Interact. 2023, 36, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Sanzo-Miró, M.; Medina, A.; Terry, L.A.; Alamar, M.C. Elucidating the Impact of Environmental Factors on the Growth of Colletotrichum coccodes Strains Isolated from Potato Tubers in Great Britain. Int. J. Food Microbiol. 2024, 423, 110843. [Google Scholar] [CrossRef] [PubMed]
- Mejía, C.; Bautista, E.J.; García, L.; Barrios Murcia, J.C.; Barrera, G. Assessment of Fungal Lytic Enzymatic Extracts Produced Under Submerged Fermentation as Enhancers of Entomopathogens’ Biological Activity. Curr. Microbiol. 2024, 81, 217. [Google Scholar] [CrossRef]
- Koshila Ravi, R.; Prema Sundara Valli, P.; Muthukumar, T. Physiological Characterization of Root Endophytic Fusarium haematococcum for Hydrolytic Enzyme Production, Nutrient Solubilization and Salinity Tolerance. Biocatal. Agric. Biotechnol. 2022, 43, 102392. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and Pathogenicity of Lasiodiplodia Species Associated with Dieback of Mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef]
- Huang, H.; Tian, C.; Huang, Y.; Huang, H. Biological Control of Poplar Anthracnose Caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. Egypt. J. Biol. Pest Control 2020, 30, 104. [Google Scholar] [CrossRef]
- Jat, L.; Sharma, P.; Gour, N. Production of Enzymes and Culture Filtrates by Colletotrichum gloeosporioides Penz. Causing Banana Fruit Rot. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 177–180. [Google Scholar] [CrossRef]
- Cruz-Davila, J.; Perez, J.V.; del Castillo, D.S.; Diez, N. Fusarium graminearum as a Producer of Xylanases with Low Cellulases When Grown on Wheat Bran. Biotechnol. Rep. 2022, 35, e00738. [Google Scholar] [CrossRef]
- Alvarez-Navarrete, M.; Alonso-Hurtado, K.L.; Flores-García, A.; Altamirano-Hernández, J.; Martínez-Pacheco, M.M.; Velázquez-Becerra, C. Comparative Study of the Extracellular Holocellulolytic Activity of Fusarium solani and Aspergillus sp. in Corn Stover. Fermentation 2024, 10, 84. [Google Scholar] [CrossRef]
- Gutiérrez-Domínguez, D.E.; Chí-Manzanero, B.; Rodríguez-Argüello, M.M.; Todd, J.N.A.; Islas-Flores, I.; Canseco-Pérez, M.Á.; Canto-Canché, B. Identification of a Novel Lipase with AHSMG Pentapeptide in Hypocreales and Glomerellales Filamentous Fungi. Int. J. Mol. Sci. 2022, 23, 9367. [Google Scholar] [CrossRef] [PubMed]




| Primers | Sequences (5′-3′) | Length (Pb) | Tm (°C) | GC (%) |
|---|---|---|---|---|
| ITS-1 | TCCGTTCCTCAACCAGCGG | 19 | 53 | 63 |
| ITS-4 | TCCTCCGCTTATTGATATGC | 20 | 50 | 45 |
| DMV2 | DMV3 | DMV5 | |||
|---|---|---|---|---|---|
| Surface (cm) | Depth (cm) | Surface (cm) | Depth (cm) | Surface (cm) | Depth (cm) |
| 6.2 ± 0.31 1 | 1.1 ± 0.13 α | 4 ± 0.11 2 | 0.97 ± 0.02 α | 7.4 ± 0.51 3 | 2.1 ± 0.3 β |
| Isolates | DMV2 | DMV3 | DMV5 | |
|---|---|---|---|---|
| Colony shape | Circular | Irregular | Circular | |
| Relief of the colony | Convex | Bomb | Flat | |
| Allure of contours | Loop | Wavy | Regular | |
| Colony surface | Rough | Dense | Smooth | |
| Mycelium color | White to orange-brown | White | White to light-cream | |
| Colony Texture | Wrinkled | Cottony | Fluffy | |
| Consistency | Dried | Mucosa | Creamy | |
| Opacity | Opaque | Opaque | Translucent | |
| Growth speed | 14.1 mm/day | 13 mm/day | 44 mm/day | |
| Type of thallus | No Septa | Septa | Septa | |
| Shape of conidia | Elongated | Fusiform | Ovoid | |
| Size of conidia | 14.3 µm | 18.7 μm | 22 μm | |
| Load of conidia | Mean | Strong | Mean | |
| Isolates | NCBI AN. of Isolates | BP | NCBI AN. of Reference Strains | Banket (%) | Similarity (%) | Species |
|---|---|---|---|---|---|---|
| DMV2 | PV576402 | 580 | FJ940734 | 100 | 100 | Colletotrichum gloeosporioides |
| KJ632401 | 97 | 100 | ||||
| MT597824 | 95 | 99.46 | ||||
| DMV3 | PV576403 | 555 | MT447515 | 99 | 99.46 | Fusarium equiseti |
| KM246255 | 99 | 99.46 | ||||
| MW580412 | 99 | 99.46 | ||||
| DMV5 | PV576404 | 555 | MH049696 | 99 | 99.82 | Lasiodiplodia theobromae |
| MN180881 | 99 | 99.64 | ||||
| MH793568 | 99 | 99.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davy, M.V.; Steve, V.O.; Sammuel, T.R.; Boat, M.A.B.; Moise, N.A.; Busuioc, A.C.; Mbondi, P.E.; Botezatu, A.V.D.; Jules, M.K.; Mihaila, M.D.I.; et al. Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon. J. Fungi 2025, 11, 385. https://doi.org/10.3390/jof11050385
Davy MV, Steve VO, Sammuel TR, Boat MAB, Moise NA, Busuioc AC, Mbondi PE, Botezatu AVD, Jules MK, Mihaila MDI, et al. Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon. Journal of Fungi. 2025; 11(5):385. https://doi.org/10.3390/jof11050385
Chicago/Turabian StyleDavy, Moussango Victor, Voundi Olugu Steve, Tchabong Raymond Sammuel, Marie Ampères Bedine Boat, Ntah Ayong Moise, Anna Cazanevscaia Busuioc, Priscile Ebong Mbondi, Andreea Veronica Dediu Botezatu, Manz Koule Jules, Maria Daniela Ionica Mihaila, and et al. 2025. "Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon" Journal of Fungi 11, no. 5: 385. https://doi.org/10.3390/jof11050385
APA StyleDavy, M. V., Steve, V. O., Sammuel, T. R., Boat, M. A. B., Moise, N. A., Busuioc, A. C., Mbondi, P. E., Botezatu, A. V. D., Jules, M. K., Mihaila, M. D. I., Dinica, R. M., & Lambert, S. M. (2025). Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon. Journal of Fungi, 11(5), 385. https://doi.org/10.3390/jof11050385










