Molecular Identification of Fusarium Isolates from Bozcaada Çavuş and Karalahna Grapes in Türkiye
Abstract
1. Introduction
2. Materials and Methods
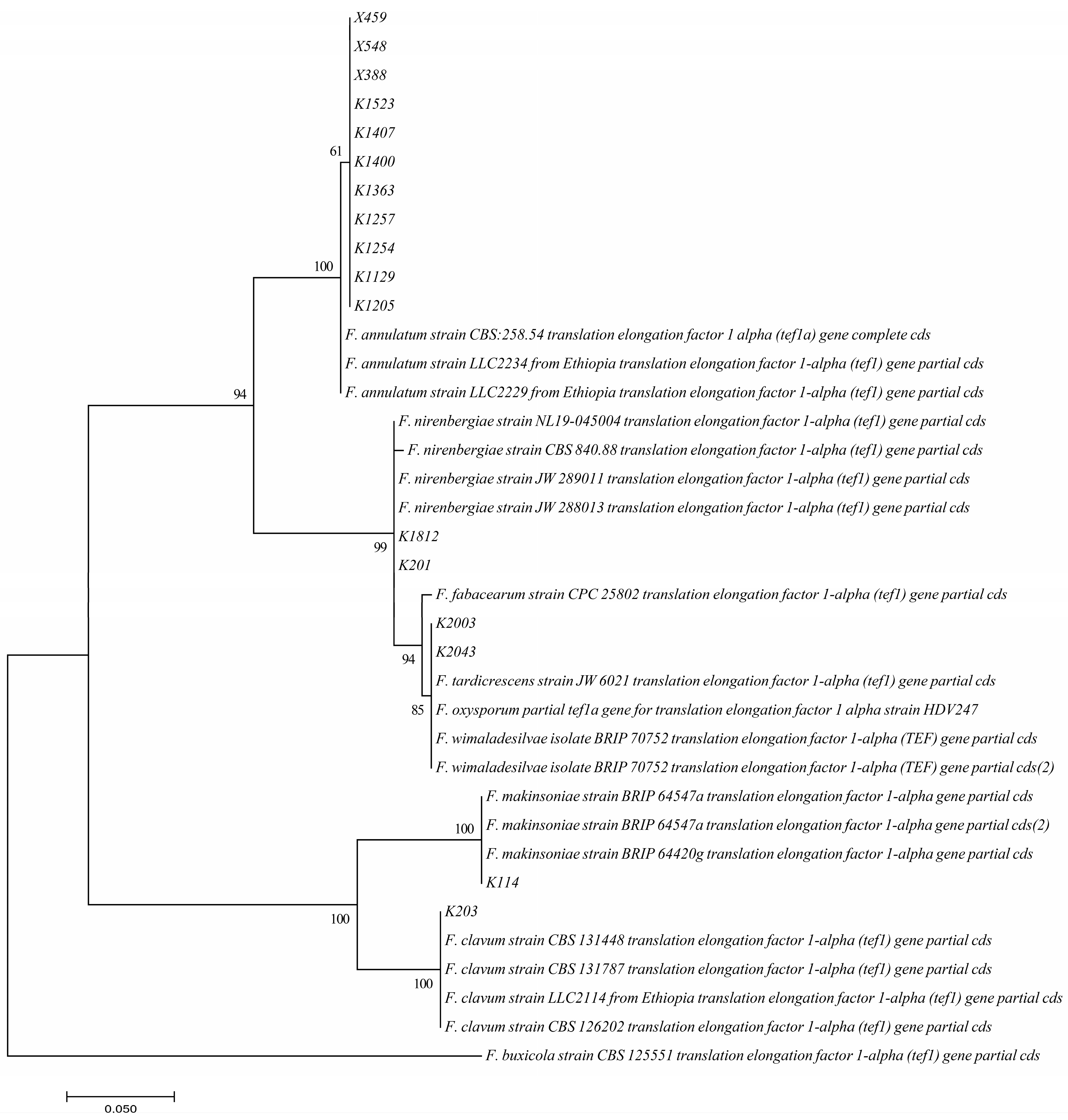
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aydoğdu, M. Türkiye’nin Bozcaada (Tenedos) Parazitoit Aleiodes Wesmael, 1838 (Hymenoptera: Braconidae: Rogadinae) Türleri. Türk. biyo. müc. derg. Haziran 2018, 9, 38–47. [Google Scholar] [CrossRef][Green Version]
- Özcan Ateş, G.; Zorba, N.N.D. Determination of mycobiota and microbial loads of grapes grown in Bozcaada, Turkey. J. Microbiol, Biotechnol. Food Sci. 2020, 9, 899–906. [Google Scholar] [CrossRef]
- Türkben, C.; Gül, F.; Uzar, Y. Türkiye’de Bağcılığın Tarım Turizmi (Agro-Turizm) İçinde Yeri ve Önemi. KMUSEKAD 2012, 2, 47–50. Available online: https://dergipark.org.tr/tr/pub/kmusekad/issue/10213/125504 (accessed on 28 March 2025).
- Şahin, E.; Dardeniz, A. Bozcaada Çavuşu ve Mevcut Tozlayıcı Çeşitleri ile Bazı Muhtemel Tozlayıcı Çeşitlerin Polen Canlılık ve Çimlenme Oranlarının Belirlenmesi. KSÜ Tar. Doga Derg. 2023, 26, 97–106. [Google Scholar] [CrossRef]
- Durmuş, E.; Yüceer, S.E.; Tan, S. Coğrafi İşaret Tescilinin Yaratacağı Sosyo-Ekonomik Etkilerin İncelenmesi: Bozcaada Çavuş Üzümü Örneği. TJAE 2022, 28, 21–29. [Google Scholar] [CrossRef]
- Çelebi Uzkuç, N.M.; Şişli, B.; Karagül Yüceer, Y.; Bayhan, A.; Kırca Toklucu, A. Soğuk Maserasyonun Karalahna Ve Cabernet Sauvıgnon Şıralarının Antosiyanin Ve Uçucu Bileşenleri Üzerine Etkisi. J. Food 2018, 43, 663–676. [Google Scholar] [CrossRef]
- Şişli, B.; Çelebi Uzkuç, N.M.; Bayhan, A.; Kırca Toklucu, A. Karalahna Şaraplarının Fenolik Bileşenleri Üzerine Fermantasyon Tekniği Ve Şişede Depolamanın Etkisi. J. Food 2023, 48, 130–143. [Google Scholar] [CrossRef]
- Tournas, V.H.; Katsoudas, E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef]
- Hocking, A.D.; Su-lin, L.L.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Fungi and mycotoxins in vineyards and grape products. Int. J. Food Microbiol. 2007, 119, 84–88. [Google Scholar] [CrossRef]
- Mikušová, P.; Ritieni, A.; Santini, A.; Juhasová, G.; Šrobárová, A. Contamination by moulds of grape berries in Slovakia. Food Add. Contam. 2010, 27, 738–747. [Google Scholar] [CrossRef]
- Tančinová, D.; Mašková, Z.; Rybárik, Ľ.; Michalová, V. Species of genera Botrytis, Fusarium and Rhizopus on grapes of the Slovak origin. Potravinarstvo 2017, 11, 403–409. [Google Scholar] [CrossRef][Green Version]
- Sharma, L.; Marques, G. Fusarium, an entomopathogen—A myth or reality? Pathogens 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Akgül, D.S.; Önder, S.; Savaş, N.G.; Yıldız, M.; Bülbül, İ.; Özarslandan, M. Molecular Identification and Pathogenicity of Fusarium Species Associated with Wood Canker, Root and Basal Rot in Turkish Grapevine Nurseries. J. Fungi 2024, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Pietri, A.; Bertuzzi, T.; Languasco, L.; Giorni, P.; Kozakiewicz, Z. Occurrence of ochratoxin A–producing fungi in grapes grown in Italy. J. Food Prot. 2003, 66, 633–636. [Google Scholar] [CrossRef]
- Chiotta, M.L.; Ponsone, M.L.; Combina, M.; Torres, A.M.; Chulze, S.N. Aspergillus section Nigri species isolated from different wine-grape growing regions in Argentina. Int. J. Food Microbiol. 2009, 136, 137–141. [Google Scholar] [CrossRef] [PubMed]
- García-Cela, E.; Crespo-Sempere, A.; Gil-Serna, J.; Porqueres, A.; Marin, S. Fungal diversity, incidence and mycotoxin contamination in grapes from two agro-climatic Spanish regions with emphasis on Aspergillus species. J. Sci. Food Agric. 2015, 95, 1716–1729. [Google Scholar] [CrossRef]
- Garmendia, G.; Vero, S. Occurrence and biodiversity of Aspergillus section Nigri on ‘Tannat’grapes in Uruguay. Int. J. Food Microbiol. 2016, 216, 31–39. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Aristeidou, E.; Lazari, M.; Tsolakidou, M.; Tsaltas, D.; Christofidou, M.; Kafouris, D.; Christou, E.; Ioannou, N. Biodiversity and ochratoxin A profile of Aspergillus section Nigri populations isolated from wine grapes in Cyprus vineyards. Food Micro. 2017, 67, 106–115. [Google Scholar] [CrossRef]
- Özcan Ateş, G.; Zorba, N.N.D.; Şen, B. Biodiversity of ochratoxigenic Aspergillus species isolated from çavuş and karalahna grapes in Bozcaada, Türkiye. Anatol. J. Bot. 2024, 8, 39–45. [Google Scholar] [CrossRef]
- Šrobárová, A.; Kakalíková, U. Fungal disease of grapevines. Eur. J. Plant Sci. Biotechnol. 2007, 1, 84–90. [Google Scholar]
- Logrieco, A.; Ferracane, R.; Haidukowsky, M.; Cozzi, G.; Visconti, A.; Ritieni, A. Fumonisin B2 production by Aspergillus niger from grapes and natural occurrence in must. Food Addit Contam Part A 2009, 26, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Mašková, Z.; Tančinová, D.; Rybárik, L.; Felšӧciová, S. Colonization of grape berries by the genus Fusarium and toxigenity of the most common representatives. J. Microb. Biotech. Food Sci. 2014, 3, 256–258. Available online: https://office2.jmbfs.org/index.php/JMBFS/article/view/7613 (accessed on 25 March 2025).
- EU Commission Regulation 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02023R0915-20250101 (accessed on 30 March 2025).
- Mikušová, P.; Šrobárová, A.; Sulyok, M.; Santini, A. Fusarium fungi and associated metabolites presence on grapes from Slovakia. Mycotoxin Res. 2013, 29, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chehri, K. Morphological and Molecular Characterisation of Campylocarpon fasciculare and Fusarium spp., the Cause of Black Disease of Grapevine in Iran. Pertanika J. Trop. Agric. Sci. 2017, 40, 587–600. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Boulé, J.; Hrycan, J.; O’Gorman, D.T. Potential role of Fusarium spp. in grapevine decline. Phytopathol. Mediterr. 2023, 62, 269–281. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, W.; Zhang, J.; Wang, H.; Peng, J.; Wang, X.; Yan, J. Belowground microbiota analysis indicates that Fusarium spp. exacerbate grapevine trunk disease. Environ. Microbiome 2023, 18, 29. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, J.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Walsh, T.J.; Hayden, R.T.; Larone, D.H. Larone’s Medically Important Fungi, 6th ed.; American Society of Microbiology: Washington, DC, USA, 2018. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 9, 2044–2049. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus fusarium are nonorthologous. Mol. Phylogeny Evol. Fungi 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–9272. [Google Scholar] [CrossRef]
- O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Rober, V.A.R.; Crous, P.W.; Johnston, P.R.; Aoki, T.; Rooney, A.P.; et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’keeffe, T.L.; Ho, Y.S.; Santillan, C.J. Occurrence of Ochratoxin A Contamination and Detection of Ochratoxigenic Aspergillus Species in Retail Samples of Dried Fruits and Nuts. J. Food Prot. 2015, 78, 836–842. [Google Scholar] [CrossRef]
- Bolton, S.L.; Brannen, P.M.; Glenn, A.E. A novel population of Fusarium fujikuroi isolated from Southeastern U.S. Winegrapes reveals the need to re-evaluate the species′ Fumonisin production. Toxins 2016, 8, 254. [Google Scholar] [CrossRef]
- Cosseboom, S.D.; Hu, M. Diversity, Pathogenicity, and Fungicide Sensitivity of Fungal Species Associated with Late-Season Rots of Wine Grape in the Mid-Atlantic United States. Plant Dis. 2021, 105, 3101–3110. [Google Scholar] [CrossRef]
- Sesli, E.; Asan, A.; Selçuk, F.; Abacı Günyar, Ö.; Akata, I.; Akgül, H.; Aktaş, S.; Alkan, S.; Allı, H.; Aydoğdu, H.; et al. Türkiye Mantarları Listesi (The Checklist of the Fungi of Turkey); Ali Nihat Gökyiğit Vakfı Yayını: İstanbul, Türkiye, 2020; pp. XVII + 1177. [Google Scholar]
- Asan, A.; Selçuk, F.; Giray, G.; Aydoğdu, H.; Ulukapı, M.; Ceylan, M.F. Türkiye mantarları listesi’ne ilaveler-1. Bağbahçe Bilim. Dergisi. 2022, 9, 65–89. [Google Scholar] [CrossRef]
- Asan, A.; Karabıyık, H.; Giray, G. Türkiye mantarları listesi’ne eklentiler-2. Bağbahçe. Bilim. Dergisi. 2024, 11, 25–43. [Google Scholar] [CrossRef]
- Bustamante, M.I.; Elfar, K.; Smith, R.; Bettiga, L.; Tian, T.; Torres-Londoño, G.A.; Eskalen, A. First report of Fusarium annulatum associated with young vine decline in California. Plant Dis. 2022, 106, 2752. [Google Scholar] [CrossRef]
- Molnár, O.; Vida, G.; Puskás, K. Fusarium species associated with Fusarium head blight in Hungarian wheat fields. Plant Dis. 2024, 108, 558–562. [Google Scholar] [CrossRef]
- Afolabi, O.O.; Bigirimana, V.D.P.; Hua, G.K.H.; Oni, F.E.; Bertier, L.; Onwughalu, J.; Oyetunji, O.E.; Ogunbayo, A.; Van De Velde, M.; Nyamangyoku, O.I.; et al. Fusarium and Sarocladium Species Associated with Rice Sheath Rot Disease in Sub-Saharan Africa. Diversity 2023, 15, 1090. [Google Scholar] [CrossRef]
- Dewing, C.; Visagie, C.M.; Steenkamp, E.T.; Wingfield, B.D.; Yilmaz, N. Three new species of Fusarium (Nectriaceae, Hypocreales) isolated from Eastern Cape dairy pastures in South Africa. MycoKeys 2025, 115, 241–271. [Google Scholar] [CrossRef]
- Gonçalves, A.; Palumbo, R.; Guimarães, A.; Gkrillas, A.; Dall’Asta, C.; Dorne, J.-L.; Battilani, P.; Venâncio, A. The route of mycotoxins in the grape food chain. Am. J. Enol. Vitic. 2020, 71, 89–104. [Google Scholar] [CrossRef]

| Title 1 | Abbreviations | Primer Sequence (5′–3′) | Reference |
|---|---|---|---|
| Translation Elongation Factor 1-alpha (tef1) | EF1-F | ATGGGTAAGGARGACAAGAC | [32] |
| EF2-R | GGARGTACCAGTSATCATG | ||
| Βeta-Tubulin (tub2) | T1-R | AACATGCGTGAGATTGTAAGT | [33] |
| T22-R | TCTGGATGTTGTTGGGAATCC |
| Isolate No. | tef1 | tub2 | Collection Date | Isolation Source | Location | ||||
|---|---|---|---|---|---|---|---|---|---|
| Closest Relative | Closest Accession Number | Identity (%) | Closest Relative | Closest Accession Number | Identity (%) | ||||
| K114 | F. makinsoniae | OQ626867.1 | 100 | F. incarnatum | MT895843.1 | 99.39 | 4 August 2015 | Çavuş grape | Bozcaada Çayır |
| F. cf. incarnatum | MW076638.1 | 100 | F. cf. ‘incarnatum-equiseti’ | PQ435149.1 | 99.39 | ||||
| F. makinsoniae | OQ626866.1 | 100 | F. cf. ‘incarnatum-equiseti’ | PQ360889.1 | 99.39 | ||||
| F. incarnatum | KP133060.1 | 99.39 | |||||||
| F. cf. ‘incarnatum-equiseti’ | PQ360887.1 | 99.39 | |||||||
| F. incarnatum | KP133061.1 | 99.39 | |||||||
| K201 | F. nirenbergiae | PQ819571.1 | 100 | F. glycines | OP642084.1 | 98.57 | 4 August 2015 | Karalahna grape | Bozcaada Sulubahçe |
| F. oxysporum | DQ016269.1 | 100 | F. oxysporum | MH827997.1 | 98.57 | ||||
| F. nirenbergiae | PP431416.1 | 100 | F. oxysporum | OP901509.1 | 98.57 | ||||
| F. nirenbergiae | MH485017.1 | 100 | F. oxysporum | PP694827.1 | 98.57 | ||||
| F. oxysporum f. sp. cepae | EU220395.1 | 100 | F. oxysporum | PQ435146.1 | 98.57 | ||||
| F. oxysporum | MF327613.1 | 100 | |||||||
| K203 | F. equiseti | MK061540.1 | 100 | F. clavum | PP796758.1 | 100 | 4 August 2015 | Karalahna grape | Bozcaada Sulubahçe |
| F. clavum | PP782146.1 | 100 | F. clavum | ON292434.1 | 100 | ||||
| F. equiseti | KF651956.1 | 100 | F. clavum | PP796769. | 100 | ||||
| Fusarium sp. NRRL 53091 | GU250573.1 | 100 | |||||||
| F. clavum | ON292415.1 | 100 | |||||||
| K1129 | F. proliferatum | PP928449.1 | 99.76 | F. fujikuroi | MK784204.1 | 99.61 | 20 June 2016 | Çavuş grape | Bozcaada Çayır |
| F. annulatum | OQ925589.1 | 99.76 | F. fujikuroi | MN896954.1 | 99.61 | ||||
| F. proliferatum | MT305198.1 | 99.76 | F. proliferatum | MZ680574.1 | 99.61 | ||||
| F. annulatum | OK888534.1 | 99.76 | F. proliferatum | ON152866.1 | 99.61 | ||||
| F. proliferatum | PP782632.1 | 99.76 | F. proliferatum | JX174034.1 | 99.61 | ||||
| K1205 | F. proliferatum | PP928449.1 | 100 | F. fujikuroi | MK784204.1 | 100 | 20 June 2016 | Çavuş grape | Bozcaada Çayır |
| F. annulatum | OQ925589.1 | 100 | F. fujikuroi | MN896954.1 | 100 | ||||
| F. proliferatum | MT305198.1 | 100 | F. proliferatum | MZ680574.1 | 100 | ||||
| F. annulatum | OK888534.1 | 100 | F. proliferatum | LC171211.1 | 100 | ||||
| F. proliferatum | PP782632.1 | 100 | F. proliferatum | ON152866.1 | 100 | ||||
| K1254 | F. proliferatum | PP928449.1 | 99.76 | F. fujikuroi | MK784204.1 | 99.61 | 20 June 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. annulatum | OQ925589.1 | 99.76 | F. fujikuroi | MN896954.1 | 99.61 | ||||
| F. proliferatum | MT305198.1 | 99.76 | F. proliferatum | MZ680574.1 | 99.61 | ||||
| F. annulatum | OK888534.1 | 99.76 | F. proliferatum | ON152866.1 | 99.61 | ||||
| F. proliferatum | PP782632.1 | 99.76 | |||||||
| K1257 | F. proliferatum | PP928449.1 | 100 | F. fujikuroi | MK784204.1 | 99.80 | 20 June 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. annulatum | OQ925589.1 | 100 | F. fujikuroi | MN896954.1 | 99.80 | ||||
| F. proliferatum | MT305198.1 | 100 | F. proliferatum | MZ680574.1 | 99.80 | ||||
| F. annulatum | OK888534.1 | 100 | F. proliferatum | LC171211.1 | 99.80 | ||||
| F. proliferatum | PP782632.1 | 100 | F. proliferatum | ON152866.1 | 99.80 | ||||
| K1363 | F. proliferatum | OP273535.1 | 99.75 | F. fujikuroi | MK784204.1 | 99.61 | 30 June 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. proliferatum | PP928449.1 | 99.75 | F. fujikuroi | MN896954.1 | 99.61 | ||||
| F. annulatum | OQ925589.1 | 99.75 | F. proliferatum | MZ680574.1 | 99.61 | ||||
| F. proliferatum | MT305198.1 | 99.75 | F. proliferatum | ON152866.1 | 99.61 | ||||
| F. annulatum | OK888534.1 | 99.75 | F. proliferatum | JX174034.1 | 99.61 | ||||
| K1400 | F. proliferatum | PP928449.1 | 99.76 | F. fujikuroi | MK784204.1 | 99.80 | 10 July 2016 | Çavuş grape | Bozcaada Çayır |
| F. annulatum | OQ925589.1 | 99.76 | F. fujikuroi | MN896954.1 | 99.80 | ||||
| F. proliferatum | MT305198.1 | 99.76 | F. proliferatum | MZ680574.1 | 99.80 | ||||
| F. annulatum | OK888534.1 | 99.76 | F. proliferatum | LC171211.1 | 99.80 | ||||
| F. proliferatum | PP782632.1 | 99.76 | F. proliferatum | ON152866. | 99.80 | ||||
| K1407 | F. proliferatum | PP928449.1 | 98.97 | F. fujikuroi | MK784204.1 | 99.80 | 10 July 2016 | Çavuş grape | Bozcaada Çayır |
| F. proliferatum | MT305198.1 | 98.97 | F. fujikuroi | MN896954.1 | 99.80 | ||||
| F. annulatum | OK888534.1 | 98.97 | F. proliferatum | MZ680574.1 | 99.80 | ||||
| F. proliferatum | PP782632.1 | 98.97 | F. proliferatum | LC171211.1 | 99.80 | ||||
| F. annulatum | OQ925612.1 | 98.97 | F. proliferatum | ON152866.1 | 99.80 | ||||
| K1523 | F. proliferatum | PP928449.1 | 100 | F. fujikuroi | MK784204.1 | 100 | 10 July 2016 | Çavuş grape | Bozcaada Çayır |
| F. annulatum | OQ925589.1 | 100 | F. fujikuroi | MN896954.1 | 100 | ||||
| F. proliferatum | MT305198.1 | 100 | F. proliferatum | MZ680574.1 | 100 | ||||
| F. annulatum | OK888534.1 | 100 | F. proliferatum | LC171211.1 | 100 | ||||
| F. proliferatum | PP782632.1 | 100 | F. proliferatum | ON152866.1 | 100 | ||||
| K1812 | F. nirenbergiae | PQ819571.1 | 100 | F. oxysporum f. sp. conglutinans | ON292451.1 | 99.81 | 1 August 2016 | Karalahna grape | Bozcaada Çayır |
| F. oxysporum | DQ016269.1 | 100 | F. avenaceum | ON292477.1 | 99.81 | ||||
| F. nirenbergiae | PP431416.1 | 100 | F. oxysporum | LC414363.1 | 99.81 | ||||
| F. nirenbergiae | MH485017.1 | 100 | F. oxysporum f. sp. conglutinans | ON292481.1 | 99.81 | ||||
| F. oxysporum f. sp. cepae | EU220395.1 | 100 | F. nirenbergiae | ON292493.1 | 99.81 | ||||
| K2003 | F. oxysporum | KP964863.1 | 100 | F. oxysporum f. sp. dianthi | LT841229.1 | 99.80 | 11August 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. oxysporum | KF537337.1 | 100 | F. oxysporum | MN451160.1 | 99.80 | ||||
| F. oxysporum | MT078506.1 | 100 | F. oxysporum f. sp. pisi | KP964945.1 | 99.80 | ||||
| F. oxysporum | MK461970.1 | 100 | F. oxysporum f. sp. vasinfectum | KT323796.1 | 99.80 | ||||
| F. oxysporum | OQ511060.1 | 100 | F. oxysporum | MN451093.1 | 99.80 | ||||
| K2043 | F. oxysporum | KP964863.1 | 99.75 | F. oxysporum f. sp. dianthi | LT841229.1 | 100 | 22 August 2016 | Karalahna grape | Bozccada Sulubahçe |
| F. oxysporum | KF537337.1 | 99.75 | F. oxysporum | MN451160.1 | 100 | ||||
| F. oxysporum | MT078506.1 | 99.75 | F. oxysporum f. sp. pisi | KP964945.1 | 100 | ||||
| F. oxysporum | MK461970.1 | 99.75 | F. oxysporum f. sp. vasinfectum | KT323796.1 | 100 | ||||
| F. oxysporum | OQ511060.1 | 99.75 | F. oxysporum | MN451093.1 | 100 | ||||
| X388 | F. proliferatum | PP928449.1 | 98.97 | F. fujikuroi | MK784204.1 | 100 | 20 July 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. proliferatum | MT305198.1 | 98.97 | F. fujikuroi | MN896954.1 | 100 | ||||
| F. annulatum | OK888534.1 | 98.97 | F. proliferatum | MZ680574.1 | 100 | ||||
| F. proliferatum | PP782632.1 | 98.97 | F. proliferatum | LC171211.1 | 100 | ||||
| F. annulatum | OQ92561 | 98.97 | F. proliferatum | ON152866.1 | 100 | ||||
| X459 | F. proliferatum | PP928449.1 | 99.76 | F. fujikuroi | MK784204.1 | 100 | 1 August 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. annulatum | OQ925589.1 | 99.76 | F. fujikuroi | MN896954.1 | 100 | ||||
| F. proliferatum | MT305198.1 | 99.76 | F. proliferatum | MZ680574.1 | 100 | ||||
| F. annulatum | OK888534.1 | 99.76 | F. proliferatum | ON152866.1 | 100 | ||||
| F. proliferatum | PP782632.1 | 99.76 | F. proliferatum | JX174034.1 | 100 | ||||
| X548 | F. proliferatum | PP928449.1 | 100 | F. fujikuroi | MK784204.1 | 100 | 11 August 2016 | Çavuş grape | Bozcaada Sulubahçe |
| F. annulatum | OQ925589.1 | 100 | F. fujikuroi | MN896954.1 | 100 | ||||
| F. proliferatum | MT305198.1 | 100 | F. proliferatum | MZ680574.1 | 100 | ||||
| F. annulatum | OK888534.1 | 100 | F. proliferatum | LC171211.1 | 100 | ||||
| F. proliferatum | PP782632.1 | 100 | F. proliferatum | ON152866.1 | 100 | ||||
| Isolate No. | Identity (%) | Closest Species | Strain Number |
|---|---|---|---|
| K114 | 100 | F. makinsoniae (possible synonym of F. guilinense) | BRIP 64420g |
| 100 | F. makinsoniae (possible synonym of F. guilinense) | BRIP 64547a | |
| K201 | 100 | F. nirenbergiae | JW 288013 |
| 100 | F. nirenbergiae | JW 289011 | |
| K203 | 100 | F. clavus (as ‘clavum’) | CBS 131448 |
| 100 | F. clavus (as ‘clavum’) | CBS 131787 | |
| K1129 | 98.74 | F. annulatum | CBS 258.54 |
| K1205 | 99.36 | F. annulatum | CBS 258.54 |
| K1254 | 99.37 | F. annulatum | CBS 258.54 |
| K1257 | 99.37 | F. annulatum | CBS 258.54 |
| K1363 | 98.71 | F. annulatum | CBS 258.54 |
| K1400 | 99.03 | F. annulatum | CBS 258.54 |
| K1407 | 98.71 | F. annulatum | CBS 258.54 |
| K1523 | 99.36 | F. annulatum | CBS 258.54 |
| K1812 | 100 | F. nirenbergiae | JW 288013 |
| 100 | F. nirenbergiae | JW 289011 | |
| K2003 | 100 | F. fabacearum | JW 6021 |
| 100 | F. fabacearum | JW 6043 | |
| 100 | F. fabacearum | NRRL 37622 | |
| 100 | F. fabacearum | LLC1367 | |
| 100 | F. fabacearum | LLC1387 | |
| 100 | F. fabacearum | LLC1410 | |
| 100 | F. fabacearum | LLC1536 | |
| 100 | F. fabacearum | LLC1682 | |
| 100 | F. fabacearum | LLC3388 | |
| 100 | F. fabacearum | LLC3481 | |
| 100 | F. wimaladesilvae | BRIP 70752a | |
| K2043 | 100 | F. fabacearum | JW 6021 |
| 100 | F. fabacearum | JW 6043 | |
| 100 | F. fabacearum | NRRL 37622 | |
| 100 | F. fabacearum | LLC1367 | |
| 100 | F. fabacearum | LLC1387 | |
| 100 | F. fabacearum | LLC1410 | |
| 100 | F. fabacearum | LLC1536 | |
| 100 | F. fabacearum | LLC1682 | |
| 100 | F. fabacearum | LLC3388 | |
| 100 | F. fabacearum | LLC3481 | |
| 100 | F. wimaladesilvae | BRIP 70752a | |
| X388 | 98.98 | F. annulatum | CBS 258.54 |
| X459 | 99.03 | F. annulatum | CBS 258.54 |
| X548 | 99.36 | F. annulatum | CBS 258.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan Ateş, G. Molecular Identification of Fusarium Isolates from Bozcaada Çavuş and Karalahna Grapes in Türkiye. J. Fungi 2025, 11, 373. https://doi.org/10.3390/jof11050373
Özcan Ateş G. Molecular Identification of Fusarium Isolates from Bozcaada Çavuş and Karalahna Grapes in Türkiye. Journal of Fungi. 2025; 11(5):373. https://doi.org/10.3390/jof11050373
Chicago/Turabian StyleÖzcan Ateş, Gülçin. 2025. "Molecular Identification of Fusarium Isolates from Bozcaada Çavuş and Karalahna Grapes in Türkiye" Journal of Fungi 11, no. 5: 373. https://doi.org/10.3390/jof11050373
APA StyleÖzcan Ateş, G. (2025). Molecular Identification of Fusarium Isolates from Bozcaada Çavuş and Karalahna Grapes in Türkiye. Journal of Fungi, 11(5), 373. https://doi.org/10.3390/jof11050373






