Microaggregates as Nutrient Reservoirs for Fungi Drive Natural Regeneration in Larch Plantation Forests
Abstract
1. Introduction
2. Materials and Methods
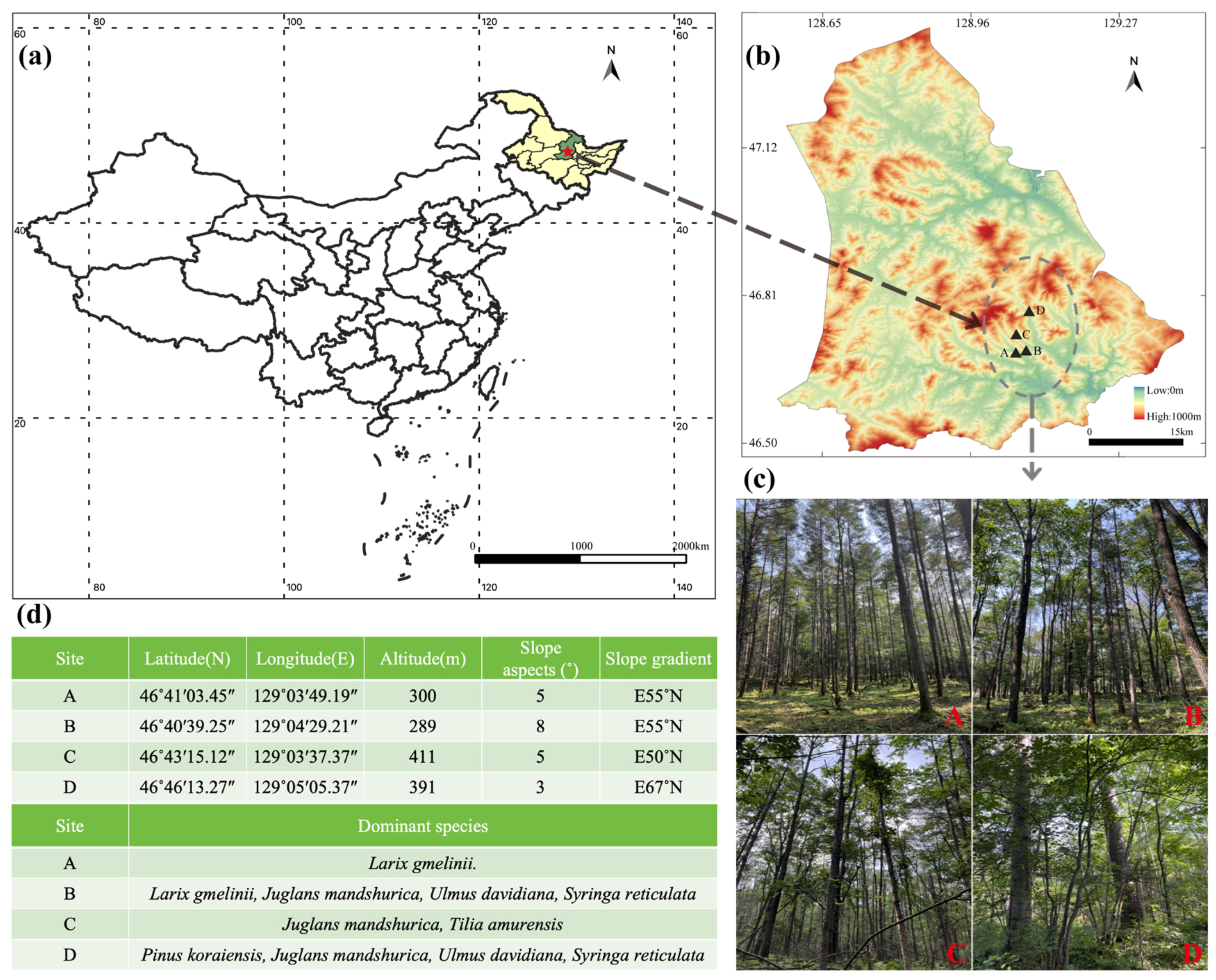
2.1. Study Site and Fieldwork Sampling
2.2. Soil Aggregate Grouping and Property Determination
2.3. DNA Extraction, PCR, and High-Throughput Sequencing
2.4. Statistical Analysis
3. Results
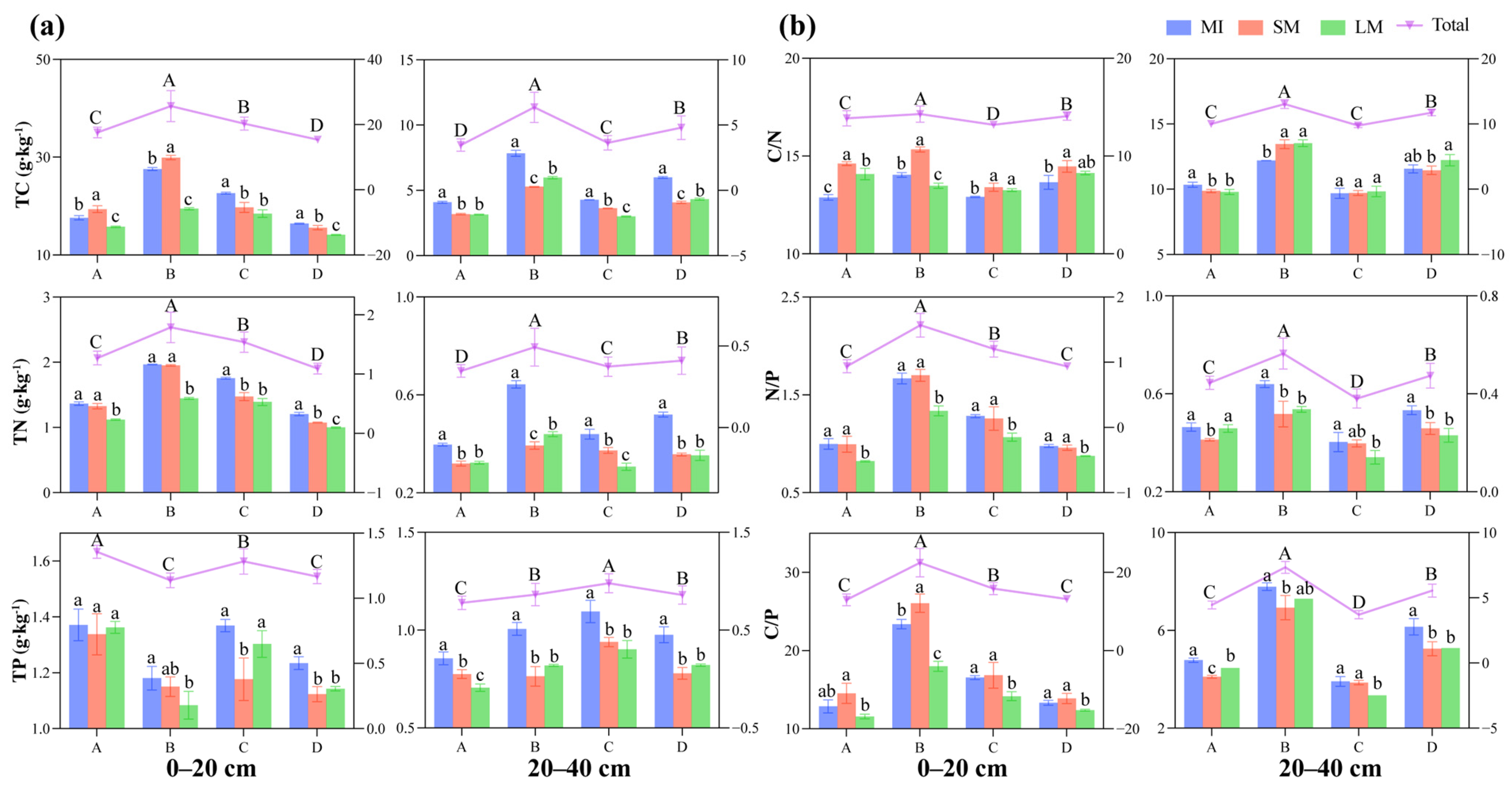
3.1. Soil Nutrients and Stoichiometric Ratios
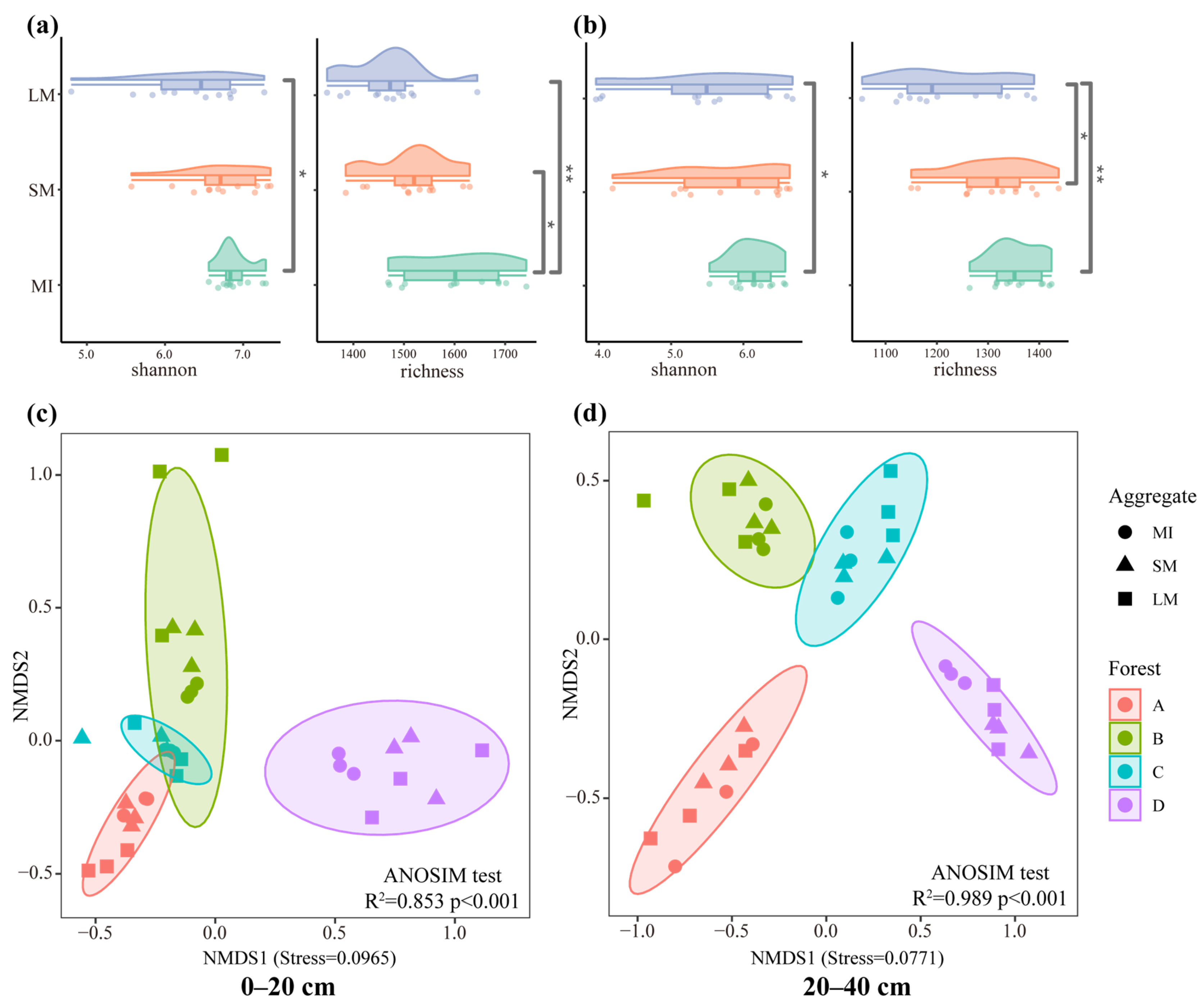
3.2. Diversity and Abundance of Fungal Communities
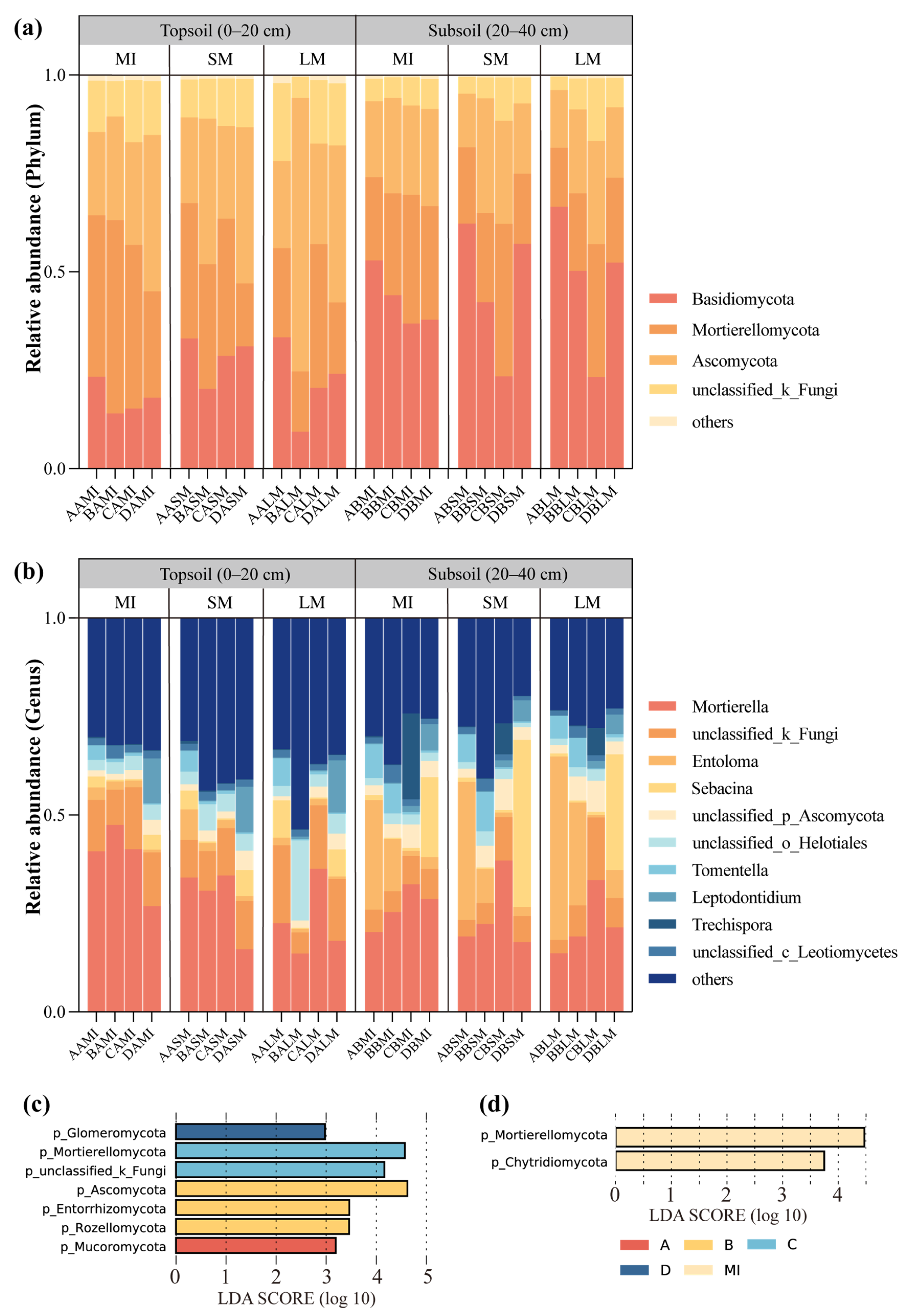
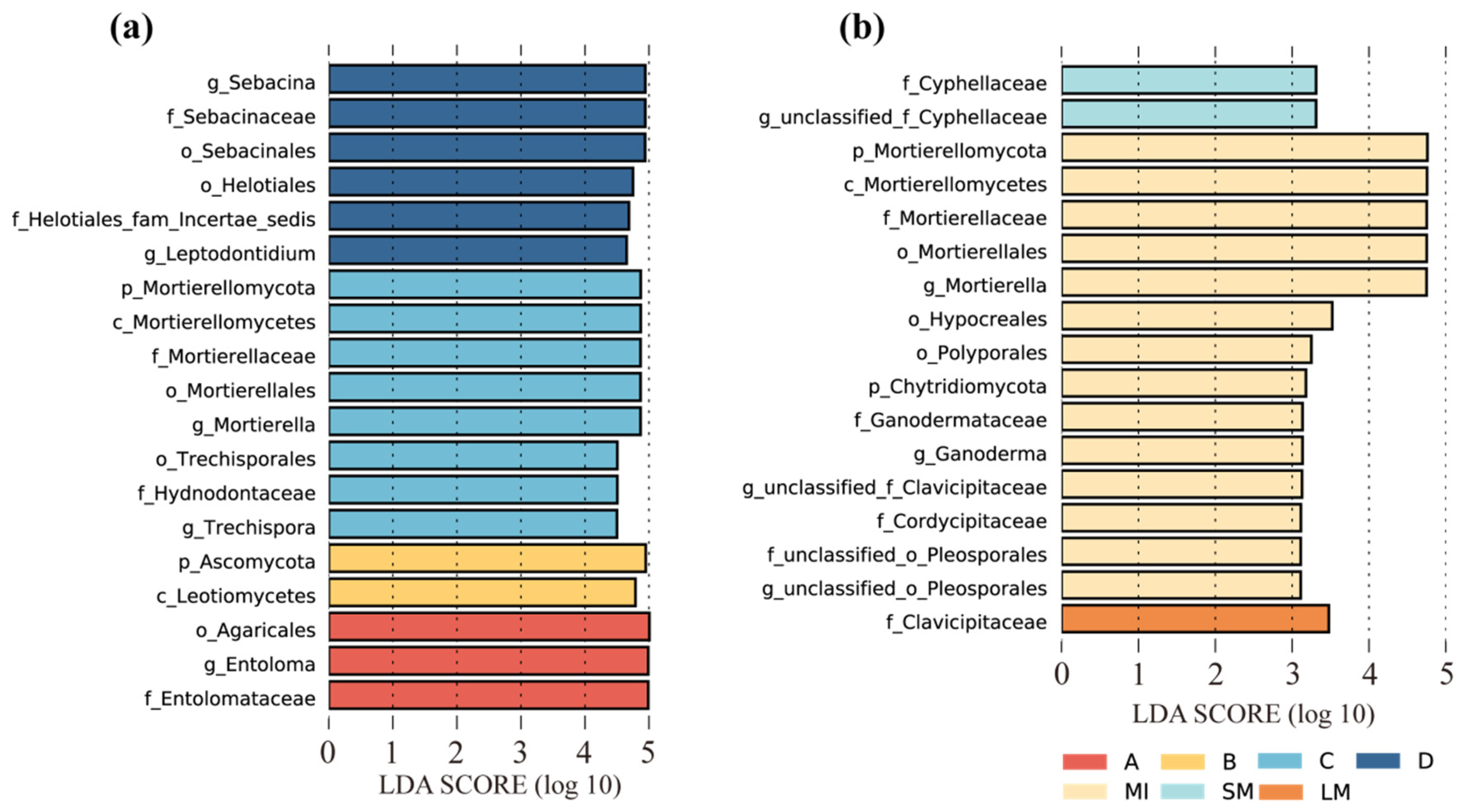
3.3. The Community Composition of Fungi
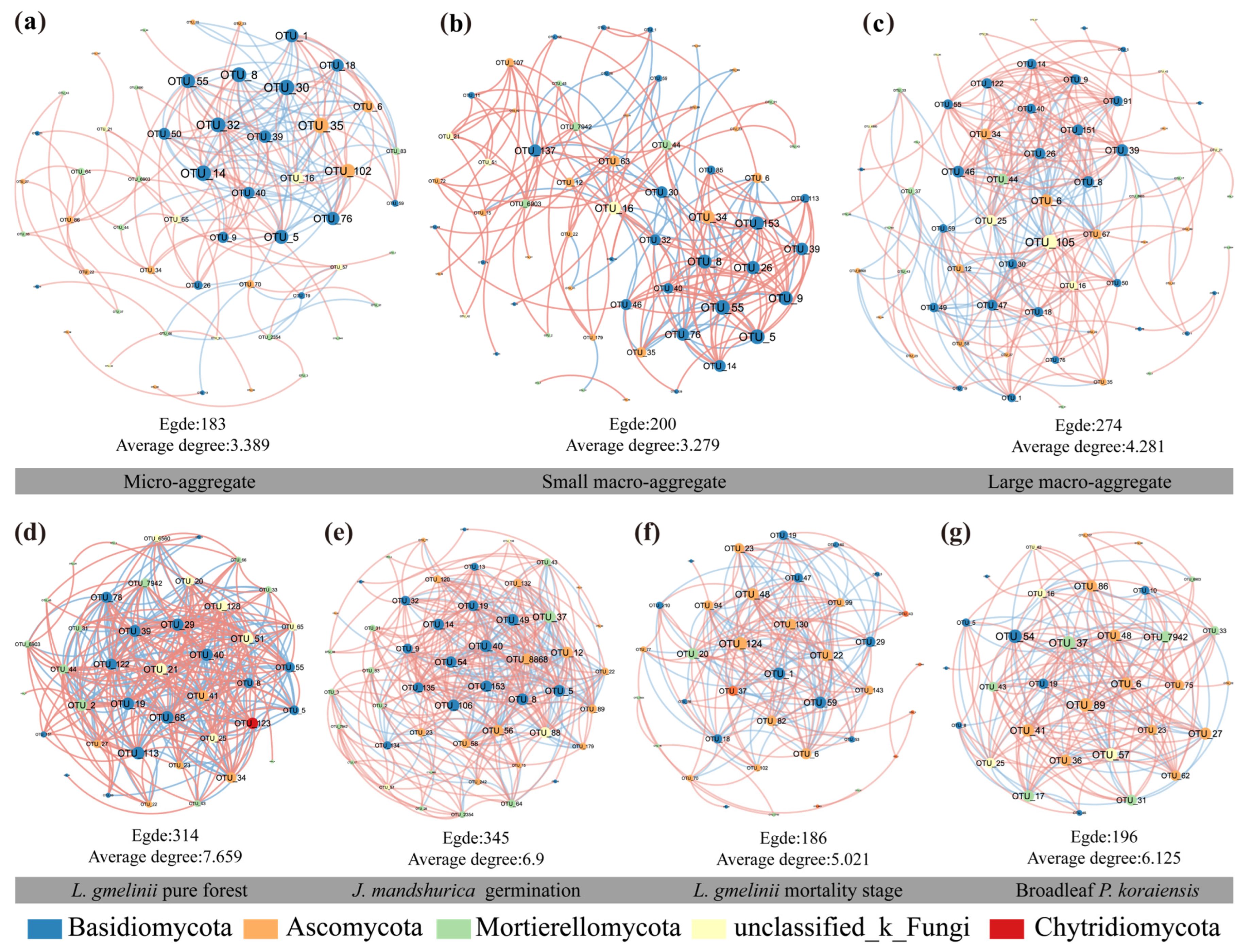
3.4. The Co-Occurrence Networks of Fungi
3.5. Drivers of Fungal Community Structure
4. Discussion
4.1. Effects of Regeneration on Nutrients in Aggregates
4.2. Effects of Forest Regeneration and Aggregate on Fungal Community Diversity and Structure
4.3. Forest Stand Renewal and Dominant Fungal Taxa in Aggregate Fractions
4.4. Interactive Effects of Forest Regeneration on Nutrient and Fungal Communities Within Different Aggregate Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, L.; Hotte, N.; Barron, S.; Cowan, J.; Sheppard, S.R.J. The Social and Economic Value of Cultural Ecosystem Services Provided by Urban Forests in North America: A Review and Suggestions for Future Research. Urban For. Urban Green. 2017, 25, 103–111. [Google Scholar] [CrossRef]
- Alkama, R.; Cescatti, A. Biophysical Climate Impacts of Recent Changes in Global Forest Cover. Science 2016, 351, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Chen, C.; Zhang, X.; Ding, C.; Yuan, M.; Shen, L.; Li, X. Biophysical Impact of Forest Age Changes on Land Surface Temperature in China. Sci. Total Environ. 2025, 964, 178445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.; Wang, X.; Quan, X. Carbon Concentration Variability of 10 Chinese Temperate Tree Species. For. Ecol. Manag. 2009, 258, 722–727. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Liu, Z. Factors Driving Native Tree Species Restoration in Plantations and Tree Structure Conversion in Chinese Temperate Forests. For. Ecol. Manag. 2022, 507, 119989. [Google Scholar] [CrossRef]
- Lozano-Baez, S.E.; Cooper, M.; Meli, P.; Ferraz, S.F.B.; Rodrigues, R.R.; Sauer, T.J. Land Restoration by Tree Planting in the Tropics and Subtropics Improves Soil Infiltration, but Some Critical Gaps Still Hinder Conclusive Results. For. Ecol. Manag. 2019, 444, 89–95. [Google Scholar] [CrossRef]
- Heilmayr, R.; Echeverria, C.; Lambin, E.F. Impacts of Chilean Forest Subsidies on Forest Cover, Carbon and Biodiversity. Nat. Sustain. 2020, 3, 701–709. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Sun, Y.; Grabosky, J. Carbon Stock in Korean Larch Plantations along a Chronosequence in the Lesser Khingan Mountains, China. J. For. Res. 2014, 25, 749–760. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill, J.F.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in Soil Fungal Community Composition Depend on Functional Group and Forest Disturbance Type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi Mediate Long Term Sequestration of Carbon and Nitrogen in Soil through Their Priming Effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Voriskova, J.; Brabcova, V.; Cajthaml, T.; Baldrian, P. Seasonal Dynamics of Fungal Communities in a Temperate Oak Forest Soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, C.; Shi, Y.; Li, H.; Tang, Y.; Chen, B.; Ke, Q.; Wu, L.; Chen, L. Short-Term Cultivation Limiting Soil Aggregate Stability and Macronutrient Accumulation Associated with Glomalin-Related Soil Protein in Eucalyptus Urophylla × Eucalyptus Grandis Plantations. Sci. Total Environ. 2023, 878, 163187. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A History of Research on the Link between (Micro)Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Nelson, S.E.; Wilkinson, K.G.; Smith, S.E.; McKenzie, B.M. Stabilisation of Soil against Wind Erosion by Six Saprotrophic Fungi. Soil Biol. Biochem. 2012, 50, 134–141. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest Soil Microbial Diversity Found in Micro-Habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Wang, J. Mechanisms of soil aggregate formation and stabilization: Research progress and prospects. J. Soil Sci. 2023, 60, 627–643. [Google Scholar]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Cao, T.; Fang, Y.; Chen, Y.; Kong, X.; Yang, J.; Alharbi, H.; Kuzyakov, Y.; Tian, X. Synergy of Saprotrophs with Mycorrhiza for Litter Decomposition and Hotspot Formation Depends on Nutrient Availability in the Rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Shi, C.; Li, X.; Qiu, Z.; Shi, F. Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus Palmeri Invasion in Northern China. J. Fungi 2023, 9, 509. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Ren, D.; Liu, C.; Zhang, Y.; Wang, S.; Li, Z.; Zhang, M. Interlinkages between Soil Properties and Keystone Taxa under Different Tillage Practices on the North China Plain. Appl. Soil Ecol. 2022, 178, 104551. [Google Scholar] [CrossRef]
- Wang, H.; Tian, D.; Liu, H.; Wang, Z.; He, Y.; Lu, J.; Zhu, Y.; Wei, S.; Wang, H.; Wu, L.; et al. Rare Rather than Abundant Taxa of Soil Bacteria and Fungi Regulate Soil Multifunctionality in Eucalyptus Plantations. Catena 2024, 245, 108303. [Google Scholar] [CrossRef]
- Cao, M.; Gong, Y.; Feng, Z.; Zhang, W.; Wang, X. Structural Characterisation of Primary Red Pine Forest in Langxiang Nature Reserve, Yichun City. For. Surv. Plan. 2012, 37, 75–80. [Google Scholar]
- Ju, W.; Fang, L.; Shen, G.; Delgado-Baquerizo, M.; Chen, J.; Zhou, G.; Ma, D.; Bing, H.; Liu, L.; Liu, J.; et al. New Perspectives on Microbiome and Nutrient Sequestration in Soil Aggregates during Long-term Grazing Exclusion. Glob. Change Biol. 2024, 30, e17027. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. Soil Aggregate Isolation Method Affects Measures of Intra-Aggregate Extracellular Enzyme Activity. Soil Biol. Biochem. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-Term Manure Application Increases Soil Organic Matter and Aggregation, and Alters Microbial Community Structure and Keystone Taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Shi, J.; Yang, L.; Liao, Y.; Li, J.; Jiao, S.; Shangguan, Z.; Deng, L. Soil Labile Organic Carbon Fractions Mediate Microbial Community Assembly Processes during Long-term Vegetation Succession in a Semiarid Region. iMeta 2023, 2, e142. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, Z.; Zhang, M.; Li, X.; Fang, X.; Zhao, M.; Shi, F. Responses of Rhizosphere Soil Chemical Properties and Bacterial Community Structure to Major Afforestation Tree Species in Xiong’an New Area. Forests 2022, 13, 1822. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, M.; Zhang, M.; Fang, X.; Wang, H.; Lv, J.; Shi, F. Topography- and Depth-Dependent Rhizosphere Microbial Community Characteristics Drive Ecosystem Multifunctionality in Juglans Mandshurica Forest. Sci. Total Environ. 2024, 949, 175070. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and Variation of C:N:P Ratios in China’s Soils: A Synthesis of Observational Data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Liu, G.-S.; Wang, X.-Z.; Zhang, Z.-Y.; Zhang, C.-H. Spatial Variability of Soil Properties in a Tobacco Field of Central China. Soil Sci. 2008, 173, 659–667. [Google Scholar] [CrossRef]
- Huang, S.; Huang, Y.; Gao, W.; Nie, S.; Cai, B.; Lin, J. Dynamics of apomictic material and nutrient return in three typical forests along an altitudinal gradient in the Wuyi Mountains. J. Trop. Subtrop. Bot. 2020, 28, 394–402. [Google Scholar]
- Zhao, Z.; Dong, P.; Fu, B.; Wu, D.; Zhao, Z. Soil Organic Carbon Distribution and Factors Affecting Carbon Accumulation in Natural and Plantation Forests in Tropical China. Ecol. Indic. 2023, 148, 110127. [Google Scholar] [CrossRef]
- Yang, Y.; Shan, C.; Shen, F.; Yang, L. The effect of apomictic litter addition on the growth of huckleberry seedlings in Chinese tulip tree and Xing’an larch. J. Appl. Ecol. 2025, 36, 703–710. [Google Scholar] [CrossRef]
- Bai, X.; Li, Y.; Hu, S.; Dong, L.; Zhang, M.; Wang, Y.; Yu, X. Effects of forest age on soil aggregates, organic carbon and bacterial communities in acacia plantation forests. J. Ecol. 2024, 44, 5259–5268. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-Associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Osborne, B.B.; Soper, F.M.; Nasto, M.K.; Bru, D.; Hwang, S.; Machmuller, M.B.; Morales, M.L.; Philippot, L.; Sullivan, B.W.; Asner, G.P.; et al. Litter Inputs Drive Patterns of Soil Nitrogen Heterogeneity in a Diverse Tropical Forest: Results from a Litter Manipulation Experiment. Soil Biol. Biochem. 2021, 158, 108247. [Google Scholar] [CrossRef]
- DeGryze, S.; Six, J.; Paustian, K.; Morris, S.J.; Paul, E.A.; Merckx, R. Soil Organic Carbon Pool Changes Following Land-use Conversions. Glob. Change Biol. 2004, 10, 1120–1132. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Huang, Y.; Yao, X.; He, X.; Ye, S. Ecological stoichiometric characteristics of soil aggregates in different fir stand types. Northwest J. Bot. 2021, 41, 1028–1035. [Google Scholar]
- Tang, L.; Wang, S. Dynamics of Soil Aggregate-Related C-N-P Stoichiometric Characteristics with Stand Age and Soil Depth in Chinese Fir Plantations. Land Degrad. Dev. 2022, 33, 1290–1306. [Google Scholar] [CrossRef]
- Spaccini, R.; Mbagwu, J.S.C.; Igwe, C.A.; Conte, P.; Piccolo, A. Carbohydrates and Aggregation in Lowland Soils of Nigeria as Influenced by Organic Inputs. Soil Tillage Res. 2004, 75, 161–172. [Google Scholar] [CrossRef]
- Mueller, M.; Oelmann, Y.; Schickhoff, U.; Boehner, J.; Scholten, T. Himalayan Treeline Soil and Foliar C:N:P Stoichiometry Indicate Nutrient Shortage with Elevation. Geoderma 2017, 291, 21–32. [Google Scholar] [CrossRef]
- Uroz, S.; Buee, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the Forest Microbiome: Highlights of Temperate and Boreal Ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Yan, W.; Zho, Y.; Shangguan, Z. Changes in Soil Microbial Community Structure during Long-Term Secondary Succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Blackwood, C.B.; Curtis, C.D.; Tilman, D. Resource Availability Controls Fungal Diversity across a Plant Diversity Gradient. Ecol. Lett. 2006, 9, 1127–1135. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Guo, C.; Bao, Y.; Lu, G.; Reinfelder, J.R.; Dang, Z. Bacterial, Archaeal, and Fungal Community Responses to Acid Mine Drainage-Laden Pollution in a Rice Paddy Soil Ecosystem. Sci. Total Environ. 2018, 616, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of Grassland Afforestation on Structure and Function of Soil Bacterial and Fungal Communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Fan, X.D.; Ren, C.J.; Zhang, L.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of the Organic Carbon Content and Stability of Soil Aggregates Affected by Soil Bacterial Community after Afforestation. Catena 2018, 171, 622–631. [Google Scholar] [CrossRef]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil Texture Affects Soil Microbial and Structural Recovery during Grassland Restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Collado, E.; Piqué, M.; Coello, J.; de-Dios-García, J.; Fuentes, C.; Coll, L. Close-to-Nature Management Effects on Tree Growth and Soil Moisture in Mediterranean Mixed Forests. For. Ecol. Manag. 2023, 549, 121457. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.; Hui, D.; Zhang, Q.; Li, M.; Zhang, Q. Soil Microbial Community and Its Interaction with Soil Carbon and Nitrogen Dynamics Following Afforestation in Central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lu, Y.; Zhang, X.; Liang, W. Contributions of Soil Biota to C Sequestration Varied with Aggregate Fractions under Different Tillage Systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Liao, Z.; Ye, S.; Wang, S. Aggregate Composition Directly Affects the Characteristics of Soil Microbial and Nematode Communities in a Chronosequence of Chinese Fir Plantations. Land Degrad. Dev. 2024, 35, 3362–3377. [Google Scholar] [CrossRef]
- Qiu, Z.; Shi, C.; Zhang, M.; Shi, F. Effects of Close-to-Nature Management of Plantation on the Structure and Ecological Functions of Soil Microorganisms with Different Habitat Specialization. Plant Soil 2023, 482, 347–367. [Google Scholar] [CrossRef]
- Hesse, C.N.; Mueller, R.C.; Vuyisich, M.; Gallegos-Graves, L.V.; Gleasner, C.D.; Zak, D.R.; Kuskel, C.R. Forest Floor Community Metatranscriptomes Identify Fungal and Bacterial Responses to N Deposition in Two Maple Forests. Front. Microbiol. 2015, 6, 337. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Li, S.; Deng, Y. Progress in molecular ecology of microbial carbon cycle based on functional genes. Microbiol. Bull. 2017, 44, 1676–1689. [Google Scholar] [CrossRef]
- Zheng, B.-X.; Hao, X.-L.; Ding, K.; Zhou, G.-W.; Chen, Q.-L.; Zhang, J.-B.; Zhu, Y.-G. Long-Term Nitrogen Fertilization Decreased the Abundance of Inorganic Phosphate Solubilizing Bacteria in an Alkaline Soil. Sci. Rep. 2017, 7, 42284. [Google Scholar] [CrossRef]
- Arias, M.; Barral, M.T.; Diaz-Fierros, F. Effects of Associations between Humic Acids and Iron or Aluminium on the Flocculation and Aggregation of Kaolin and Quartz. Eur. J. Soil Sci. 1996, 47, 335–343. [Google Scholar] [CrossRef]
- Akter, S.; Kamruzzaman, M.D.; Sarder, M.D.P.; Amin, M.D.S.; Joardar, J.C.; Islam, M.D.S.; Nasrin, S.; Islam, M.U.; Islam, F.; Rabbi, S.; et al. Mycorrhizal Fungi Increase Plant Nutrient Uptake, Aggregate Stability and Microbial Biomass in the Clay Soil. Symbiosis 2024, 93, 163–176. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and Soil Structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Li, Y.; Nakasone, K.K.; He, S.-H. Stratocorticium Sinensis Gen. et Sp. Nov. and Cericium Gloeocystidiatum Sp. Nov. (Cyphellaceae, Agaricales) from East Asia. J. Fungi 2024, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Jiang, Y.; Chen, F. Polyunsaturated Fatty Acids (PUFAs) Content of the Fungus Mortierella Alpina Isolated from Soil. J. Agric. Food Chem. 2007, 55, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Sterkenburg, E.; Bahr, A.; Durling, M.B.; Clemmensen, K.E.; Lindahl, B.D. Changes in Fungal Communities along a Boreal Forest Soil Fertility Gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef]
- Lei, J.; Wu, H.; Li, X.; Guo, W.; Duan, A.; Zhang, J. Response of Rhizosphere Bacterial Communities to Near-Natural Forest Management and Tree Species within Chinese Fir Plantations. Microbiol. Spectr. 2023, 11, e02328-22. [Google Scholar] [CrossRef]
- Toju, H.; Sato, H. Root-Associated Fungi Shared Between Arbuscular Mycorrhizal and Ectomycorrhizal Conifers in a Temperate Forest. Front. Microbiol. 2018, 9, 433. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Hannula, S.E.; Morrien, E. Will Fungi Solve the Carbon Dilemma? Geoderma 2022, 413, 115767. [Google Scholar] [CrossRef]
- Liu, S.; Garcia-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype Diversity within Soil Fungal Functional Groups Drives Ecosystem Stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Wang, B.; Waythomas, C.; Rainey, F.; Talbot, S.L. Organic Matter Quantity and Source Affects Microbial Community Structure and Function Following Volcanic Eruption on Kasatochi Island, Alaska. Environ. Microbiol. 2016, 18, 146–158. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential Growth Responses of Soil Bacterial Taxa to Carbon Substrates of Varying Chemical Recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil Bacterial Community Dynamics Reflect Changes in Plant Community and Soil Properties during the Secondary Succession of Abandoned Farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Stursova, M.; Zifcakova, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose Utilization in Forest Litter and Soil: Identification of Bacterial and Fungal Decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who Is Who in Litter Decomposition? Metaproteomics Reveals Major Microbial Players and Their Biogeochemical Functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef]
- Yang, L. Bioassay of chemosensitisation effect of Larch aqueous extracts on Liriodendron amurense. J. Northeast. For. Univ. 2006, 2, 15–17. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Wang, K.; Wang, Z.; Fang, X.; Wang, H.; Li, N.; Shi, C.; Shi, F. Microaggregates as Nutrient Reservoirs for Fungi Drive Natural Regeneration in Larch Plantation Forests. J. Fungi 2025, 11, 316. https://doi.org/10.3390/jof11040316
Lin Y, Wang K, Wang Z, Fang X, Wang H, Li N, Shi C, Shi F. Microaggregates as Nutrient Reservoirs for Fungi Drive Natural Regeneration in Larch Plantation Forests. Journal of Fungi. 2025; 11(4):316. https://doi.org/10.3390/jof11040316
Chicago/Turabian StyleLin, Yiping, Kefan Wang, Zilu Wang, Xin Fang, Haomin Wang, Nuo Li, Cong Shi, and Fuchen Shi. 2025. "Microaggregates as Nutrient Reservoirs for Fungi Drive Natural Regeneration in Larch Plantation Forests" Journal of Fungi 11, no. 4: 316. https://doi.org/10.3390/jof11040316
APA StyleLin, Y., Wang, K., Wang, Z., Fang, X., Wang, H., Li, N., Shi, C., & Shi, F. (2025). Microaggregates as Nutrient Reservoirs for Fungi Drive Natural Regeneration in Larch Plantation Forests. Journal of Fungi, 11(4), 316. https://doi.org/10.3390/jof11040316




