Gene Editing in Ganoderma lucidum: Development, Challenges, and Future Prospects
Abstract
1. Introduction
2. The Development of Gene-Editing Technology
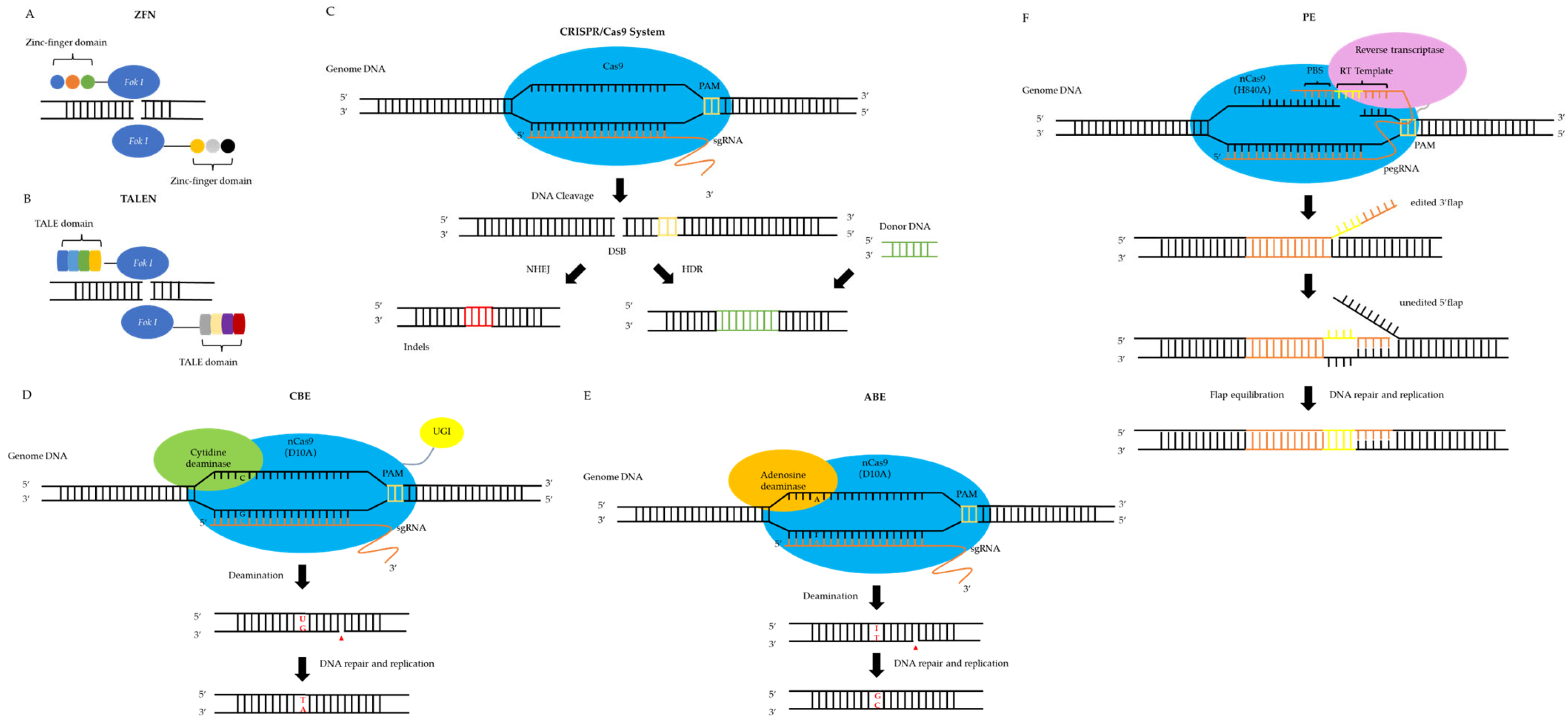
2.1. First Generation: Zinc Finger Nucleases (ZFNs)
2.2. Second Generation: Transcription Activator-like Effector Nucleases (TALENs)
2.3. Third Generation: Clusters of Regularly Spaced Short Palindromic Repeats/Cas9 Protein Systems (CRISPR/Cas9 Systems)
2.4. Precision Gene Editing Tools
3. Application of CRISPR/Cas9 System in G. lucidum
4. The Limitations of Gene-Editing Technology in Fungi at Present
4.1. Low Genetic Transformation Efficiency
4.2. Low Efficiency of Gene Editing
4.3. Low Precision of DNA Repair
5. Optimization Strategies to Overcome the Limitations of Gene Editing in G. lucidum
5.1. Gene Silencing
5.1.1. Lift the Restriction of PAM
5.1.2. Improve Gene Editing Efficiency
5.1.3. Reduce Off-Target Effects
5.2. Gene Overexpression
5.2.1. Construct Overexpression Vectors to Improve Gene Expression Level
5.2.2. Develop CRISPR Activation System for Overexpression
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.-W.; Su, K.-Q.; Zhang, Y.-M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2011, 93, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Lin, X.; Chen, Y.; Zhong, Y.; Zhong, X.; Wang, B.; Liang, X. Application research progress of Ganoderma lucidum breeding technology. Agric. Eng. 2024, 14, 29–34. [Google Scholar] [CrossRef]
- Tang, M.; Yao, Y.; Rong, D.; Jiang, M. Research Progress of Edible Fungi Breeding Technology. Edible Fungi China 2022, 41, 1–6+10. [Google Scholar] [CrossRef]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef]
- Porteus, M.H.; Carroll, D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005, 23, 967–973. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2012, 14, 49–55. [Google Scholar] [CrossRef]
- Wood, A.J.; Lo, T.-W.; Zeitler, B.; Pickle, C.S.; Ralston, E.J.; Lee, A.H.; Amora, R.; Miller, J.C.; Leung, E.; Meng, X.; et al. Targeted Genome Editing Across Species Using ZFNs and TALENs. Science 2011, 333, 307. [Google Scholar] [CrossRef]
- Boch, J.; Bonas, U. Xanthomonas AvrBs3 Family-Type III Effectors: Discovery and Function. Annu. Rev. Phytopathol. 2010, 48, 419–436. [Google Scholar] [CrossRef]
- Chandrasegaran, S.; Carroll, D. Origins of Programmable Nucleases for Genome Engineering. J. Mol. Biol. 2016, 428, 963–989. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Yeh, C.D.; Richardson, C.D.; Corn, J.E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 2019, 21, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Solá-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.-J.; Liquori, A.J.; et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Chen, P.J.; Hussmann, J.A.; Yan, J.; Knipping, F.; Ravisankar, P.; Chen, P.-F.; Chen, C.; Nelson, J.W.; Newby, G.A.; Sahin, M.; et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 2021, 184, 5635–5652.e29. [Google Scholar] [CrossRef]
- Yan, J.; Oyler-Castrillo, P.; Ravisankar, P.; Ward, C.C.; Levesque, S.; Jing, Y.; Simpson, D.; Zhao, A.; Li, H.; Yan, W.; et al. Improving prime editing with an endogenous small RNA-binding protein. Nature 2024, 628, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Doman, J.L.; Pandey, S.; Neugebauer, M.E.; An, M.; Davis, J.R.; Randolph, P.B.; McElroy, A.; Gao, X.D.; Raguram, A.; Richter, M.F.; et al. Phage-assisted evolution and protein engineering yield compact, efficient prime editors. Cell 2023, 186, 3983–4002.e26. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2022, 24, 161–177. [Google Scholar] [CrossRef]
- Blaeschke, F.; Chen, Y.Y.; Apathy, R.; Daniel, B.; Chen, A.Y.; Chen, P.A.; Sandor, K.; Zhang, W.; Li, Z.; Mowery, C.T.; et al. Modular pooled discovery of synthetic knockin sequences to program durable cell therapies. Cell 2023, 186, 4216–4234.e33. [Google Scholar] [CrossRef]
- Haley, B.; Roudnicky, F. Functional Genomics for Cancer Drug Target Discovery. Cancer Cell 2020, 38, 31–43. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, Y.; Xue, C.; Li, B.; Li, X.; Wang, Y.; Li, J.; Liu, G.; Huang, X.; Cao, X.; et al. An engineered prime editor with enhanced editing efficiency in plants. Nat. Biotechnol. 2022, 40, 1394–1402. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Z.; Xu, Y.; Wang, F.; Jiang, Y.; Fan, F.; Li, W.; Zhu, J.; Li, X.; Wang, J.; et al. CRISPR-mediated targeted mutagenesis for improving nitrogen use efficiency in japonica rice. Plant Commun. 2025, 6, 101189. [Google Scholar] [CrossRef]
- Srila, W.; Pangjantuk, A.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. Development of CRISRP/Cas9-based TP53-knockout pig muscle stem cells for use in the cultured meat industry. 3 Biotech 2025, 15, 92. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Mani, M.; Kandavelou, K.; Dy, F.J.; Durai, S.; Chandrasegaran, S. Design, engineering, and characterization of zinc finger nucleases. Biochem. Biophys. Res. Commun. 2005, 335, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Geurts, A.M.; Cost, G.J.; Freyvert, Y.; Zeitler, B.; Miller, J.C.; Choi, V.M.; Jenkins, S.S.; Wood, A.; Cui, X.; Meng, X.; et al. Knockout Rats via Embryo Microinjection of Zinc-Finger Nucleases. Science 2009, 325, 433. [Google Scholar] [CrossRef]
- Curtin, S.J.; Zhang, F.; Sander, J.D.; Haun, W.J.; Starker, C.; Baltes, N.J.; Reyon, D.; Dahlborg, E.J.; Goodwin, M.J.; Coffman, A.P.; et al. Targeted Mutagenesis of Duplicated Genes in Soybean with Zinc-Finger Nucleases. Plant Physiol. 2011, 156, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef]
- Carlson, D.F.; Tan, W.; Lillico, S.G.; Stverakova, D.; Proudfoot, C.; Christian, M.; Voytas, D.F.; Long, C.R.; Whitelaw, C.B.A.; Fahrenkrug, S.C. Efficient TALEN-mediated gene knockout in livestock. Proc. Natl. Acad. Sci. USA 2012, 109, 17382–17387. [Google Scholar] [CrossRef]
- Mussolino, C.; Morbitzer, R.; Lütge, F.; Dannemann, N.; Lahaye, T.; Cathomen, T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011, 39, 9283–9293. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, H.; Zou, G.; Zhou, Z.; Zhong, J.-J. CRISPR-Cas9 assisted gene disruption in the higher fungus Ganoderma species. Process Biochem. 2017, 56, 57–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zou, G.; Tang, C.; Feng, J.; Bao, D.; Chen, J.; Tan, Y. Construction of a CRISPR/Cas9-Based Genome Editing System in Ganoderma lucidum ‘Hunong No.1’ Cultivar. Acta Edulis Fungi 2023, 30, 9–18. [Google Scholar] [CrossRef]
- Gao, Z.; Herrera-Carrillo, E.; Berkhout, B. Delineation of the Exact Transcription Termination Signal for Type 3 Polymerase III. Mol. Ther. Nucleic Acids 2018, 10, 36–44. [Google Scholar] [CrossRef]
- Wang, P.-A.; Xiao, H.; Zhong, J.-J. CRISPR-Cas9 assisted functional gene editing in the mushroom Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2019, 104, 1661–1671. [Google Scholar] [CrossRef]
- Li, H.; Zhong, J.-J. Role of calcineurin-responsive transcription factor CRZ1 in ganoderic acid biosynthesis by Ganoderma lucidum. Process Biochem. 2020, 95, 166–173. [Google Scholar] [CrossRef]
- Liu, K.; Sun, B.; You, H.; Tu, J.L.; Yu, X.; Zhao, P.; Xu, J.W. Dual sgRNA-directed gene deletion in basidiomycete Ganoderma lucidum using the CRISPR/Cas9 system. Microb. Biotechnol. 2020, 13, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-A.; Zhang, J.-M.; Zhong, J.-J. CRISPR-Cas9 assisted in-situ complementation of functional genes in the basidiomycete Ganoderma lucidum. Process Biochem. 2022, 121, 689–697. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, X.; Zhang, Z.; Tian, J.; Feng, N.; Tang, C.; Zou, G.; Zhang, J. An Efficient CRISPR/Cas9 Genome Editing System for a Ganoderma lucidum Cultivated Strain by Ribonucleoprotein Method. J. Fungi 2023, 9, 1170. [Google Scholar] [CrossRef]
- Eom, H.; Choi, Y.-J.; Nandre, R.; Han, H.-G.; Kim, S.; Kim, M.; Oh, Y.-L.; Nakazawa, T.; Honda, Y.; Ro, H.-S. The Cas9-gRNA ribonucleoprotein complex-mediated editing of pyrG in Ganoderma lucidum and unexpected insertion of contaminated DNA fragments. Sci. Rep. 2023, 13, 11133. [Google Scholar] [CrossRef]
- Kim, M.; Oh, M.J.; Im, J.-H.; Lee, E.-J.; Woo, S.-I.; Oh, Y.-L. Optimization of RNP/Nanoparticle Systems for Enhanced CRISPR/Cas9-Based Gene Editing in Ganoderma lucidum. J. Mushrooms 2024, 22, 231–235. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Eom, H.; Nandre, R.; Kim, M.; Oh, Y.-L.; Kim, S.; Ro, H.-S. Simultaneous gene editing of both nuclei in a dikaryotic strain of Ganoderma lucidum using Cas9-gRNA ribonucleoprotein. J. Microbiol. 2025, 63, e.2409006. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Liu, D.; Wu, M.; Tian, C. Advances in metabolic engineering of filamentous fungi. Chin. J. Biotechnol. 2021, 37, 1637–1658. [Google Scholar] [CrossRef]
- Liu, J.; Song, C.; Li, Q.; Xu, Z.; Zhang, D.; Zhang, M.; Tan, Q.; Shang, X. A colonized millet grain method for Agrobacterium-mediated transformation of the button mushroom Agaricus bisporus. J. Microbiol. Methods 2018, 152, 148–153. [Google Scholar] [CrossRef]
- Zhang, J.j.; Shi, L.; Chen, H.; Sun, Y.q.; Zhao, M.w.; Ren, A.; Chen, M.j.; Wang, H.; Feng, Z.y. An efficient Agrobacterium-mediated transformation method for the edible mushroom Hypsizygus marmoreus. Microbiol. Res. 2014, 169, 741–748. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, M.; Chai, S.; Zhu, Z.; Wang, Y.; Zhou, Z. Efficient genome editing in filamentous fungi via an improved CRISPR-Cas9 ribonucleoprotein method facilitated by chemical reagents. Microb. Biotechnol. 2020, 14, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, Z.; Xue, W.; Li, Y.; Tang, G.; Wang, A.; Zhang, Y.; Wang, H. Ku80 Gene is Related to Non-Homologous End-Joining and Genome Stability in Aspergillus niger. Curr. Microbiol. 2011, 62, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.S.; Suzuki, H.; Shimokita, E.; Chiba, H.; Noji, S.; Osakabe, Y.; Osakabe, K. Genome editing in the mushroom-forming basidiomycete Coprinopsis cinerea, optimized by a high-throughput transformation system. Sci. Rep. 2017, 7, 1260. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Lin, S.; Staahl, B.T.; Alla, R.K.; Doudna, J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 2014, 3, e04766. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Kwaku Dad, A.-B.; Beloor, J.; Gopalappa, R.; Lee, S.-K.; Kim, H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef]
- Boontawon, T.; Nakazawa, T.; Xu, H.; Kawauchi, M.; Sakamoto, M.; Honda, Y. Gene targeting using pre-assembled Cas9 ribonucleoprotein and split-marker recombination in Pleurotus ostreatus. FEMS Microbiol. Lett. 2021, 368, fnab080. [Google Scholar] [CrossRef]
- Kück, U.; Hoff, B. New tools for the genetic manipulation of filamentous fungi. Appl. Microbiol. Biotechnol. 2010, 86, 51–62. [Google Scholar] [CrossRef]
- Tu, J.L.; Bai, X.Y.; Xu, Y.L.; Li, N.; Xu, J.W. Targeted Gene Insertion and Replacement in the Basidiomycete by Inactivation of Nonhomologous End Joining Using CRISPR/Cas9. Appl. Environ. Microbiol. 2021, 87, e0151021. [Google Scholar] [CrossRef]
- De Jong, J.F.; Ohm, R.A.; De Bekker, C.; Wösten, H.A.B.; Lugones, L.G. Inactivation of ku80 in the mushroom-forming fungus Schizophyllum commune increases the relative incidence of homologous recombination. FEMS Microbiol. Lett. 2010, 310, 91–95. [Google Scholar] [CrossRef]
- Eom, H.; Choi, Y.-J.; Nandre, R.; Kim, M.; Oh, Y.-L.; Kim, S.; Nakazawa, T.; Honda, Y.; Ro, H.-S. Targeted insertion of heterogenous DNA using Cas9-gRNA ribonucleoprotein-mediated gene editing in Ganoderma lucidum. Bioengineered 2025, 16, 2458376. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Zhao, D.; Pang, C.; Li, J.; Li, S.; Qiao, W.; Tan, J.; Bi, C.; Zhang, X. Mismatch prime editing gRNA increased efficiency and reduced indels. Nat. Commun. 2025, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Huai, G.; Wang, Y.; Du, J.; Cheng, Z.; Xie, Y.; Zhou, J.; Tang, H.; Jiang, Y.; Xing, X.; Deng, S.; et al. The generation and evaluation of TKO/hCD55/hTM/hEPCR gene-modified pigs for clinical organ xenotransplantation. Front. Immunol. 2025, 15, 1488552. [Google Scholar] [CrossRef] [PubMed]
- Konishi, C.T.; Mulaiese, N.; Butola, T.; Zhang, Q.; Kagan, D.; Yang, Q.; Pressler, M.; Dirvin, B.G.; Devinsky, O.; Basu, J.; et al. Modeling and correction of protein conformational disease in iPSC-derived neurons through personalized base editing. Mol. Ther. Nucleic Acids 2025, 36, 102441. [Google Scholar] [CrossRef]
- Tan, J.J.; Zhang, F.; Karcher, D.; Bock, R. Expanding the genome-targeting scope and the site selectivity of high-precision base editors. Nat. Commun. 2020, 11, 629. [Google Scholar] [CrossRef]
- Mitsui, R.; Yamada, R.; Ogino, H. CRISPR system in the yeast Saccharomyces cerevisiae and its application in the bioproduction of useful chemicals. World J. Microbiol. Biotechnol. 2019, 35, 111. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, F.; Karcher, D.; Bock, R. Engineering of high-precision base editors for site-specific single nucleotide replacement. Nat. Commun. 2019, 10, 439. [Google Scholar] [CrossRef]
- Si, X.; Zhang, H.; Wang, Y.; Chen, K.; Gao, C. Manipulating gene translation in plants by CRISPR–Cas9-mediated genome editing of upstream open reading frames. Nat. Protoc. 2020, 15, 338–363. [Google Scholar] [CrossRef]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef]
- Papikian, A.; Liu, W.; Gallego-Bartolomé, J.; Jacobsen, S.E. Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat. Commun. 2019, 10, 729. [Google Scholar] [CrossRef]
- Molla, K.A.; Shih, J.; Wheatley, M.S.; Yang, Y. Predictable NHEJ Insertion and Assessment of HDR Editing Strategies in Plants. Front. Genome Ed. 2022, 4, 825236. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Cheng, Q.; Li, W.; Kan, M.; Zhang, Y.; Meng, X.; Guo, H.; Jing, Y.; Chen, M.; Liu, G.; et al. Creation of high-resistant starch rice through systematic editing of amylopectin biosynthetic genes in rs4. Plant Biotechnol. J. 2024, 23, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-b.; Zhou, R.; Wang, H.-m.; Zhang, P.-p.; Yang, Z.-f.; Xuan, D.-d.; Zhang, Y.-x.; Zhan, X.-d.; Cao, L.-y.; Cheng, S.-h.; et al. OsLAP3/OsSTRL2, encoding a rice strictosidine synthase, is required for anther cuticle formation and pollen exine patterning in rice. Front. Plant Sci. 2025, 15, 1508828. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xie, H.; Song, M.; Zhao, L.; Liu, H.; Li, G.; Zhu, J.K. Simple method for transformation and gene editing in medicinal plants. J. Integr. Plant Biol. 2024, 66, 17–19. [Google Scholar] [CrossRef]
- Fang, X.; Shi, L.; Xu, Y.; Zhao, M. Cloning of a sterol 14α-demethylase gene and the effects of over-expression of the gene on biological synthesis of triterpenes in Ganoderma lucidum. Mycosystema 2011, 30, 242–248. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.Y.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.W.; Li, Z.; Peterson, R.T.; Yeh, J.-R.J.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhao, S.; Pi, Y.; Chen, W.; Chen, C.; Liu, Q.; Li, M.; Han, D.; Ji, Q. Highly efficient base editing in Staphylococcus aureus using an engineered CRISPR RNA-guided cytidine deaminase. Chem. Sci. 2018, 9, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Topkar, V.V.; Zheng, Z.; Joung, J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 2015, 33, 1293–1298. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Liu, Y.; Yang, B.; Wang, X.; Wei, J.; Lu, Z.; Zhang, Y.; Wu, J.; Huang, X.; et al. Base editing with a Cpf1–cytidine deaminase fusion. Nat. Biotechnol. 2018, 36, 324–327. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR–Cas12b enables efficient plant genome engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Jiang, Y.; Zhou, Z.; Zou, G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Tan, J.; Forner, J.; Karcher, D.; Bock, R. DNA base editing in nuclear and organellar genomes. Trends Genet. 2022, 38, 1147–1169. [Google Scholar] [CrossRef]
- Peng, R.; Lin, G.; Li, J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2015, 283, 1218–1231. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Zheng, Z.; Nguyen, N.T.; Liebers, M.; Topkar, V.V.; Thapar, V.; Wyvekens, N.; Khayter, C.; Iafrate, A.J.; Le, L.P.; et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2014, 33, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bae, S.; Park, J.; Kim, E.; Kim, S.; Yu, H.R.; Hwang, J.; Kim, J.-I.; Kim, J.-S. Digenome-seq: Genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 2015, 12, 237–243. [Google Scholar] [CrossRef]
- Kruminis-Kaszkiel, E.; Juranek, J.; Maksymowicz, W.; Wojtkiewicz, J. CRISPR/Cas9 Technology as an Emerging Tool for Targeting Amyotrophic Lateral Sclerosis (ALS). Int. J. Mol. Sci. 2018, 19, 906. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Xu, J.-W.; Xu, Y.-N.; Zhong, J.-J. Enhancement of Ganoderic Acid Accumulation by Overexpression of an N-Terminally Truncated 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Gene in the Basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 2012, 78, 7968–7976. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhang, T.J.; Lu, X.X.; Ma, B.L.; Ren, A.; Shi, L.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Membrane fluidity is involved in the regulation of heat stress induced secondary metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Li, N.; Yu, X.; Zhao, P.; Li, T.; Xu, J.-W. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynthesis in Ganoderma lingzhi. Phytochemistry 2017, 134, 46–53. [Google Scholar] [CrossRef]
- Fang, X.; Shi, L.; Ren, A.; Jiang, A.-L.; Wu, F.-L.; Zhao, M.-W. The cloning, characterization and functional analysis of a gene encoding an acetyl-CoA acetyltransferase involved in triterpene biosynthesis in Ganoderma lucidum. Mycoscience 2013, 54, 100–105. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Gu, Z.; Li, Y.-R.; Shi, G.; Ding, Z. Heterologous expression and characterization of the key enzymes involved in sugar donor synthesis of polysaccharide in Ganoderma lucidum. Microbiol. China 2019, 46, 3233–3247. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Zhang, D.; Yue, T.; LIi, N.; Xu, J. Heterologous Expression of Vitreoscilla Hemoglobin in Ganoderma lingzhi for Increased Exopolysaccharide Production. Acta Edulis Fungi 2017, 24, 35–41. [Google Scholar] [CrossRef]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iyer, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J.; et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Malzahn, A.; Zhang, Y.; Qi, Y. CRISPR-Act2.0: An Improved Multiplexed System for Plant Transcriptional Activation. In Plant Genome Editing with CRISPR Systems; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 83–93. [Google Scholar] [CrossRef]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Sretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR–Act3.0 for highly efficient multiplexed gene activation in plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Wang, Z.; Chen, W.; Lin, D.; Xu, P. Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 2024, 42, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Li, M.; Chen, G.; Qi, L.; Song, S.; Li, Z.; Yan, C. Alcoholic Extracts from the Ganoderma Lucidum Fermentation Product Alleviated Ethanol-Induced Liver Injury, Gut Leakiness, and Gut Dysbiosis in Mice. Plant Foods Hum. Nutr. 2024, 80, 2. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Wang, W.; Liu, Y.; Meng, Y.; Wang, Y.; Fan, M.; Cai, C. Ganoderma lucidum polysaccharide alleviates cognitive dysfunction by inhibiting neuroinflammation via NLRP3/NF-κB signaling pathway. J. Ethnopharmacol. 2025, 338, 119065. [Google Scholar] [CrossRef]
- Chen, S. Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments. Toxins 2012, 4, 913–939. [Google Scholar] [CrossRef]
- Wang, W.F.; Xiao, H.; Zhong, J.J. Biosynthesis of a novel ganoderic acid by expressing CYP genes from Ganoderma lucidum in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2022, 106, 523–534. [Google Scholar] [CrossRef]
- Lan, X.; Yuan, W.; Wang, M.; Xiao, H. Efficient biosynthesis of antitumor ganoderic acid HLDOA using a dual tunable system for optimizing the expression of CYP5150L8 and a Ganoderma P450 reductase. Biotechnol. Bioeng. 2019, 116, 3301–3311. [Google Scholar] [CrossRef]
- Azi, F.; Hong, Y.; Wu, Z.; Xu, P. Synthetic consortium of Ganoderma lucidum and Lactobacillus plantarum for enhanced natural products biosynthesis. Biochem. Eng. J. 2023, 196, 108950. [Google Scholar] [CrossRef]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.-T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nat. Commun. 2021, 12, 1384. [Google Scholar] [CrossRef]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2020, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, X.; Liu, Y.; Liu, N.; Li, Y.; Luo, J.; Ma, Q.; Wu, D.; Li, J.; Xu, C.; et al. Programmable A-to-Y base editing by fusing an adenine base editor with an N-methylpurine DNA glycosylase. Nat. Biotechnol. 2023, 41, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, Y.; Sretenovic, S.; Liu, S.; Cheng, Y.; Han, Y.; Liu, G.; Bao, Y.; Fang, Q.; Zheng, X.; et al. CRISPR-BETS: A base-editing design tool for generating stop codons. Plant Biotechnol. J. 2021, 20, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Qiu, F.; Wang, Y.; Li, B.; Zhao, K.T.; Chen, K.; Gao, C. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat. Biotechnol. 2023, 41, 1758–1764. [Google Scholar] [CrossRef]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 2019, 571, 219–225. [Google Scholar] [CrossRef]
| Gene-Editing Technology | Working Principle | Advantage | Disadvantage | Application |
|---|---|---|---|---|
| ZFNs | Zinc finger domains recognize and bind to specific DNA sequences, followed by dimerization of the Fok I endonuclease domain to execute cleavage activity. | Specific, targeting specific sequences for cleavage. | Constrained targeting range due to sequence recognition preferences; Complex design requirements for multi-finger arrays; cytotoxicity from excessive DNA damage response activation; and off-target effects from promiscuous heterodimer formation. | Human disease treatment and crop trait improvement, etc. [31,32,33]. |
| TALENs | TALE domain specifically recognizes and binds to target DNA sequences, followed by dimerization of the Fok I nuclease domain to induce site-specific DNA cleavage at the predetermined genomic locus. | Simpler for design requirements and higher targeting specificity. | Complexity in design and construction; prohibitive production costs; low delivery efficiency of TALENs systems; and cytotoxic effects. | Disease modeling, plants, and livestock improvement, etc. [34,35,36]. |
| CRISPR/Cas9 | The target DNA sequence is recognized and bound by the Cas protein under the guidance of sgRNA, resulting in the induction of double-strand breaks at the designated genomic locus. | Higher specificity and simpler design. | High dependency on PAM sequences; off-target effects; and inability to achieve precise single-base editing. | Crop trait improvement, drug development, and disease treatment, etc. [23,24,25,26,27,28,29,30]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Liu, Y.; Zhang, Z.; Cai, M.; Hao, Y.; Hu, H. Gene Editing in Ganoderma lucidum: Development, Challenges, and Future Prospects. J. Fungi 2025, 11, 310. https://doi.org/10.3390/jof11040310
He S, Liu Y, Zhang Z, Cai M, Hao Y, Hu H. Gene Editing in Ganoderma lucidum: Development, Challenges, and Future Prospects. Journal of Fungi. 2025; 11(4):310. https://doi.org/10.3390/jof11040310
Chicago/Turabian StyleHe, Shiqi, Yuanchao Liu, Zhi Zhang, Manjun Cai, Yufan Hao, and Huiping Hu. 2025. "Gene Editing in Ganoderma lucidum: Development, Challenges, and Future Prospects" Journal of Fungi 11, no. 4: 310. https://doi.org/10.3390/jof11040310
APA StyleHe, S., Liu, Y., Zhang, Z., Cai, M., Hao, Y., & Hu, H. (2025). Gene Editing in Ganoderma lucidum: Development, Challenges, and Future Prospects. Journal of Fungi, 11(4), 310. https://doi.org/10.3390/jof11040310






