A Novel Effector FoUpe9 Enhances the Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4 by Inhibiting Plant Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Plant Materials, and Growth Conditions
2.2. Bioinformatic Analysis
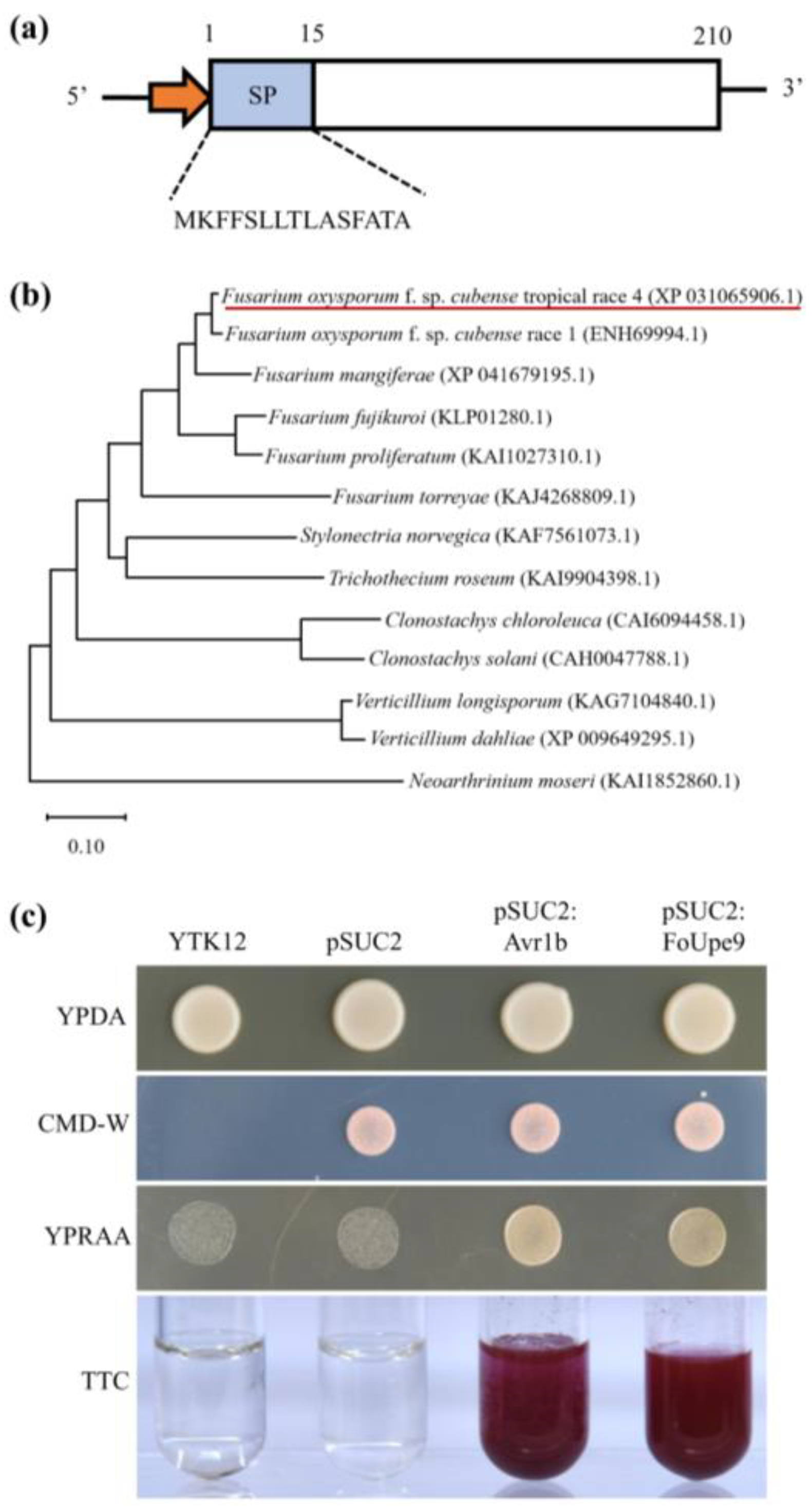
2.3. Yeast Signal Sequence Trap System
2.4. Real-Time Quantitative PCR (RT-qPCR) Assays
2.5. Agroinfiltration Assays
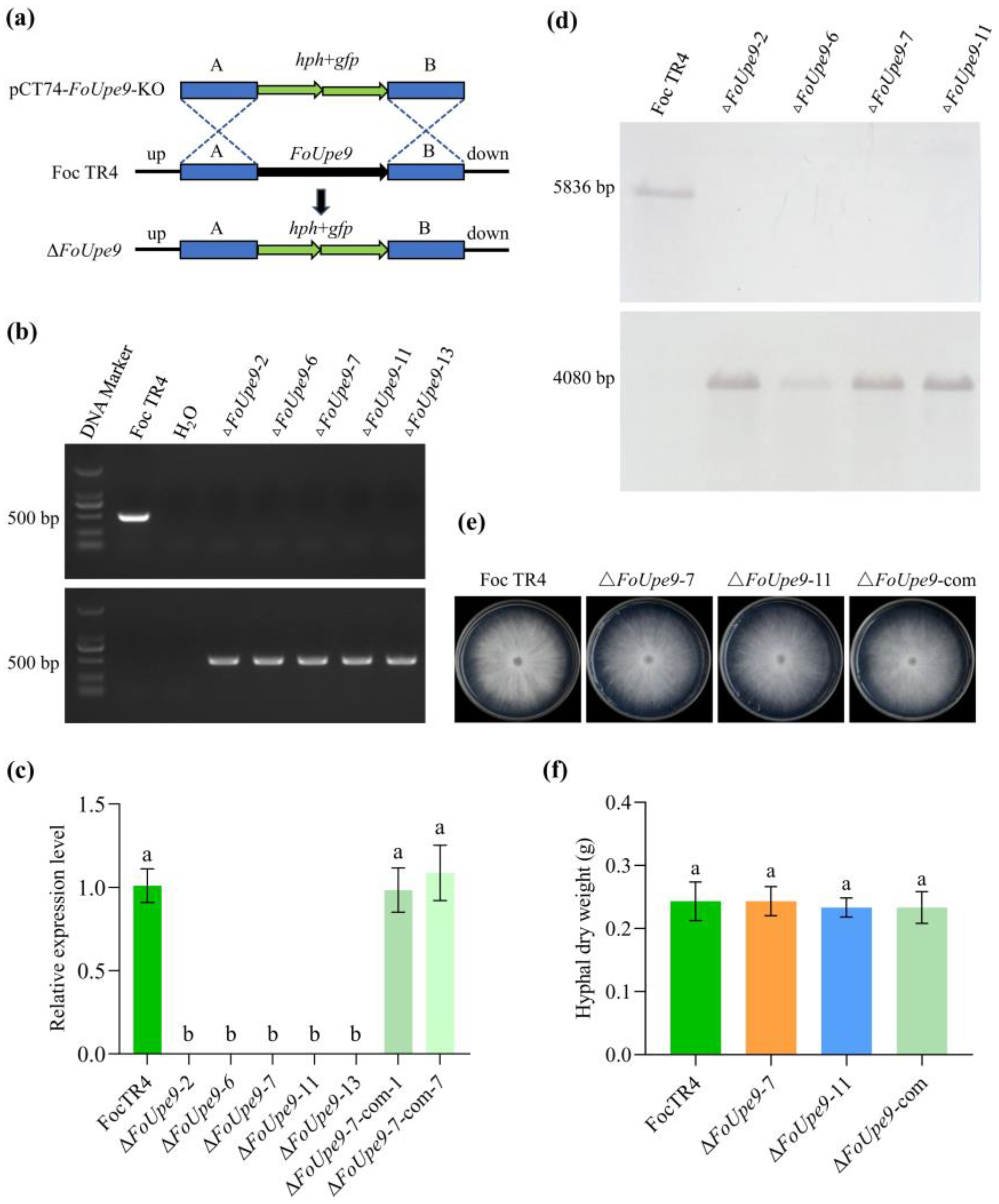
2.6. Deletion and Complementation of FoUpe9
2.7. Stress Sensitivity Assays
2.8. Pathogenicity Tests
2.9. Subcellular Localization
2.10. DAB Staining and H2O2 Measurements
2.11. Statistical Analysis
3. Results
3.1. FoUpe9 Is Highly Conserved in Fusarium Genus
3.2. FoUpe9 Contains a Functional Signal Peptide
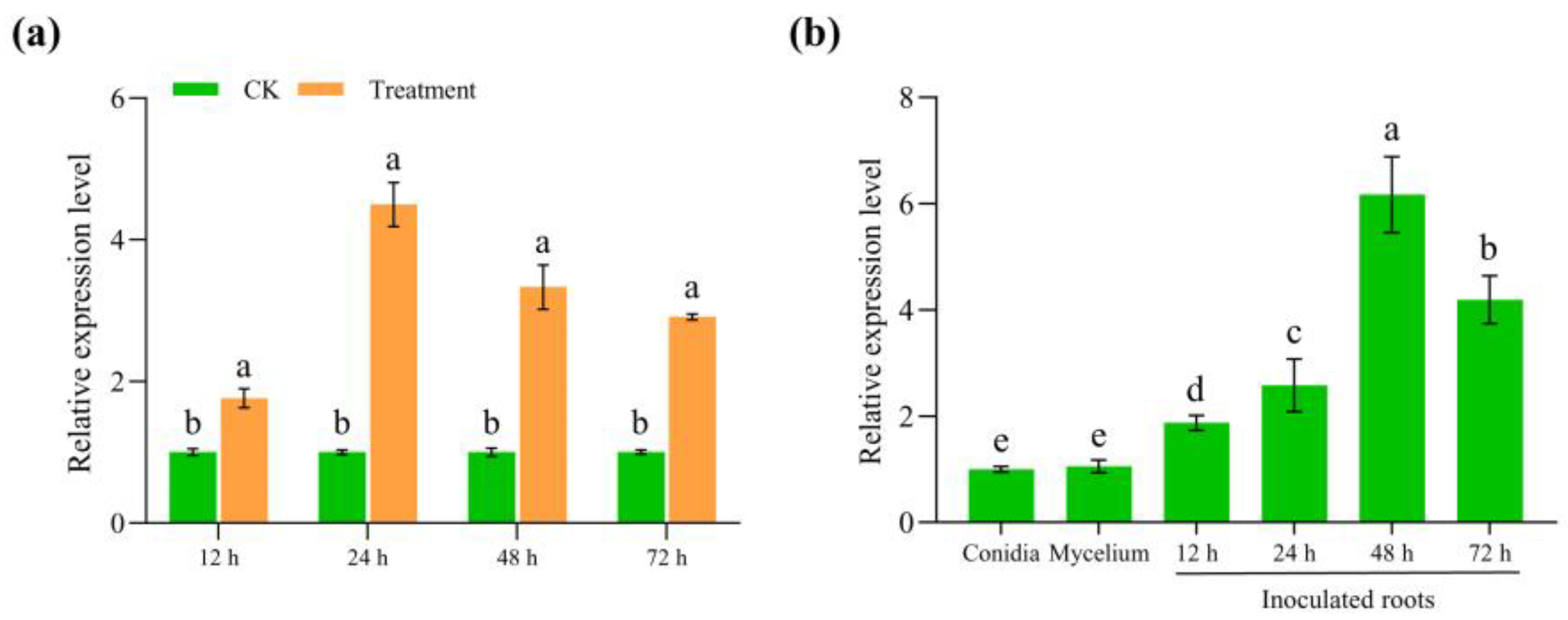
3.3. FoUpe9 Is Highly Expressed During the Early Infection Stage
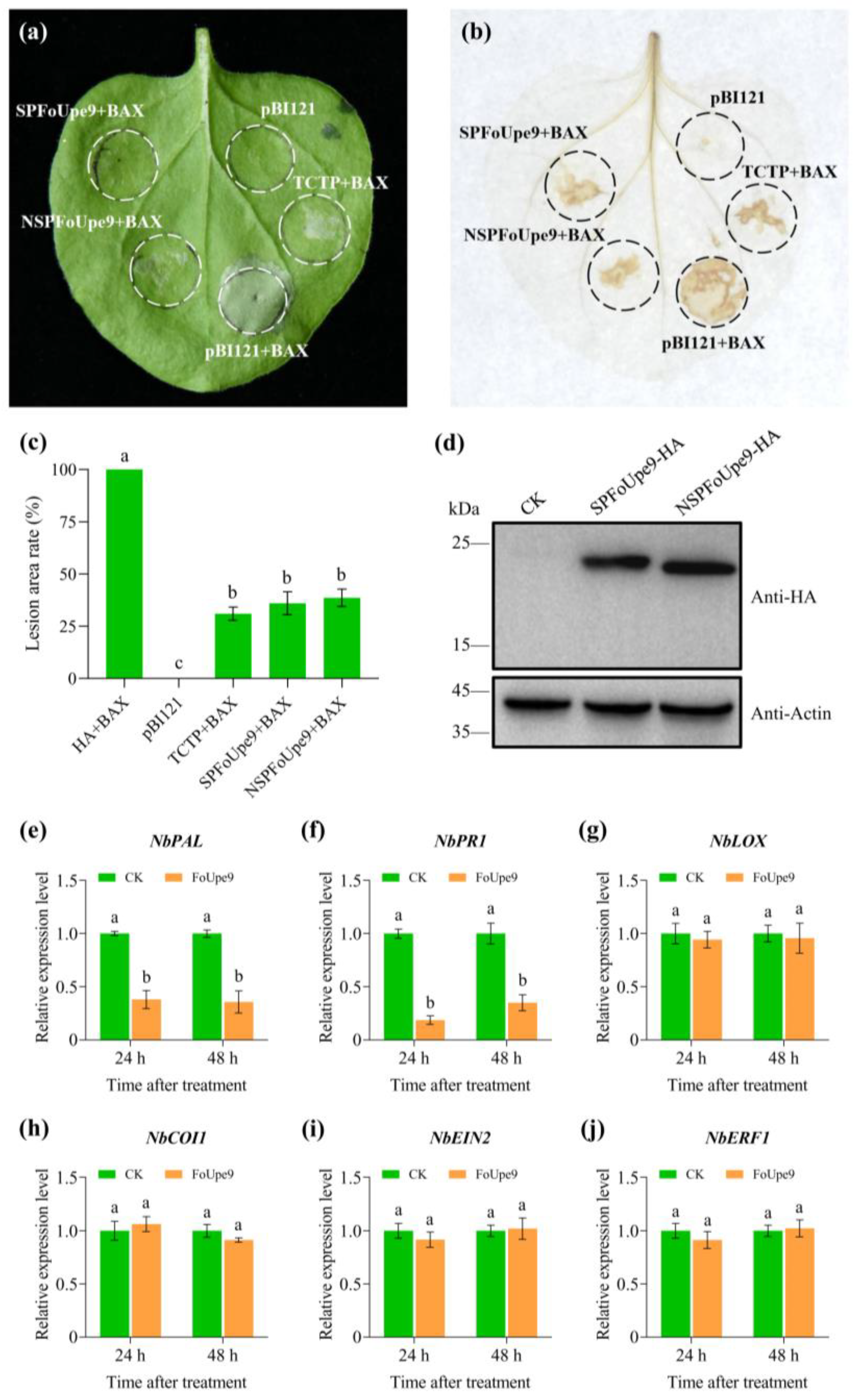
3.4. FoUpe9 Could Inhibit Plant Immune Responses in Nicotiana benthamiana
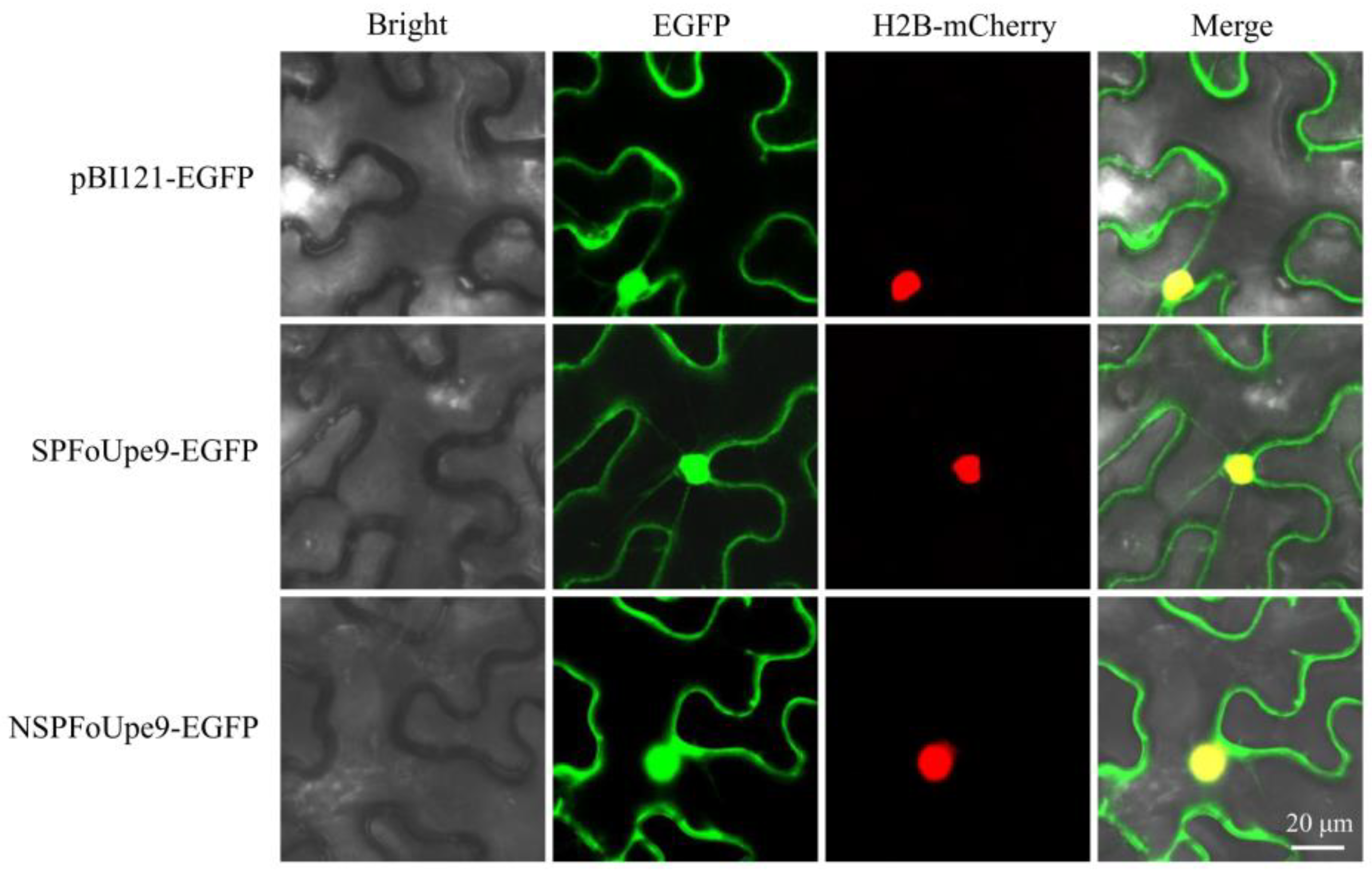
3.5. FoUpe9 Protein Is Localized in the Nucleus and Cytoplasm
3.6. FoUpe9 Is Dispensable in Mycelial Growth and Conidiation
3.7. FoUpe9 Has No Effect on Sensitivity to Various Stresses
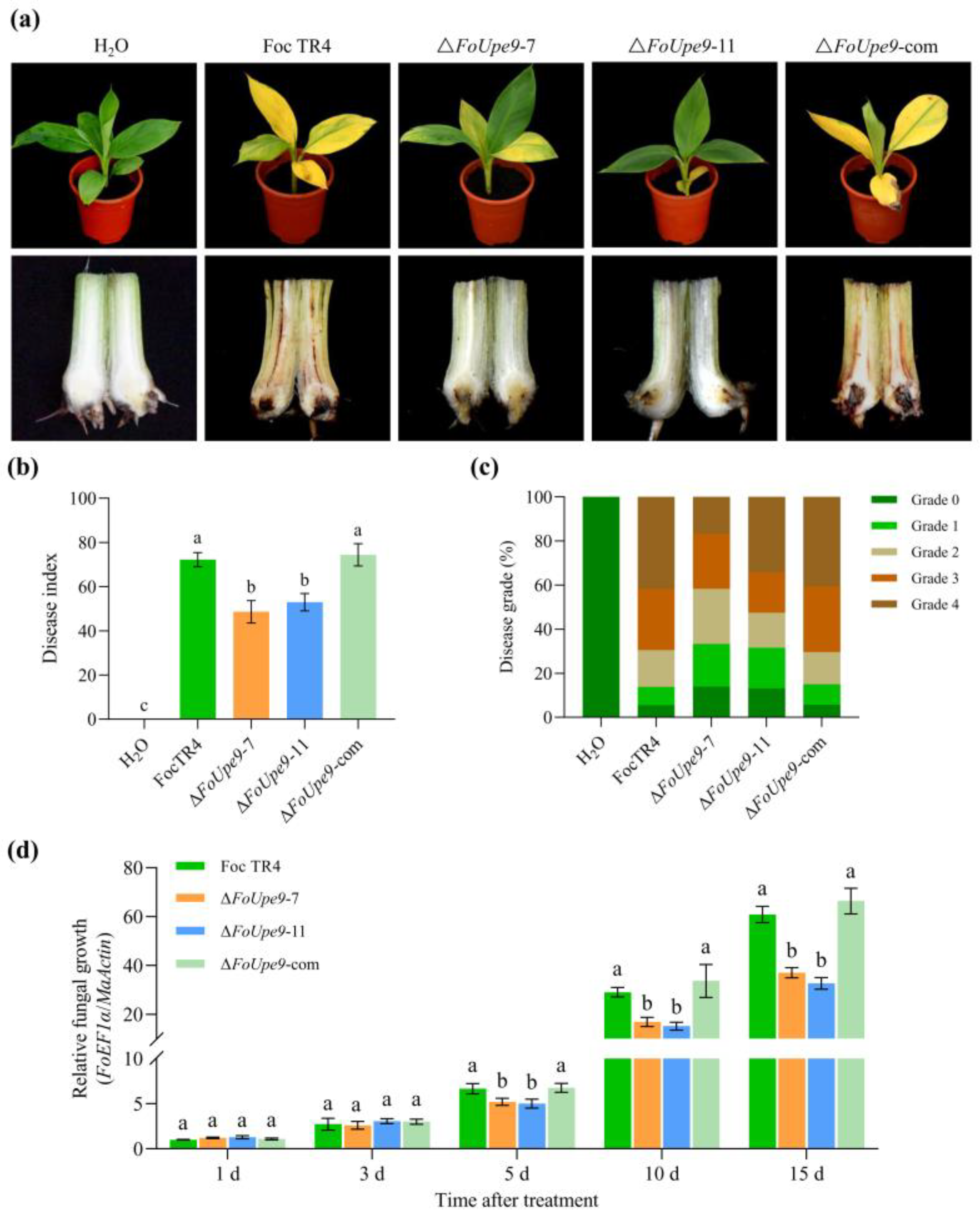
3.8. FoUpe9 Is Essential for the Full Virulence of Foc TR4
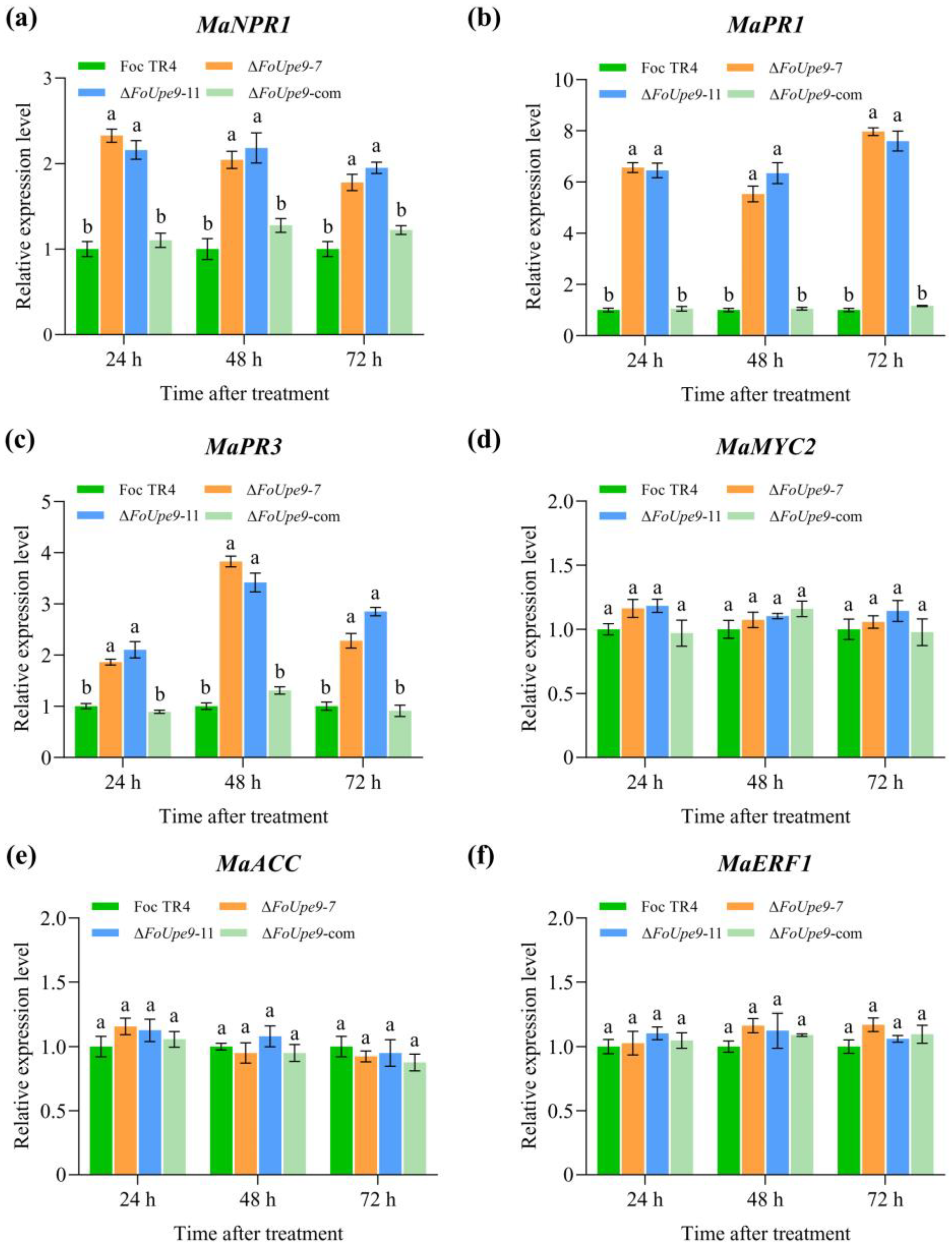
3.9. FoUpe9 Suppressed ROS Accumulation and Immune Response in Banana Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAX | Bcl2-associated X protein |
| CFW | Calcofluor white |
| CR | Congo red |
| EGFP | Enhanced green fluorescent protein |
| hph | Hygromycin B phosphotransferase |
| PEG | Polyethylene glycol |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| TCTP | Translationally controlled tumor protein |
| TTC | 2,3,5-triphenyl tetrazolium chloride |
| YPDA | Yeast peptone dextrose adenine |
References
- Ploetz, R.C. Fusarium Wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The Epidemiology of Fusarium Wilt of Banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; Macleod, A.; Magnusson, C.S.; Milonas, P.; et al. Pest categorisation of Fusarium oxysporum f. sp. cubense tropical race 4. EFSA J. 2022, 20, e07092. [Google Scholar] [PubMed]
- Li, W.; Wang, X.; Li, C.; Sun, J.; Li, S.; Peng, M. Dual species transcript profiling during the interaction between banana (Musa acuminata) and the fungal pathogen Fusarium oxysporum f. sp. cubense. BMC Genom. 2019, 20, 519. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fan, H.; Lei, Z.; Wu, C.; Zhou, D.; Li, H. Histological and gene expression analyses in banana reveals the pathogenic differences between races 1 and 4 of banana Fusarium wilt pathogen. Phytopathology 2019, 109, 1029–1042. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Ordonez, N.; Seidl, M.F.; Waalwijk, C.; Drenth, A.; Kilian, A.; Thomma, B.P.H.J.; Ploetz, R.C.; Kema, G.H.J. Worse comes to worst: Bananas and Panama disease—When plant and pathogen clones meet. PLoS Pathog. 2015, 11, e1005197. [Google Scholar] [CrossRef]
- Thangavelu, R.; Amaresh, H.; Gopi, M.; Loganathan, M.; Nithya, B.; Ganga Devi, P.; Anuradha, C.; Thirugnanavel, A.; Patil, K.B.; Blomme, G.; et al. Geographical distribution, host range and genetic diversity of Fusarium oxysporum f. sp. cubense causing Fusarium wilt of banana in India. J. Fungi 2024, 10, 887. [Google Scholar]
- Yang, D.; Du, C.; Zhang, J.; Pan, L.; Wei, S.; Jiang, S.; Li, C.; San, C.C.; Huy, N.D.; Ye, Y.; et al. Validation and application of a molecular detection system for Fusarium wilt of banana in China. Plant Dis. 2023, 107, 3687–3692. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Liu, Z.; Qian, Y.; Li, Y.; Yang, L.; Liu, S.; Liang, W.; Li, J. The secreted FolAsp aspartic protease facilitates the virulence of Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2023, 14, 1103418. [Google Scholar] [CrossRef]
- Kim, C.; Song, H.; Lee, Y. Ambivalent response in pathogen defense: A double-edged sword? Plant Commun. 2022, 3, 100415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pruitt, R.N.; Nurnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kong, L.; Chen, H.; Lin, Y.; Tu, S.; Wang, L.; Chen, Z.; Zeng, M.; Xiao, J.; Yuan, P.; et al. The Phytophthora sojae nuclear effector PsAvh110 targets a host transcriptional complex to modulate plant immunity. Plant Cell 2023, 35, 574–597. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Staskawicz, B.J.; Dangl, J.L. The plant immune system: From discovery to deployment. Cell 2024, 187, 2095–2116. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Li, J.; Li, H.; Li, Y.; Nie, Y. In Vitro secretome analysis suggests differential pathogenic mechanisms between Fusarium oxysporum f. sp. cubense race 1 and race 4. Biomolecules 2021, 11, 1353. [Google Scholar]
- Guo, L.; Wang, J.; Liang, C.; Yang, L.; Zhou, Y.; Liu, L.; Huang, J. Fosp9, a novel secreted protein, is essential for the full virulence of Fusarium oxysporum f. sp. cubense on banana (Musa spp.). Appl. Environ. Microbiol. 2022, 88, e0060421. [Google Scholar]
- Yang, Y.; An, B.; Guo, Y.; Luo, H.; He, C.; Wang, Q. A novel effector, FSE1, regulates the pathogenicity of Fusarium oxysporum f. sp. cubense tropical race 4 to banana by targeting the MYB transcription factor MaEFM-like. J. Fungi 2023, 9, 472. [Google Scholar]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f.sp. cubense tropical race 4 to Cavendish banana. Fungal Biol. 2019, 123, 423–430. [Google Scholar]
- Yan, T.; Zhou, X.; Li, J.; Li, G.; Zhao, Y.; Wang, H.; Li, H.; Nie, Y.; Li, Y. FoCupin1, a Cupin_1 domain-containing protein, is necessary for the virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Front. Microbiol. 2022, 13, 1001540. [Google Scholar]
- He, Y.; Li, P.; Zhou, X.; Ali, S.; Zhu, J.; Ma, Y.; Li, J.; Zhang, N.; Li, H.; Li, Y.; et al. A ribonucleaseT2 protein FocRnt2 contributes to the virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Mol. Plant Pathol. 2024, 25, e13502. [Google Scholar] [CrossRef]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. A small secreted virulence-related protein is essential for the necrotrophic interactions of Sclerotinia sclerotiorum with its host plants. PLoS Pathog. 2016, 12, e1005435. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Guo, Z.; Wang, N.; Wegner, A.; Wang, J.; Zou, X.; Hu, J.; Liu, M.; Zhang, H.; et al. MoIug4 is a novel secreted effector promoting rice blast by counteracting host OsAHL1-regulated ethylene gene transcription. New Phytol. 2022, 235, 1163–1178. [Google Scholar] [CrossRef]
- Li, G.; Shi, Q.; He, Y.; Zhu, J.; Zhong, M.; Tong, L.; Li, H.; Nie, Y.; Li, Y. Screening of candidate effectors from Magnaporthe oryzae by in vitro secretomic analysis. Int. J. Mol. Sci. 2023, 24, 3189. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Chen, T.; Lin, Y.; Luo, C. Functional evaluation of the signal peptides of secreted proteins. Bio-Protocol 2018, 8, e2839. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lukasik, E.; Gawehns, F.; Takken, F.L.W. The use of agroinfiltration for transient expression of plant resistance and fungal effector proteins in Nicotiana benthamiana leaves. Methods Mol. Biol. 2012, 835, 61–74. [Google Scholar]
- Dai, Y.; Cao, Z.; Huang, L.; Liu, S.; Shen, Z.; Wang, Y.; Wang, H.; Zhang, H.; Li, D.; Song, F. CCR4-not complex subunit Not2 plays critical roles in vegetative growth, conidiation and virulence in watermelon Fusarium wilt pathogen Fusarium oxysporum f. sp. niveum. Front. Microbiol. 2016, 7, 1449. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Chen, X.; Duan, Y.; Qiao, F.; Liu, H.; Huang, J.; Luo, C.; Chen, X.; Li, G.; Xie, K.; Hsiang, T.; et al. A secreted fungal effector suppresses rice immunity through host histone hypoacetylation. New Phytol. 2022, 235, 1977–1994. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, J.; Choi, J.; Cheong, K.; Song, H.; Choi, G.; Kang, S.; Lee, Y. Kingdom-wide analysis of fungal small secreted proteins (SSPs) reveals their potential role in host association. Front. Plant Sci. 2016, 7, 186. [Google Scholar] [CrossRef]
- Ma, M.; Tang, L.; Sun, R.; Lyu, X.; Xie, J.; Fu, Y.; Li, B.; Chen, T.; Lin, Y.; Yu, X.; et al. An effector SsCVNH promotes the virulence of Sclerotinia sclerotiorum through targeting classIII peroxidase AtPRX71. Mol. Plant Pathol. 2024, 25, e13464. [Google Scholar] [CrossRef]
- Hoang, C.V.; Bhaskar, C.K.; Ma, L. A Novel Core Effector Vp1 Promotes Fungal colonization and virulence of Ustilago maydis. J. Fungi 2021, 7, 589. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Tian, M.; Dai, K.; Zheng, W.; Liu, Z.; Yang, S.; Liu, X.; Shi, D.; Zhang, H.; et al. Fg12 ribonuclease secretion contributes to Fusarium graminearum virulence and induces plant cell death. J. Integr. Plant Biol. 2021, 63, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Redkar, A.; Sabale, M.; Schudoma, C.; Zechmann, B.; Gupta, Y.K.; López-Berges, M.S.; Venturini, G.; Gimenez-Ibanez, S.; Turrà, D.; Solano, R.; et al. Conserved secreted effectors contribute to endophytic growth and multihost plant compatibility in a vascular wilt fungus. Plant Cell 2022, 34, 3214–3232. [Google Scholar] [CrossRef]
- Bindics, J.; Khan, M.; Uhse, S.; Kogelmann, B.; Baggely, L.; Reumann, D.; Ingole, K.D.; Stirnberg, A.; Rybecky, A.; Darino, M.; et al. Many ways to TOPLESS—manipulation of plant auxin signalling by a cluster of fungal effectors. New Phytol. 2022, 236, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Liang, X.; Liu, G.; Du, Y.; Zhang, S.; Meng, Y.; Zhu, J.; Rollins, J.A.; Zhang, R.; Sun, G. A fungal effector suppresses plant immunity by manipulating DAHPS-mediated metabolic flux in chloroplasts. New Phytol. 2024, 244, 1552–1569. [Google Scholar] [CrossRef]
- Shang, S.; Liu, G.; Zhang, S.; Liang, X.; Zhang, R.; Sun, G. A fungal CFEM-containing effector targets NPR1 regulator NIMIN2 to suppress plant immunity. Plant Biotechnol. J. 2024, 22, 82–97. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, S.; Zhao, Y.; Meng, X.; Song, L.; Feng, H.; Huang, L. Hce2 domain-containing effectors contribute to the full virulence of Valsa mali in a redundant manner. Mol. Plant Pathol. 2019, 20, 843–856. [Google Scholar] [CrossRef]
- Yang, B.; Yang, S.; Guo, B.; Wang, Y.; Zheng, W.; Tian, M.; Dai, K.; Liu, Z.; Wang, H.; Ma, Z.; et al. The Phytophthora effector Avh241 interacts with host NDR1-like proteins to manipulate plant immunity. J. Integr. Plant Biol. 2021, 63, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, S.; Bai, H.; Fan, J.; Batool, W.; Shabbir, A.; Han, Y.; Zheng, H.; Lu, G.; Lin, L.; et al. A nonclassically secreted effector of Magnaporthe oryzae targets host nuclei and plays important roles in fungal growth and plant infection. Mol. Plant Pathol. 2023, 24, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Wu, B.; Xie, J.; Viljoen, A.; Wang, W.; Mostert, D.; Xie, Y.; Fu, G.; Xiang, D.; et al. The M35 metalloprotease effector FocM35_1 is required for full virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Pathogens 2021, 10, 670. [Google Scholar]
- Wang, Y.; Zhang, X.; Wang, T.; Zhou, S.; Liang, X.; Xie, C.; Kang, Z.; Chen, D.; Zheng, L. The small secreted protein FoSsp1 elicits plant defenses and negatively regulates pathogenesis in Fusarium oxysporum f. sp. cubense (Foc4). Front. Plant Sci. 2022, 13, 873451. [Google Scholar] [CrossRef]
- Zhao, S.; An, B.; Guo, Y.; Hou, X.; Luo, H.; He, C.; Wang, Q. Label free proteomics and systematic analysis of secretome reveals effector candidates regulated by SGE1 and FTF1 in the plant pathogen Fusarium oxysporum f. sp. cubense tropical race 4. BMC Genom. 2020, 21, 275. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Zhao, Y.; Liang, X.; Liu, S.; Zhang, Y.; Kang, Z.; Chen, D.; Zheng, L. Systemic screening of Fusarium oxysporum candidate effectors reveals FoSSP17 that suppresses plant immunity and contributes to virulence. Phytopathol. Res. 2023, 5, 42. [Google Scholar] [CrossRef]
- Situ, J.; Jiang, L.; Fan, X.; Yang, W.; Li, W.; Xi, P.; Deng, Y.; Kong, G.; Jiang, Z. An RXLR effector PlAvh142 from Peronophythora litchii triggers plant cell death and contributes to virulence. Mol. Plant Pathol. 2020, 21, 415–428. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Z.; Gu, B.; Zhao, H.; Jia, J.; Fan, G.; Meng, Y.; Du, Y.; Shan, W. An RXLR effector secreted by Phytophthora parasitica is a virulence factor and triggers cell death in various plants. Mol. Plant Pathol. 2019, 20, 356–371. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Sun, Y.; Xu, Y.; Zhou, S.; Luo, P.; Wang, Z.; Chen, D.; Liang, X.; Kang, Z.; et al. A novel key virulence factor, FoSSP71, inhibits plant immunity and promotes pathogenesis in Fusarium oxysporum f. sp. cubense. Microbiol. Spectr. 2025, 25, e0294024. [Google Scholar] [CrossRef]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Garcia, E.; Tamang, T.M.; Park, J.; Dalby, M.; Martin-Urdiroz, M.; Rodriguez Herrero, C.; Vu, A.H.; Park, S.; Talbot, N.J.; Valent, B. Clathrin-mediated endocytosis facilitates the internalization of Magnaporthe oryzae effectors into rice cells. Plant Cell 2023, 35, 2527–2551. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef]
- Wang, S.; Boevink, P.C.; Welsh, L.; Zhang, R.; Whisson, S.C.; Birch, P.R.J. Delivery of cytoplasmic and apoplastic effectors from Phytophthora infestans haustoria by distinct secretion pathways. New Phytol. 2017, 216, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Dalio, R.J.; Maximo, H.J.; Roma-Almeida, R.; Barretta, J.N.; José, E.M.; Vitti, A.J.; Blachinsky, D.; Reuveni, M.; Pascholati, S.F. Tea tree oil induces systemic resistance against Fusarium wilt in banana and xanthomonas infection in tomato plants. Plants 2020, 9, 1137. [Google Scholar] [CrossRef]
- Liu, S.; Wu, B.; Yang, J.; Bi, F.; Dong, T.; Yang, Q.; Hu, C.; Xiang, D.; Chen, H.; Huang, H.; et al. A cerato-platanin family protein FocCP1 is essential for the penetration and virulence of Fusarium oxysporum f. sp. cubense tropical race 4. Int. J. Mol. Sci. 2019, 20, 3785. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, B.; Li, X.; Chen, H.; Takáč, T.; Šamaj, J.; Xu, C. Comparative digital gene expression analysis of tissue-cultured plantlets of highly resistant and susceptible banana cultivarsin response to Fusarium oxysporum. Int. J. Mol. Sci. 2018, 19, 350. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, H.; Du, X.; Wang, S.; Ma, X.W.; Nürnberger, T.; Guo, H.S.; Hua, C. The Verticillium-specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytol. 2017, 215, 368–381. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, Z.; Ma, Y.; Zeng, L.; Wu, Y.; Chen, Y.; Liang, L.; Zhu, J.; Li, H.; Nie, Y.; Li, Y. A Novel Effector FoUpe9 Enhances the Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4 by Inhibiting Plant Immunity. J. Fungi 2025, 11, 308. https://doi.org/10.3390/jof11040308
Cong Z, Ma Y, Zeng L, Wu Y, Chen Y, Liang L, Zhu J, Li H, Nie Y, Li Y. A Novel Effector FoUpe9 Enhances the Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4 by Inhibiting Plant Immunity. Journal of Fungi. 2025; 11(4):308. https://doi.org/10.3390/jof11040308
Chicago/Turabian StyleCong, Zheng, Yini Ma, Lisha Zeng, Yaoyao Wu, Yaojun Chen, Ludan Liang, Jie Zhu, Huaping Li, Yanfang Nie, and Yunfeng Li. 2025. "A Novel Effector FoUpe9 Enhances the Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4 by Inhibiting Plant Immunity" Journal of Fungi 11, no. 4: 308. https://doi.org/10.3390/jof11040308
APA StyleCong, Z., Ma, Y., Zeng, L., Wu, Y., Chen, Y., Liang, L., Zhu, J., Li, H., Nie, Y., & Li, Y. (2025). A Novel Effector FoUpe9 Enhances the Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4 by Inhibiting Plant Immunity. Journal of Fungi, 11(4), 308. https://doi.org/10.3390/jof11040308




