The Role of Oral Yeasts in the Development and Progression of Oral Squamous Cell Carcinoma: A Scoping Review
Abstract
1. Introduction
- The techniques and methodologies used to investigate oral yeast colonisation and/or infection in the context of oral epithelial carcinogenesis.
- The role of Candida spp. colonisation and/or infection in oral epithelial carcinogenesis.
- The role of non-Candida yeast species colonisation and/or infection in oral epithelial carcinogenesis.
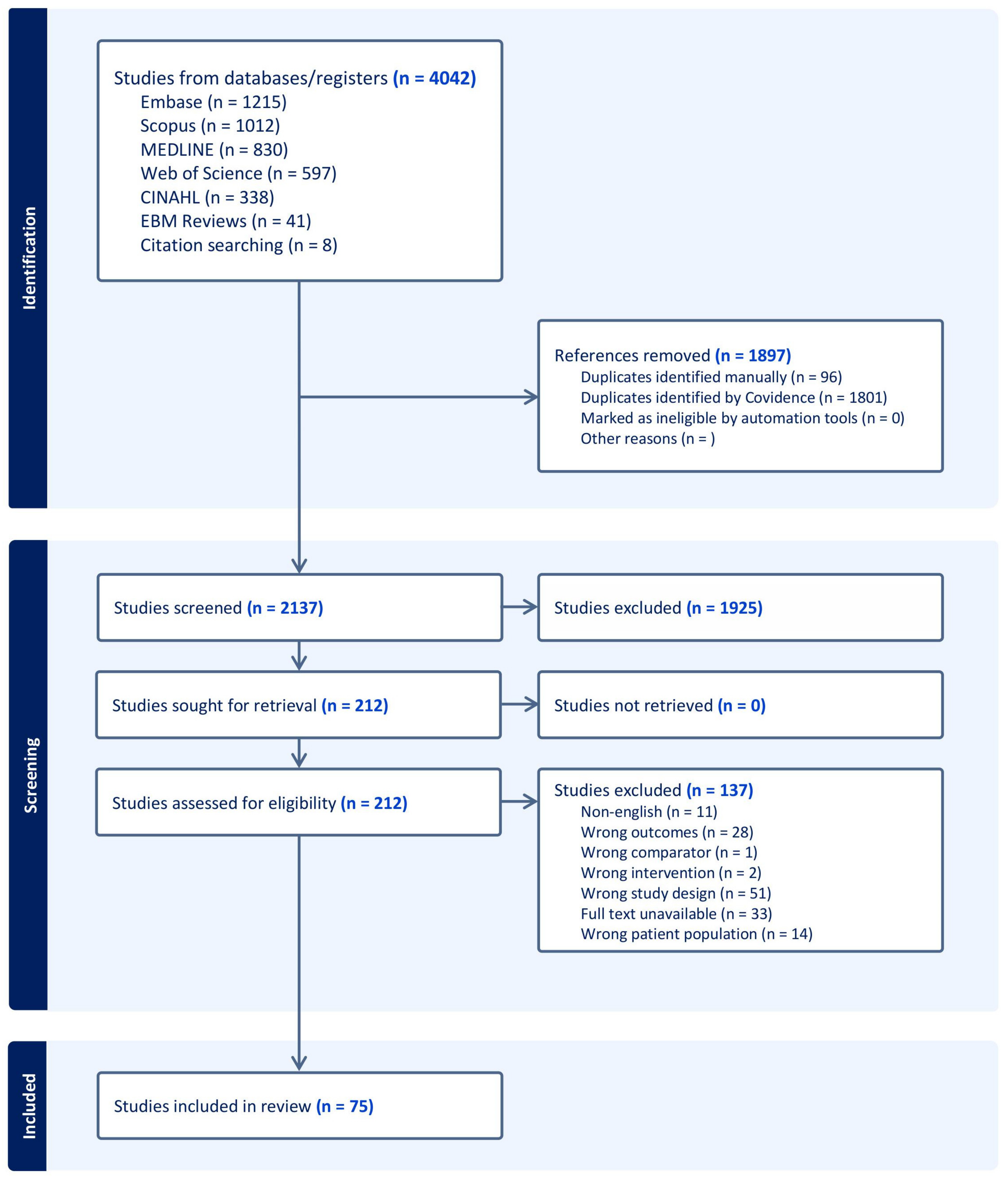
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Selection of Sources of Evidence
2.4. Data Items and Data Charting Process
2.5. Synthesis of Results
3. Results
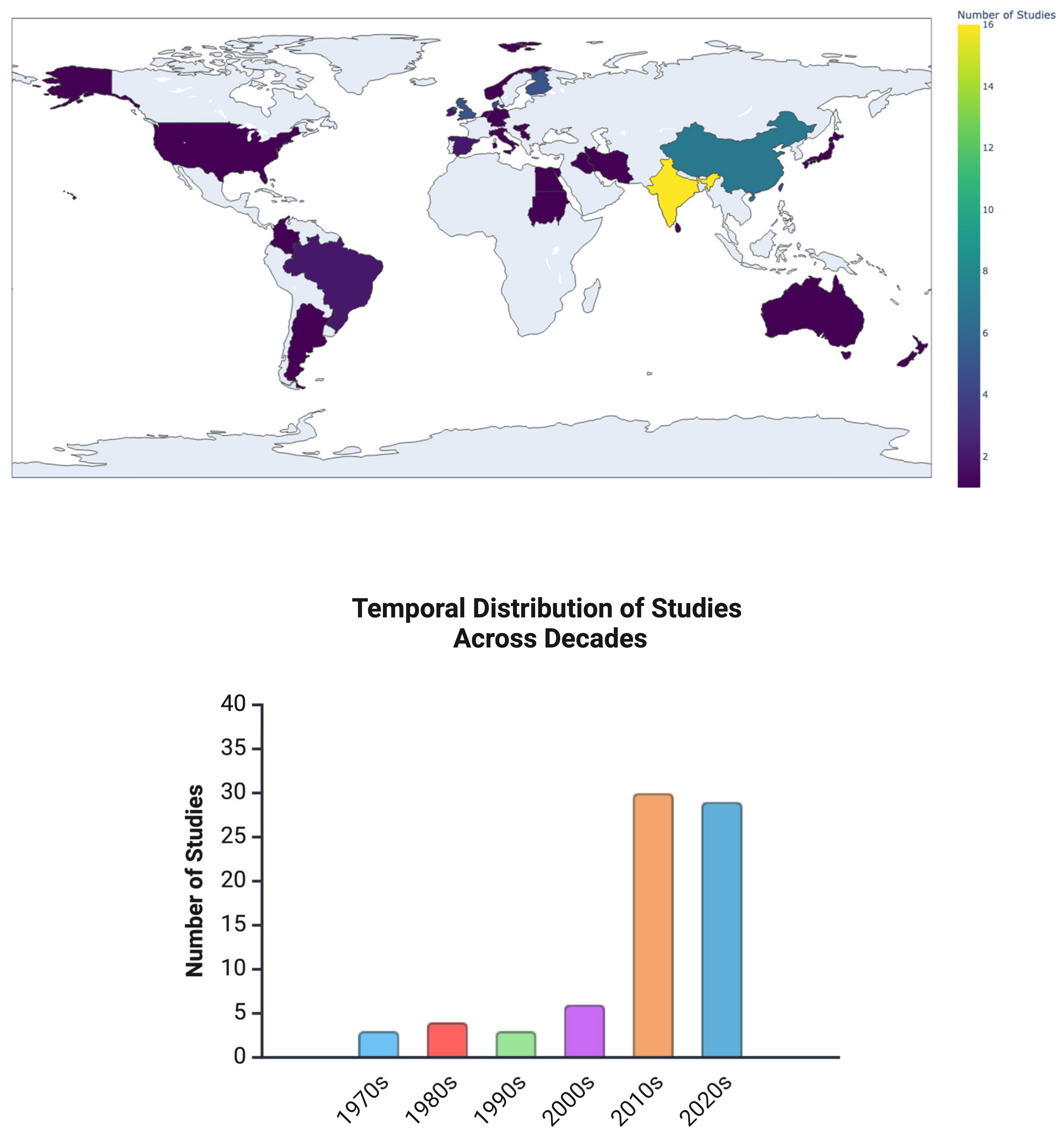
3.1. Study Characteristics
3.2. Objective 1: The Techniques and Methodologies Used to Investigate Oral Yeast Colonisation and/or Infection in the Context of Oral Epithelial Carcinogenesis
3.2.1. Culture-Based and Phenotype-Based Identification Techniques
3.2.2. Molecular and Genotypic Characterisation
3.2.3. High-Throughput Sequencing and Metagenomic Analyses
3.2.4. Fungal–Bacterial Interaction Studies
3.2.5. Proteomic and Metabolomic Approaches
3.2.6. In Vitro and In Vivo Experimental Models
- In Vitro Studies: Oral squamous cell carcinoma cell lines (e.g., SCC25, SCC15, CAL27) and a dysplastic cell model (i.e., DOK) have been co-cultured with Candida spp. to examine their effects on cell proliferation, migration, cytokine secretion, and oncogenic signalling [14,36,56,69,73,74,76,77]. Additionally, co-culture models have been developed to study the effects of Candidal biofilm or combined fungal–bacterial biofilm on OSCC cell lines and oral epithelial cells [56,76].
- In Vivo Studies: OSCC models (e.g., 4NQO-induced and xenografted) have been used to assess the impact of fungal colonisation on tumour development. Studies have shown that C. albicans-colonised animals develop more severe dysplasia and tumours, accompanied by increased immune suppression and PD-L1 expression. These findings indicate that Candida spp. may contribute to immune evasion and tumour progression.
3.3. Objective 2: The Role of Candida spp. Colonisation and/or Infection in Oral Epithelial Carcinogenesis
3.3.1. Prevalence of Candida spp. in OSCC and OPMDs and Its Clinical Implications
3.3.2. Clinical Impact of Candida spp. Colonisation
3.3.3. Prevalence of Non-albicans Candida spp. (NAC) Species in OSCC and OPMDs
3.3.4. Mechanisms of Candida spp. in Oral Epithelial Carcinogenesis
3.3.5. Acetaldehyde Production and Carcinogenicity
3.3.6. Virulence Factors and Tissue Invasion
3.3.7. Immunomodulatory Properties
3.3.8. Fungal–Bacterial Interactions in OSCC Progression
3.4. Objective 3: The Role of Non-Candida Yeast Species Colonisation and/or Infection in Oral Epithelial Carcinogenesis
4. Discussion
Limitations of This Review
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-Forming Unit |
| DNA | Deoxyribonucleic Acid |
| EBM | Evidence-Based Medicine |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| GMS | Gomori Methenamine Silver |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HRP | Horseradish Peroxidase |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin-6 |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LFC | Logarithmic Fold Change |
| MMP | Matrix Metalloproteinase |
| mRNA | Messenger Ribonucleic Acid |
| NAC | Non-albicans Candida |
| NF-κB | Nuclear Factor Kappa B |
| OED | Oral epithelial dysplasia |
| OPMD | Oral potentially malignant disorder |
| OSCC | Oral squamous cell carcinoma |
| OTSCC | Oral tongue squamous cell carcinoma |
| PAS | Periodic Acid–Schiff |
| PCR | Polymerase Chain Reaction |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews |
| qPCR | Quantitative Polymerase Chain Reaction |
| RPKM | Reads Per Kilobase of Exon Model per Million Mapped Reads |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| SDA | Sabouraud’s Dextrose Agar |
| scSeq | Single-Cell Sequencing |
| TLR | Toll-like receptor |
| TNF-α | Tumour Necrosis Factor Alpha |
| TGF-β | Transforming Growth Factor Beta |
| URM | Upstream Regulatory Mechanism |
| Wnt | Wingless-Related Integration Site (Wnt Signalling Pathway) |
References
- Lythgoe, M.P.; Mullish, B.H.; Frampton, A.E.; Krell, J. Polymorphic Microbes: A New Emerging Hallmark of Cancer. Trends Microbiol. 2022, 30, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Renstrup, G. Occurrence of Candida in Oral Leukoplakias. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 1970, 78, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Roed-Petersen, B.; Renstrup, G.; Pindborg, J.J. Candida in Oral Leukoplakias A Histologic and Exfoliative Cytologic Study. Eur. J. Oral Sci. 1970, 78, 323–328. [Google Scholar] [CrossRef]
- Daftary, D.K.; Mehta, F.S.; Gupta, P.C.; Pindborg, J.J. The Presence of Candida in 723 Oral Leukoplakias among Indian Villagers. Eur. J. Oral Sci. 1972, 80, 75–79. [Google Scholar] [CrossRef]
- Hornstein, O.P.; Gräßel, R.; Schirner, E. Prevalence Rates of Candidosis in Leukoplakias and Carcinomas of the Oral Cavity. Arch. Dermatol. Res. 1979, 266, 99–102. [Google Scholar] [CrossRef]
- Silverman, S., Jr.; Gorsky, M.; Ms, F.L. Dds, Oral Leukoplakia and Malignant Transformation. A Follow-up Study of 257 Patients. Cancer 1984, 53, 563–568. [Google Scholar] [CrossRef]
- Krogh, P.; Holmstrup, P.; Thorn, J.J.; Vedtofte, P.; Pindborg, J.J. Yeast Species and Biotypes Associated with Oral Leukoplakia and Lichen Planus. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 48–54. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K.; Kumar, V.N. A Comparative Study of Candida Species Diversity among Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. BMC Res. Notes 2020, 13, 488. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K. Oral Candidal Carriage among Patients with Oral Squamous Cell Carcinoma: A Case-Control Study. J. Orofac. Sci. 2019, 11, 55–58. [Google Scholar] [CrossRef]
- McCullough, M.; Jaber, M.; Barrett, A.W.; Bain, L.; Speight, P.M.; Porter, S.R. Oral Yeast Carriage Correlates with Presence of Oral Epithelial Dysplasia. Oral Oncol. 2002, 38, 391–393. [Google Scholar] [CrossRef]
- Tamgadge, S.; Tamgadge, A.; Pillai, A.; Chande, M.; Acharya, S.; Kamat, N. Association of Candida Sp. with the Degrees of Dysplasia and Oral Cancer: A Study by Calcofluor White under Fluorescent Microscopy. Iran. J. Pathol. 2017, 12, 348–355. [Google Scholar] [PubMed]
- Hebbar, P.; Pai, A.; Sujatha, D. Mycological and Histological Associations of Candida in Oral Mucosal Lesions. J. Oral Sci. 2013, 55, 157–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiu, C.-T.; Li, C.-F.; Li, J.-R.; Wang, J.; Chuang, C.-Y.; Chiang, W.-F.; Huang, S.-C.; Chang, S.-W. Candida Invasion and Influences in Smoking Patients with Multiple Oral Leucoplakias—A Retrospective Study. Mycoses 2011, 54, e377–e383. [Google Scholar] [CrossRef] [PubMed]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2022, 13, e03144-21. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wu, W.; Wu, S.; Young, A.; Yan, Z. Is Candida albicans a Contributor to Cancer? A Critical Review Based on the Current Evidence. Microbiol. Res. 2023, 272, 127370. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida Virulence and Ethanol-Derived Acetaldehyde Production in Oral Cancer and Non-Cancer Subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Shukla, K.; Vun, I.; Lov, I.; Laparidis, G.; McCamley, C.; Ariyawardana, A. Role of Candida Infection in the Malignant Transformation of Oral Leukoplakia: A Systematic Review of Observational Studies. Transl. Res. Oral Oncol. 2019, 4, 2057178X1982822. [Google Scholar] [CrossRef]
- Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida albicans in Oral Carcinogenesis. Pathophysiology 2022, 29, 650–662. [Google Scholar] [CrossRef]
- Navgire, G.S.; Goel, N.; Sawhney, G.; Sharma, M.; Kaushik, P.; Mohanta, Y.K.; Mohanta, T.K.; Al-Harrasi, A. Analysis and Interpretation of Metagenomics Data: An Approach. Biol. Proced. Online 2022, 24, 18. [Google Scholar] [CrossRef]
- He, S.; Chakraborty, R.; Ranganathan, S. Metaproteomic Analysis of an Oral Squamous Cell Carcinoma Dataset Suggests Diagnostic Potential of the Mycobiome. Int. J. Mol. Sci. 2023, 24, 1050. [Google Scholar] [CrossRef]
- Mohamed, N.; Litlekalsøy, J.; Ahmed, I.A.; Martinsen, E.M.H.; Furriol, J.; Javier-Lopez, R.; Elsheikh, M.; Gaafar, N.M.; Morgado, L.; Mundra, S.; et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients from Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front. Cell. Infect. Microbiol. 2021, 11, 673465. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. A Dysbiotic Mycobiome Dominated by Candida albicans Is Identified within Oral Squamous-Cell Carcinomas. J. Oral Microbiol. 2017, 9, 1385369. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Peck, K.N.; Shih, N.; Chalian, A.A.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; Alwine, J.; et al. Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 4036. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Barrett, A.W.; Kingsmill, V.J.; Speight, P.M. The Frequency of Fungal Infection in Biopsies of Oral Mucosal Lesions. Oral Dis. 1998, 4, 26–31. [Google Scholar] [CrossRef]
- Vuckovic, N.; Bokor-Bratic, M.; Vuckovic, D.; Picuric, I. Presence of Candida albicans in Potentially Malignant Oral Mucosal Lesions. Arch. Oncol. 2004, 12, 51–54. [Google Scholar] [CrossRef]
- Dany, A.; Kurian, K.; Shanmugam, S. Association of Candida in Different Stages of Oral Leukoplakia. J. Indian Acad. Oral Med. Radiol. 2011, 23, 14–16. [Google Scholar] [CrossRef]
- Odedra, S.; Chawda, J.; Rupapara, R.; Rupakar, P. Presence of Candida in Oral Dysplastic Lesions—A Casual Involvement or a Causal Role? Indian J. Public Health Res. Dev. 2013, 4, 267–271. [Google Scholar] [CrossRef]
- Wu, L.; Feng, J.; Shi, L.; Shen, X.; Liu, W.; Zhou, Z. Candidal Infection in Oral Leukoplakia: A Clinicopathologic Study of 396 Patients from Eastern China. Ann. Diagn. Pathol. 2013, 17, 37–40. [Google Scholar] [CrossRef]
- Bakri, M.M.; Cannon, R.D.; Holmes, A.R.; Rich, A.M. Detection of Candida albicans ADH1 and ADH2 mRNAs in Human Archival Oral Biopsy Samples. J. Oral Pathol. Med. 2014, 43, 704–710. [Google Scholar] [CrossRef]
- Singh, S.K.; Gupta, A.; Rajan, S.Y.; Padmavathi, B.N.; Mamatha, G.P.; Mathur, H.; Bhuvaneshwari, S.; Soundarya, S. Correlation of Presence of Candida and Epithelial Dysplasia in Oral Mucosal Lesions. J. Clin. Diagn. Res. JCDR 2014, 8, ZC31–ZC35. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Rathod, G.P. Clinicopathologic Assessment of Candida Colonization of Oral Leukoplakia. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Brouns, E.; Baart, J.A.; Karagozoglu, K.H.; Aartman, I.H.A.; Bloemena, E.; Van der Waal, I. Malignant Transformation of Oral Leukoplakia in a Well-Defined Cohort of 144 Patients. Oral Dis. 2014, 20, e19–e24. [Google Scholar] [CrossRef]
- Hongal, B.; Kulkarni, V.; Deshmukh, R.; Joshi, P.; Karande, P.; Shroff, A. Prevalence of Fungal Hyphae in Potentially Malignant Lesions and Conditions-Does Its Occurrence Play a Role in Epithelial Dysplasia? J. Oral Maxillofac. Pathol. 2015, 19, 10. [Google Scholar] [CrossRef]
- Hafed, L.; Farag, H.; El-Rouby, D.; Shaker, O.; Shabaan, H.-A. Candida albicans Alcohol Dehydrogenase 1 Gene in Oral Dysplasia and Oral Squamous Cell Carcinoma. Pol. J. Pathol. 2019, 70, 210–216. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, P.-F.; Liu, K.-J.; Chung, H.-L.; Yang, W.-C.; Hsu, K.-C.; Fong, T.-H.; Lo, H.-J.; Chen, Y.-P.; Yang, J.-R.; et al. LDOC1 Suppresses Microbe-Induced Production of IL-1β in Human Normal and Cancerous Oral Cells through the PI3K/Akt/GSK-3β Axis. Cancers 2020, 12, 3148. [Google Scholar] [CrossRef]
- Erira, A.T.; Navarro, A.F.R.; Robayo, D.A.G. Human Papillomavirus, Epstein-Barr Virus, and Candida albicans Co-Infection in Oral Leukoplakia with Different Degrees of Dysplasia. Clin. Exp. Dent. Res. 2021, 7, 914–923. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, S.; Wang, X.; Gao, Y.; Yan, Z. Malignant Transformation and Treatment Recommendations of Chronic Hyperplastic Candidiasis-A Six-Year Retrospective Cohort Study. Mycoses 2021, 64, 1422–1428. [Google Scholar] [CrossRef]
- Yang, S.-W.; Lee, Y.-C.; Lee, Y.-S.; Chang, L.-C.; Lai, Y.-R. Risk Assessment of Malignant Transformation of Oral Leukoplakia in Patients with Previous Oral Squamous Cell Carcinoma. Int. J. Oral Maxillofac. Surg. 2022, 51, 1394–1400. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Beirami, M.; Zareshahrabadi, Z.; Sedarat, H.; Zomorodian, K. Evaluation of the Distribution of Candida Species in Patients with Dysplastic and Nondysplastic Oral Lichen Planus Lesions. BioMed Res. Int. 2022, 2022, 8100352. [Google Scholar] [CrossRef]
- Saraneva, O.; Furuholm, J.; Hagström, J.; Sorsa, T.; Rita, V.; Tervahartiala, T.; Välimaa, H.; Ruokonen, H. Oral Potentially Malignant Disorders and Candida in Oral Tongue Squamous Cell Carcinoma Patients. Dent. J. 2023, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Bhargava, A.; Mehra, S.K.; Dakwala, F. Identification of Candida albicans by Using Different Culture Medias and Its Association in Potentially Malignant and Malignant Lesions. Contemp. Clin. Dent. 2011, 2, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gall, F.; Colella, G.; Di Onofrio, V.; Rossiello, R.; Angelillo, I.F.; Liguori, G. Candida Spp. in Oral Cancer and Oral Precancerous Lesions. New Microbiol. 2013, 36, 283–288. [Google Scholar] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida Colonization in Oral Cancer Patients and Its Relationship with Traditional Risk Factors of Oral Cancer: A Matched Case-Control Study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Hulimane, S.; Maluvadi-Krishnappa, R.; Mulki, S.; Rai, H.; Dayakar, A.; Kabbinahalli, M. Speciation of Candida Using CHROMagar in Cases with Oral Epithelial Dysplasia and Squamous Cell Carcinoma. J. Clin. Exp. Dent. 2018, 10, e657–e660. [Google Scholar] [CrossRef]
- Bansal, R.; Pallagatti, S.; Sheikh, S.; Aggarwal, A.; Gupta, D.; Singh, R. Candidal Species Identification in Malignant and Potentially Malignant Oral Lesions with Antifungal Resistance Patterns. Contemp. Clin. Dent. 2018, 9, S309–S313. [Google Scholar] [CrossRef]
- Makinen, A.; Nawaz, A.; Makitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida albicans in Oral Squamous Cell Cancer Patients. J. Oral Maxillofac. Surg. 2018, 76, 2564–2571. [Google Scholar] [CrossRef]
- Saxena, A.; Nagi, R.; Sandeep, T.; Patil, D.J.; Choudhary, R.; Kaur, A. Identification of Candida albicans and Non-albicans Candida Resistant Species in Tobacco Users and Oral Squamous Cell Carcinoma Patients: Comparison of HiCrome Agar and Automated VITEK 2 System. J. Oral Maxillofac. Pathol. JOMFP 2021, 25, 551–552. [Google Scholar] [CrossRef]
- Abidullah, M.; Bhosle, S.; Komire, B.; Sharma, P.; Swathi, K.; Karthik, L. Investigation of Candidal Species among People Who Suffer from Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. J. Pharm. Bioallied Sci. 2021, 13, S1050–S1054. [Google Scholar] [CrossRef]
- Ilhan, B.; Vural, C.; Gurhan, C.; Vural, C.; Veral, A.; Wilder-Smith, P.; Ozdemir, G.; Guneri, P. Real-Time PCR Detection of Candida Species in Biopsy Samples from Non-Smokers with Oral Dysplasia and Oral Squamous Cell Cancer: A Retrospective Archive Study. Cancers 2023, 15, 5251. [Google Scholar] [CrossRef]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible Mycological Etiology of Oral Mucosal Cancer: Catalytic Potential of Infecting Candida albicans and Other Yeasts in Production of N-Nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Rindum, J.L.; Stenderup, A.; Holmstrup, P. Identification of Candida albicans Types Related to Healthy and Pathological Oral Mucosa. J. Oral Pathol. Med. 1994, 23, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Bartie, K.L.; Potts, A.J.; Wilson, M.J.; Fardy, M.J.; Lewis, M.A. Strain Persistence of Invasive Candida albicans in Chronic Hyperplastic Candidosis That Underwent Malignant Change. Gerodontology 2001, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahim, M.H.; McManus, B.A.; Flint, S.R.; Coleman, D.C. Genotyping Candida albicans from Candida Leukoplakia and Non-Candida Leukoplakia Shows No Enrichment of Multilocus Sequence Typing Clades but Enrichment of ABC Genotype C in Candida Leukoplakia. PLoS ONE 2013, 8, e73738. [Google Scholar] [CrossRef]
- Weerasekera, M.-M.; Wijesinghe, G.-K.; Sampath, A.; Dilhari, A.; Madhumal, T.; Dilrukshi, R.; Willaddara, R.; Karunathilaka, S.; Gunasekara, C.; Fernando, N.; et al. The Genotypes and Virulence Attributes of C. albicans Isolates from Oral Leukoplakia. Med. Oral Patol. Oral Cirugia Bucal 2021, 26, e786–e794. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Cirillo, N.; Lenzo, J.C.; Catmull, D.V.; O’Brien-Simpson, N.; Reynolds, E.C.; Dashper, S.; McCullough, M. Monospecies and Polymicrobial Biofilms Differentially Regulate the Phenotype of Genotype-Specific Oral Cancer Cells. Carcinogenesis 2019, 40, 184–193. [Google Scholar] [CrossRef]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of Carcinogenic Acetaldehyde by Candida albicans from Patients with Potentially Malignant Oral Mucosal Disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Marttila, E.; Bowyer, P.; Sanglard, D.; Uittamo, J.; Kaihovaara, P.; Salaspuro, M.; Richardson, M.; Rautemaa, R. Fermentative 2-Carbon Metabolism Produces Carcinogenic Levels of Acetaldehyde in Candida albicans. Mol. ORAL Microbiol. 2013, 28, 281–291. [Google Scholar] [CrossRef]
- Rehani, S.; Rao, N.N.; Rao, A.; Carnelio, S.; Ramakrishnaiah, S.H.; Prakash, P.Y. Spectrophotometric Analysis of the Expression of Secreted Aspartyl Proteinases from Candida in Leukoplakia and Oral Squamous Cell Carcinoma. J. Oral Sci. 2011, 53, 421–425. [Google Scholar] [CrossRef][Green Version]
- Berkovits, C.; Toth, A.; Szenzenstein, J.; Deak, T.; Urban, E.; Gacser, A.; Nagy, K. Analysis of Oral Yeast Microflora in Patients with Oral Squamous Cell Carcinoma. SpringerPlus 2016, 5, 1257. [Google Scholar] [CrossRef]
- Nawaz, A.; Mäkinen, A.; Pärnänen, P.; Meurman, J. Proteolytic Activity of Non-Albicans Candida and Candida albicans in Oral Cancer Patients. New Microbiol. 2018, 41, 296–301. [Google Scholar] [PubMed]
- Castillo, G.D.V.; de Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of Virulence Factor of Candida Species in Oral Lesions and Its Association with Potentially Malignant and Malignant Lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Schneider, J.; Moragues, M.D.; Martinez-Conde, R.; Ponton, J.; Aguirre, J.M. Cross-Reactivity between Candida albicans and Oral Squamous Cell Carcinoma Revealed by Monoclonal Antibody C7. Anticancer Res. 2007, 27, 3639–3643. [Google Scholar] [PubMed]
- Hsieh, Y.-P.; Wu, Y.-H.; Cheng, S.-M.; Lin, F.-K.; Hwang, D.-Y.; Jiang, S.-S.; Chen, K.-C.; Chen, M.-Y.; Chiang, W.-F.; Liu, K.-J.; et al. Single-Cell RNA Sequencing Analysis for Oncogenic Mechanisms Underlying Oral Squamous Cell Carcinoma Carcinogenesis with Candida albicans Infection. Int. J. Mol. Sci. 2022, 23, 4833. [Google Scholar] [CrossRef] [PubMed]
- Rusanen, P.; Marttila, E.; Amatya, S.B.; Hagstrom, J.; Uittamo, J.; Reunanen, J.; Rautemaa-Richardson, R.; Salo, T. Expression of Toll-like Receptors in Oral Squamous Cell Carcinoma. PLoS ONE 2024, 19, e0300437. [Google Scholar] [CrossRef]
- Franklin, C.D.; Martin, M.V. The Effects of Candida albicans on Turpentine-Induced Hyperplasia of Hamster Cheek Pouch Epithelium. J. Med. Vet. Mycol. 1986, 24, 281–287. [Google Scholar]
- O’Grady, J.; Reade, P. Candida albicans as A Promoter of Oral Mucosal Neoplasia. Carcinogenesis 1992, 13, 783–786. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mallya, S.; Dongari-Bagtzoglou, A. A Novel Immunocompetent Murine Model for Candida albicans-Promoted Oral Epithelial Dysplasia. Med. Mycol. 2009, 47, 157–167. [Google Scholar] [CrossRef]
- Chen, X.; Luo, Q.; Ding, J.; Yang, M.; Zhang, R.; Chen, F. Zymosan Promotes Proliferation, Candida albicans Adhesion and IL-1β Production of Oral Squamous Cell Carcinoma in Vitro. Infect. Agent. Cancer 2020, 15, 51. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Jayaraman, S.; Quigley, C.; Mamileti, P.; Ghannoum, M.; Weinberg, A.; Thuener, J.; Pan, Q.; Pandiyan, P. The Role of Dectin-1 Signaling in Altering Tumor Immune Microenvironment in the Context of Aging. Front. Oncol. 2021, 11, 669066. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wu, W.; Zhang, W.; Li, L.; Liu, Q.; Yan, Z. Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio 2023, 14, e0044723. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Wu, S.; Li, L.; Cai, X.; Chen, F.; Yan, Z. Candida albicans-Myeloid Cells-T Lymphocytes Axis in the Tumor Microenvironment of Oral Tumor-Bearing Mice. Cancer Lett. 2024, 588, 216814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Zhang, W.; Wu, S.; Yan, Z. Candida albicans Induces Upregulation of Programmed Death Ligand 1 in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2022, 51, 444–453. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, I.; O’Sullivan, J. Alcohol Consumption Modulates Candida albicans-Induced Oral Carcinogenesis and Progression. J. Oral Biosci. 2023, 65, 293–304. [Google Scholar] [CrossRef]
- Nakazawa, K.; Fifita, S.; Kuyama, K. The Cytological Findings of Oral Inflammatory Lesions, Lichen Planus and Leukoplakia Coexisted with and without Candida: With Special Reference to Clinical, Histopathological, Immunohistochemical and Flow Cytometrical Analyses. Int. J. Oral-Med. Sci. 2007, 6, 81–90. [Google Scholar] [CrossRef][Green Version]
- Amaya Arbeláez, M.I.; De Paula E Silva, A.C.A.; Navegante, G.; Valente, V.; Barbugli, P.A.; Vergani, C.E. Proto-Oncogenes and Cell Cycle Gene Expression in Normal and Neoplastic Oral Epithelial Cells Stimulated With Soluble Factors From Single and Dual Biofilms of Candida albicans and Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 627043. [Google Scholar] [CrossRef]
- Marin-dett, F.; Campanella, J.; Trovatti, E.; Bertolini, M.; Vergani, C.; Barbugli, P. Extracellular Lipids of Candida albicans Biofilm Induce Lipid Droplet Formation and Decreased Response to a Topoisomerase I Inhibitor in Dysplastic and Neoplastic Oral Cells. J. Appl. ORAL Sci. 2022, 30, e20220319. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and Mycobiome Associations in Oral Tongue Cancer. Oncotarget 2017, 8, 97273–97289. [Google Scholar] [CrossRef]
- Jain, V.; Baraniya, D.; El-Hadedy, D.E.; Chen, T.; Slifker, M.; Alakwaa, F.; Cai, K.Q.; Chitrala, K.N.; Fundakowski, C.; Al-Hebshi, N.N. Integrative Metatranscriptomic Analysis Reveals Disease-Specific Microbiome–Host Interactions in Oral Squamous Cell Carcinoma. Cancer Res. Commun. 2023, 3, 807–820. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Ryan, C.A.; Stanton, C.; Patangia, D.; Ross, R.P. Altered Oral Microbiome in Sudanese Toombak Smokeless Tobacco Users Carries a Newly Emerging Risk of Squamous Cell Carcinoma Development and Progression. Sci. Rep. 2023, 13, 6645. [Google Scholar] [CrossRef]
- Heng, W.; Wang, W.; Dai, T.; Jiang, P.; Lu, Y.; Li, R.; Zhang, M.; Xie, R.; Zhou, Y.; Zhao, M.; et al. Oral Bacteriome and Mycobiome across Stages of Oral Carcinogenesis. Microbiol. Spectr. 2022, 10, e0273722. [Google Scholar] [CrossRef]
- Krogh, P. The Role of Yeasts in Oral Cancer by Means of Endogenous Nitrosation. Acta Odontol. Scand. 1990, 48, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.; Ji, N.; Li, J.; Chen, Q. The Evil Companion of OSCC: Candida albicans. Oral Dis. 2024, 30, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Theofilou, V.I.; Alfaifi, A.; Montelongo-Jauregui, D.; Jabra-Rizk, M.-A. Is Candida albicans an Opportunistic Oncogenic Pathogen? PLoS Pathog. 2022, 18, e1010413. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Kaushik, K.; de Arruda, J.A.A.; Georgakopoulou, E.; Vieira, A.T.; Silva, T.A.; Devadiga, D.; Anyanechi, C.E.; Shetty, S. Fungal Footprints in Oral Cancer: Unveiling the Oral Mycobiome. Front. Oral Health 2024, 5, 1360340. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wicaksono, S.; Ngokwe, Z.B.; McCullough, M.; Yap, T. The Role of Oral Yeasts in the Development and Progression of Oral Squamous Cell Carcinoma: A Scoping Review. J. Fungi 2025, 11, 260. https://doi.org/10.3390/jof11040260
Wicaksono S, Ngokwe ZB, McCullough M, Yap T. The Role of Oral Yeasts in the Development and Progression of Oral Squamous Cell Carcinoma: A Scoping Review. Journal of Fungi. 2025; 11(4):260. https://doi.org/10.3390/jof11040260
Chicago/Turabian StyleWicaksono, Satutya, Zilefac Brian Ngokwe, Michael McCullough, and Tami Yap. 2025. "The Role of Oral Yeasts in the Development and Progression of Oral Squamous Cell Carcinoma: A Scoping Review" Journal of Fungi 11, no. 4: 260. https://doi.org/10.3390/jof11040260
APA StyleWicaksono, S., Ngokwe, Z. B., McCullough, M., & Yap, T. (2025). The Role of Oral Yeasts in the Development and Progression of Oral Squamous Cell Carcinoma: A Scoping Review. Journal of Fungi, 11(4), 260. https://doi.org/10.3390/jof11040260





