Characterization of Virulence Factors, Cellular Stress Response, and Antifungal Susceptibility Testing of Trichosporon spp. Isolated from Northeast Brazilian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Used in the Present Study
2.2. Strain Reactivation and Preliminary Identification
2.3. DNA Extraction
2.4. PCR Assay and Sequencing of the IGS1 Region
2.5. Trichosporon spp. Molecular Identification and Genotyping
2.6. Trichosporon spp. Inoculum Standardization
2.7. Trichosporon spp. Adhesion to Human Buccal Epithelial Cells (HBECs)
2.8. Trichosporon spp. Biofilm Formation
2.9. Trichosporon spp. Cell Surface Hydrophobicity (CSH)
2.10. Trichosporon spp. Hemolytic Activity
2.11. Trichosporon spp. Phospholipase Activity
2.12. Trichosporon spp. DNAse Activity
2.13. Trichosporon spp. Cell-Wall and Plasma-Membrane Damage in the Presence of Cellular Stressors
2.14. Trichosporon spp. Antifungal Susceptibility Testing
2.15. Statistical Analysis
3. Results
3.1. Trichosporon spp. Phenotypic Screening and Molecular Identification
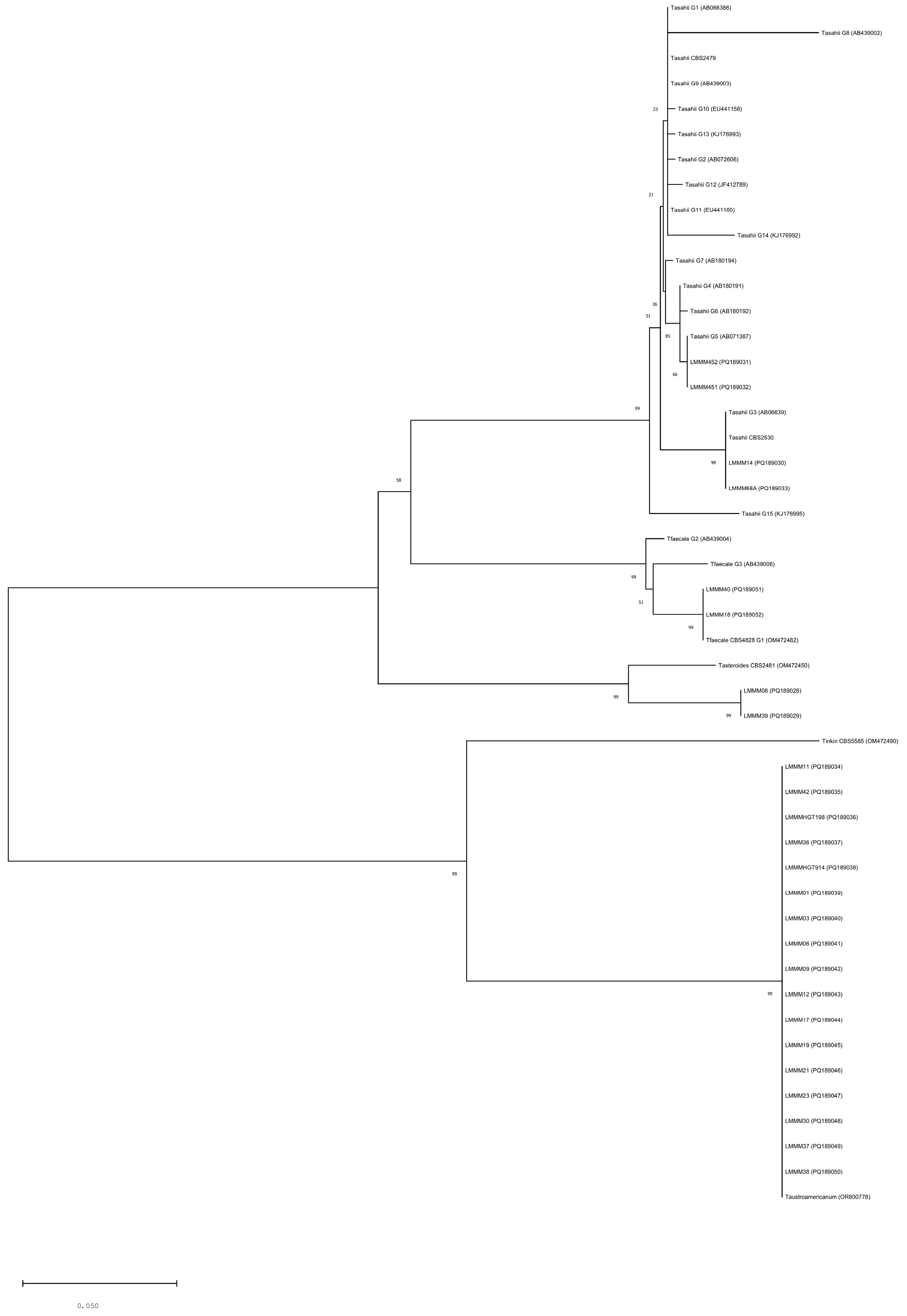
3.2. Phylogenetic Analysis of Trichosporon spp. and Genotyping of Trichosporon asahii
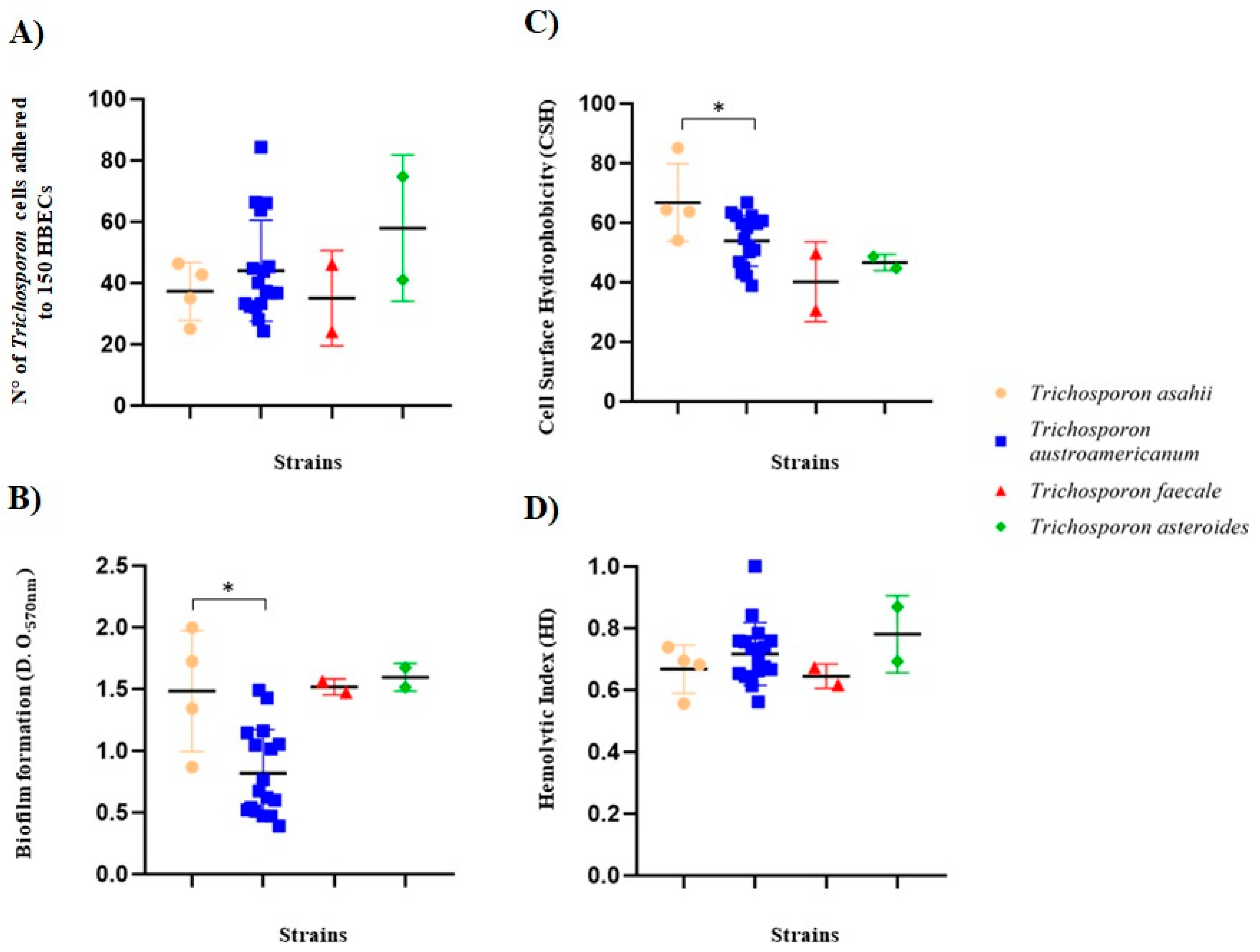
3.3. Trichosporon spp. Adhesion to Human Buccal Epithelial Cells (HBECs)
3.4. Trichosporon spp. Biofilm Formation
3.5. Trichosporon spp. Cell Surface Hydrophobicity (CSH)
3.6. Trichosporon spp. Hemolytic Activity
3.7. Trichosporon spp. Phospholipase and DNAse Activity
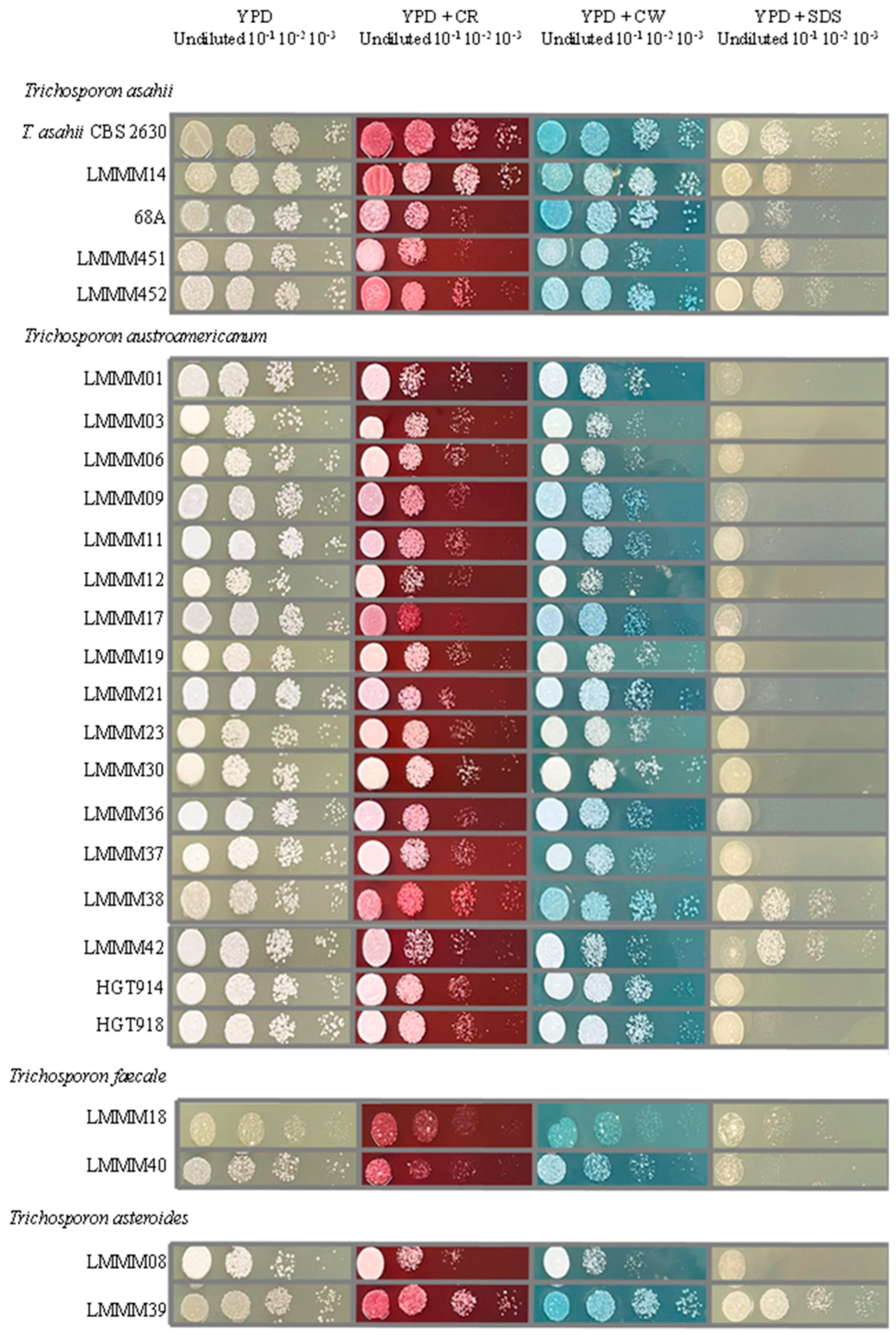
3.8. Trichosporon spp. Cell-Wall and Membrane Stressors
3.9. Trichosporon spp. Antifungal Susceptibility Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumoto, Y.; Yoshikawa, A.; Nagamachi, T.; Sugiyama, Y.; Yamada, T.; Sugita, T. A Critical Role of Calcineurin in Stress Responses, Hyphal Formation, and Virulence of the Pathogenic Fungus Trichosporon asahii. Sci. Rep. 2022, 12, 16126. [Google Scholar] [CrossRef]
- Lara, B.R.; De Camargo, B.B.; Paula, C.R.; Junior, D.P.L.; Garces, H.G.; Arnoni, M.V.; Silveira, M.; Gimenes, V.M.F.; Siqueira, L.P.M.H.; Takahashi, J.P.F.; et al. Comparing the Phenotypic, Genotypic, and Proteomic Identification of Trichosporon Species: A Globally Emerging Yeast of Medical Importance. Med. Mycol. 2021, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, I.B.; de Sousa Araújo, G.R.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; Zancopé-Oliveira, R.M.; Frases, S.; Almeida-Paes, R. Comparative Biophysical and Ultrastructural Analysis of Melanins Produced by Clinical Strains of Different Species From the Trichosporonaceae Family. Front. Microbiol. 2022, 13, 876611. [Google Scholar] [CrossRef]
- Colombo, A.L.; Padovan, A.C.B.; Chaves, G.M. Current Knowledge of Trichosporon Spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011, 24, 682–700. [Google Scholar] [PubMed]
- Mariné, M.; Brown, N.A.; Riaño-Pachón, D.M.; Goldman, G.H. On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Trichosporon Species. PLoS Pathog. 2015, 11, e1004982. [Google Scholar] [CrossRef]
- Parashar, A.; Rastogi, V.; Rudramurthy, S.M.; Ghosh, A.K.; Chander, J.; Kindo, A.J. Faster and Accurate Identification of Clinically Important Trichosporon Using MALDI TOF MS. Indian J. Med. Microbiol. 2022, 40, 359–364. [Google Scholar] [CrossRef]
- Chagas-Neto, T.C.; Chaves, G.M.; Colombo, A.L. Update on the Genus Trichosporon. Mycopathologia 2008, 166, 121–132. [Google Scholar]
- de Magalhães, A.R.; Nishikawa, M.M.; de Mondino, S.S.B.; de Macedo, H.W.; da Silva da Rocha, E.M.; de Souza Baptista, A.R. Trichosporon Isolation from Human Ungueal Infections: Is There a Pathogenic Role? An. Bras. Dermatol. 2016, 91, 173–179. [Google Scholar] [CrossRef]
- Inácio, C.P.; Rocha, A.P.S.; Barbosa, R.d.N.; Oliveira, N.T.; Silva, J.C.; de Lima-Neto, R.G.; Macêdo, D.P.C.; Neves, R.P. Experimental White Piedra: A Robust Approach to Ultrastructural Analysis, Scanning Electron Microscopy and Etiological Discoveries. Exp. Dermatol. 2016, 25, 79–81. [Google Scholar] [CrossRef]
- de Carvalho Ribeiro, C.S.; Zaitz, C.; de Souza Framil, V.M.; de Carvalho Ottoboni, T.S.; de Carvalho Tonoli, M.S.; Ribeiro, R.P. Descriptive Study of Onychomycosis in a Hospital in São Paulo. Braz. J. Microbiol. 2015, 46, 485–492. [Google Scholar] [CrossRef]
- Milan, E.P.; Silva-Rocha, W.P.; De Almeida, J.J.S.; Fernandes, T.U.G.; De Araújo Prudente, A.L.; De Azevedo, M.F.; Francisco, E.C.; De Azevedo Melo, A.S.; Colombo, A.L.; Chaves, G.M. Trichosporon inkin Meningitis in Northeast Brazil: First Case Report and Review of the Literature. BMC Infect. Dis. 2018, 18, 470. [Google Scholar] [CrossRef]
- da Silva Pontes, Z.B.V.; Ramos, A.L.; de Oliveira Lima, E.; de Fátima de Lacerda Guerra, M.; Oliveira, N.M.C.; dos Santos, J.P. Clinical and Mycological Study of Scalp White Piedra in the State of Paraíba, Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 747–750. [Google Scholar]
- Iturrieta-González, I.A.; Padovan, A.C.B.; Bizerra, F.C.; Hahn, R.C.; Colombo, A.L. Multiple Species of Trichosporon Produce Biofilms Highly Resistant to Triazoles and Amphotericin B. PLoS ONE 2014, 9, e109553. [Google Scholar] [CrossRef]
- Sun, W.; Su, J.; Xu, S.; Yan, D. Trichosporon asahii Causing Nosocomial Urinary Tract Infections in Intensive Care Unit Patients: Genotypes, Virulence Factors and Antifungal Susceptibility Testing. J. Med. Microbiol. 2012, 61, 1750–1757. [Google Scholar] [CrossRef][Green Version]
- Pfaller, M.A.; Diekema, D.J. Rare and Emerging Opportunistic Fungal Pathogens: Concern for Resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar]
- Montoya, A.M.; González, A.S.; Palma-Nicolás, J.P.; Gómez-Treviño, A.; González, J.G.; González, G.M. Genotyping, Extracellular Compounds, and Antifungal Susceptibility Testing of Trichosporon asahii Isolated from Mexican Patients. Med. Mycol. 2015, 53, 505–511. [Google Scholar] [CrossRef]
- Rastogi, V.; Honnavar, P.; Rudramurthy, S.M.; Pamidi, U.; Ghosh, A.; Chakrabarti, A. Molecular Characterisation and Antifungal Susceptibility of Clinical Trichosporon Isolates in India. Mycoses 2016, 59, 528–534. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, Q.M.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Boekhout, T.; et al. Towards an Integrated Phylogenetic Classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [PubMed]
- Wang, L.; Wang, Q.M. Molecular Phylogenetic Analysis of Ballistoconidium-Forming Yeasts in Trichosporonales (Tremellomycetes): A Proposal for Takashimella gen. nov. and Cryptotrichosporon tibetense sp. nov. PLoS ONE 2015, 10, e0132653. [Google Scholar] [CrossRef][Green Version]
- Francisco, E.C.; Desnos-Ollivier, M.; Dieleman, C.; Boekhout, T.; Santos, D.W.d.C.L.; Medina-Pestana, J.O.; Colombo, A.L.; Hagen, F. Unveiling Trichosporon austroamericanum sp. nov.: A Novel Emerging Opportunistic Basidiomycetous Yeast Species. Mycopathologia 2024, 189, 43. [Google Scholar] [CrossRef]
- Caggiano, G.; Iatta, R.; Laneve, A.; Manca, F.; Montagna, M.T. Observational Study on Candidaemia at a University Hospital in Southern Italy from 1998 to 2004. Mycoses 2008, 51, 123–128. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Frases, S.; Casadevall, A.; Fischman-Gompertz, O.; Nimrichter, L.; Rodrigues, M.L. Structural and Functional Properties of the Trichosporon asahii Glucuronoxylomannan. Fungal Genet. Biol. 2009, 46, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Nayyar, C.; Gulati, N.; Singla, N.; Rai, S.; Chandar, J. A Comprehensive Review of Trichosporon spp.: An Invasive and Emerging Fungus. Cureus 2021, 13, e17345. [Google Scholar] [CrossRef]
- Caira, M.; Trecarichi, E.M.; Tumbarello, M.; Leone, G.; Pagano, L. Uncommon Yeast Infections in Hematological Patients: From Diagnosis to Treatment. Expert Rev. Anti-Infect. Ther. 2011, 9, 1067–1075. [Google Scholar] [PubMed]
- Rodriguez-Tudela, J.L.; Diaz-Guerra, T.M.; Mellado, E.; Cano, V.; Tapia, C.; Perkins, A.; Gomez-Lopez, A.; Rodero, L.; Cuenca-Estrella, M. Susceptibility Patterns and Molecular Identification of Trichosporon Species. Antimicrob. Agents Chemother. 2005, 49, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Messias, A.; Junior, S.; Bandeira, M.A.; Miranda, R.; de Camargo, Z.P. Trichosporon Species Isolated from the Perigenital Region, Urine and Catheters of a Brazilian Population. Braz. J. Microbiol. 2010, 41, 628–634. [Google Scholar]
- Diaz, M.R.; Fell, J.W. High-Throughput Detection of Pathogenic Yeasts of the Genus Trichosporon. J. Clin. Microbiol. 2004, 42, 3696–3706. [Google Scholar] [CrossRef]
- Francisco, E.C.; de Almeida Junior, J.N.; de Queiroz Telles, F.; Aquino, V.R.; Mendes, A.V.A.; de Andrade Barberino, M.G.M.; de Tarso, P.; Guimarães, T.; Hahn, R.C.; Padovan, A.C.B.; et al. Species Distribution and Antifungal Susceptibility of 358 Trichosporon Clinical Isolates Collected in 24 Medical Centres. Clin. Microbiol. Infect. 2019, 25, 909.e1–909.e5. [Google Scholar] [CrossRef]
- Guo, L.N.; Yu, S.Y.; Hsueh, P.R.; Al-Hatmi, A.M.S.; Meis, J.F.; Hagen, F.; Xiao, M.; Wang, H.; Barresi, C.; Zhou, M.L.; et al. Invasive Infections Due to Trichosporon: Species Distribution, Genotyping, and Antifungal Susceptibilities from a Multicenter Study in China. J. Clin. Microbiol. 2019, 57, e01505-18. [Google Scholar] [CrossRef]
- Matsue, K.; Uryu, H.; Koseki, M.; Asada, N.; Takeuchi, M. Breakthrough Trichosporonosis in Patients with Hematologic Malignancies Receiving Micafungin. Clin. Infect. Dis. 2006, 42, 753–757. [Google Scholar]
- Fischman, O.; Bezerra, F.C.; Francisco, E.C.; da Silva, F.C.; Nishikaku, A.S.; Cavalcanti, S.D.B.; de Azevedo Melo, A.S.; Bentubo, H.D.L.; Petri, V. Trichosporon inkin: An Uncommon Agent of Scalp White Piedra. Report of Four Cases in Brazilian Children. Mycopathologia 2014, 178, 85–89. [Google Scholar] [CrossRef]
- Silva-Rocha, W.P.; de Azevedo, M.F.; Chaves, G.M. Épidémiologie et Distribution Des Espèces Fongiques Des Mycoses Superficielles Dans Le Nord-Est Du Brésil. J. Mycol. Med. 2017, 27, 57–64. [Google Scholar] [CrossRef]
- Sugita, T.; Nakajima, M.; Ikeda, R.; Matsushima, T.; Shinoda, T. Sequence Analysis of the Ribosomal DNA Intergenic Spacer 1 Regions of Trichosporon Species. J. Clin. Microbiol. 2002, 40, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D. Viewing and Editing Assembled Sequences Using Consed. Curr. Protoc. Bioinform. 2003, 2, 11.2.1–11.2.43. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Base-Calling of Automated Sequencer Traces Using Phred. II. Error Probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Francisco, E.C.; de Almeida Junior, J.N.; Queiroz-Telles, F.; Aquino, V.R.; Mendes, A.V.A.; de Oliveira Silva, M.; Castro, P.d.T.O.e.; Guimarães, T.; Ponzio, V.; Hahn, R.C.; et al. Correlation of Trichosporon asahii Genotypes with Anatomical Sites and Antifungal Susceptibility Profiles: Data Analyses from 284 Isolates Collected in the Last 22 Years across 24 Medical Centers. Antimicrob. Agents Chemother. 2021, 65, e01104-20. [Google Scholar]
- Chaves, G.M.; Bates, S.; MacCallum, D.M.; Odds, F.C. Candida albicans GRX2, Encoding a Putative Glutaredoxin, Is Required for Virulence in a Murine Model. Genet. Mol. Res. 2007, 6, 1051–1063. [Google Scholar] [PubMed]
- Bates, S.; MacCallum, D.M.; Bertram, G.; Munro, C.A.; Hughes, H.B.; Buurman, E.T.; Brown, A.J.P.; Odds, F.C.; Gow, N.A.R. Candida albicans Pmr1p, a Secretory Pathway P-Type Ca2+/Mn2+-ATPase, Is Required for Glycosylation and Virulence. J. Biol. Chem. 2005, 280, 23408–23415. [Google Scholar] [CrossRef]
- Jin, Y.; Yip, H.K.; Samaranayake, Y.H.; Yau, J.Y.; Samaranayake, L.P. Biofilm-Forming Ability of Candida albicans Is Unlikely to Contribute to High Levels of Oral Yeast Carriage in Cases of Human Immunodeficiency Virus Infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Muadcheingka, T.; Tantivitayakul, P. Distribution of Candida albicans and Non-Albicans Candida Species in Oral Candidiasis Patients: Correlation between Cell Surface Hydrophobicity and Biofilm Forming Activities. Arch. Oral Biol. 2015, 60, 894–901. [Google Scholar] [CrossRef]
- Luo, G.; Samaranayake, L.P.; Yau, J.Y.Y. Candida Species Exhibit Differential in Vitro Hemolytic Activities. J. Clin. Microbiol. 2001, 39, 2971–2974. [Google Scholar] [CrossRef]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate Method for Detection of Phospholipase Activity in Candida albicans. Med. Mycol. 1982, 20, 7–14. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Parra-Giraldo, C.M.; Hernáez, M.L.; Reales-Calderon, J.A.; Solis, N.V.; Filler, S.G.; Monteoliva, L.; Gil, C. Candida albicans Cell Shaving Uncovers New Proteins Involved in Cell Wall Integrity, Yeast to Hypha Transition, Stress Response and Host-Pathogen Interaction. J. Proteom. 2015, 127, 340–351. [Google Scholar] [CrossRef]
- CLSI Document M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- CLSI Document M27-S4; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32.
- Takashima, M.; Manabe, R.-i.; Nishimura, Y.; Endoh, R.; Ohkuma, M.; Sriswasdi, S.; Sugita, T.; Iwasaki, W. Recognition and Delineation of Yeast Genera Based on Genomic Data: Lessons from Trichosporonales. Fungal Genet. Biol. 2019, 130, 31–42. [Google Scholar] [CrossRef]
- Arastehfar, A.; de Almeida Júnior, J.N.; Perlin, D.S.; Ilkit, M.; Boekhout, T.; Colombo, A.L. Multidrug-Resistant Trichosporon Species: Underestimated Fungal Pathogens Posing Imminent Threats in Clinical Settings. Crit. Rev. Microbiol. 2021, 47, 679–698. [Google Scholar]
- Takashima, M.; Sugita, T. Draft Genome Analysis of Trichosporonales Species That Contribute to the Taxonomy of the Genus Trichosporon and Related Taxa. Med. Mycol. J. 2019, 60, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Robles-Tenorio, A.; Lepe-Moreno, K.Y.; Mayorga-Rodríguez, J. White Piedra, a Rare Superficial Mycosis: An Update. Curr. Fungal Infect. Rep. 2020, 14, 197–202. [Google Scholar] [CrossRef]
- Martínez-Herrera, E.; Duarte-Escalante, E.; del Rocío Reyes-Montes, M.; Arenas, R.; Acosta-Altamirano, G.; Moreno-Coutiño, G.; Vite-Garín, T.M.; Meza-Robles, A.; Frías-De-León, M.G. Molecular Identification of Yeasts from the Order Trichosporonales Causing Superficial Infections. Rev. Iberoam. Micol. 2021, 38, 119–124. [Google Scholar] [CrossRef]
- Guerrero-Ponce, A.E.; Araiza, J.; Tirado-Sánchez, A.; Bonifaz, A. Review Article White Piedra: Review of 131 Cases. Mycoses 2024, 67, e13668. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Gomez-Lopez, A.; Alastruey-Izquierdo, A.; Mellado, E.; Bernal-Martinez, L.; Cuenca-Estrella, M. Genotype Distribution of Clinical Isolates of Trichosporon asahii Based on Sequencing of Intergenic Spacer 1. Diagn. Microbiol. Infect. Dis. 2007, 58, 435–440. [Google Scholar] [CrossRef] [PubMed]
- do Espírito Santo, E.P.T.; Monteiro, R.C.; da Costa, A.R.F.; Marques-da-Silva, S.H. Molecular Identification, Genotyping, Phenotyping, and Antifungal Susceptibilities of Medically Important Trichosporon, Apiotrichum, and Cutaneotrichosporon Species. Mycopathologia 2020, 185, 307–317. [Google Scholar] [CrossRef]
- Maza, P.K.; Bonfim-Melo, A.; Padovan, A.C.B.; Mortara, R.A.; Orikaza, C.M.; Ramos, L.M.D.; Moura, T.R.; Soriani, F.M.; Almeida, R.S.; Suzuki, E.; et al. Candida albicans: The Ability to Invade Epithelial Cells and Survive under Oxidative Stress Is Unlinked to Hyphal Length. Front. Microbiol. 2017, 8, 1235. [Google Scholar] [CrossRef]
- Tumbarello, M.; Fiori, B.; Trecarichi, E.M.; Posteraro, P.; Losito, A.R.; de Luca, A.; Sanguinetti, M.; Fadda, G.; Cauda, R.; Posteraro, B. Risk Factors and Outcomes of Candidemia Caused by Biofilm-Forming Isolates in a Tertiary Care Hospital. PLoS ONE 2012, 7, e33705. [Google Scholar] [CrossRef]
- Yang, S.; Liao, Y.; Cong, L.; Lu, X.; Yang, R. In Vitro Interactions between Non-Steroidal Anti-Inflammatory Drugs and Antifungal Agents against Planktonic and Biofilm Forms of Trichosporon asahii. PLoS ONE 2016, 11, e0157047. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Pompilio, A.; Picciani, C.; Iezzi, M.; D’Antonio, D.; Piccolomini, R. Biofilm Formation by the Emerging Fungal Pathogen Trichosporon asahii: Development, Architecture, and Antifungal Resistance. Antimicrob. Agents Chemother. 2006, 50, 3269–3276. [Google Scholar] [CrossRef]
- Cordeiro, R.d.A.; Aguiar, A.L.R.; da Silva, B.N.; Pereira, L.M.G.; Portela, F.V.M.; de Camargo, Z.P.; de Lima-Neto, R.G.; Castelo-Branco, D.d.S.C.M.; Rocha, M.F.G.; Sidrim, J.J.C. Trichosporon asahii and Trichosporon inkin Biofilms Produce Antifungal-Tolerant Persister Cells. Front. Cell. Infect. Microbiol. 2021, 11, 645812. [Google Scholar] [CrossRef]
- Liao, Y.; Zhao, H.; Lu, X.; Yang, S.; Zhou, J.; Yang, R. Efficacy of Ethanol against Trichosporon asahii Biofilm in Vitro. Med. Mycol. 2015, 53, 396–404. [Google Scholar] [CrossRef]
- Kurakado, S.; Miyashita, T.; Chiba, R.; Sato, C.; Matsumoto, Y.; Sugita, T. Role of Arthroconidia in Biofilm Formation by Trichosporon asahii. Mycoses 2021, 64, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, H.; Liu, Z.; Wang, Y.; Zhong, Z.; Peng, G.; Gu, Y. Trichosporon asahii PLA2 Gene Enhances Drug Resistance to Azoles by Improving Drug Efflux and Biofilm Formation. Int. J. Mol. Sci. 2023, 24, 8855. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.R.; de Camargo, B.B.; Paula, C.R.; Monari, G.P.d.M.; Garces, H.G.; Arnoni, M.V.; Silveira, M.; Gimenes, V.M.F.; Leite Junior, D.P.; Bonfietti, L.X.; et al. Aspects Related to Biofilm Production and Antifungal Susceptibility of Clinically Relevant Yeasts of the Genus Trichosporon. Med. Mycol. 2023, 61, myad022. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Kawauchi, M.; Abe, K. Cell Wall Integrity and Its Industrial Applications in Filamentous Fungi. J. Fungi 2022, 8, 435. [Google Scholar] [CrossRef]
- Hazen, K.C.; Lay, J.-G.; Hazen, B.W.; Fu, R.C.; Seetha, A. Partial Biochemical Characterization of Cell Surface Hydrophobicity and Hydrophilicity of Candida albicans. Infect. Immun. 1990, 58, 3469–3476. [Google Scholar]
- Dabiri, S.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Comparative Analysis of Proteinase, Phospholipase, Hydrophobicity and Biofilm Forming Ability in Candida Species Isolated from Clinical Specimens. J. Mycol. Med. 2018, 28, 437–442. [Google Scholar] [CrossRef]
- Galán-Ladero, M.A.; Blanco-Blanco, M.T.; Hurtado, C.; Pérez-Giraldo, C.; Blanco, M.T.; Gómez-García, A.C. Determination of Biofilm Production by Candida Tropicalis Isolated from Hospitalized Patients and Its Relation to Cellular Surface Hydrophobicity, Plastic Adherence and Filamentation Ability. Yeast 2013, 30, 331–339. [Google Scholar] [CrossRef]
- de Andrade, I.B.; Figueiredo-Carvalho, M.H.G.; Chaves, A.L.d.S.; Coelho, R.A.; Almeida-Silva, F.; Zancopé-Oliveira, R.M.; Frases, S.; Brito-Santos, F.; Almeida-Paes, R. Metabolic and Phenotypic Plasticity May Contribute for the Higher Virulence of Trichosporon asahii over Other Trichosporonaceae Members. Mycoses 2023, 66, 430–440. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hirata, C.; Takei, M.; Tagami, N.; Murasawa, H.; Ikeda, R. Cell Surface Hydrophobicity and Colony Morphology of Trichosporon asahii Clinical Isolates. Yeast 2017, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.F.J.; Klis, F.M. Identification of Fungal Cell Wall Mutants Using Susceptibility Assays Based on Calcofluor White and Congo Red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Wood, P.J. Specificity in the interaction of direct dys with polysaccharides. Carbohydr. Res. 1980, 85, 271–287. [Google Scholar]
- Liu, Z.; Raj, S.; van Rhijn, N.; Fraczek, M.; Michel, J.P.; Sismeiro, O.; Legendre, R.; Varet, H.; Fontaine, T.; Bromley, M.; et al. Functional Genomic and Biochemical Analysis Reveals Pleiotropic Effect of Congo Red on Aspergillus fumigatus. MBio 2021, 12, e00863-21. [Google Scholar] [CrossRef]
- Ellepola, A.N.B.; Samaranayake, L.P.; Khan, Z.U. Extracellular Phospholipase Production of Oral Candida albicans Isolates from Smokers, Diabetics, Asthmatics, Denture Wearers and Healthy Individuals Following Brief Exposure to Polyene, Echinocandin and Azole Antimycotics. Braz. J. Microbiol. 2016, 47, 911–916. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus Aureus: DNase and Mannitol Salt Agar Improve the Efficiency of the Tube Coagulase Test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 23. [Google Scholar] [CrossRef]
- Sánchez, M.; Colom, F. Extracellular DNase Activity of Cryptococcus neoformans and Cryptococcus gattii. Rev. Iberoam. Micol. 2010, 27, 10–13. [Google Scholar] [CrossRef]
- Bentubo, H.D.L.; Gompertz, O.F. Effects of Temperature and Incubation Time on the in vitro Expression of Proteases, Phospholipases, Lipases and DNases by Different Species of Trichosporon. Springerplus 2014, 3, 377. [Google Scholar] [CrossRef]
- Sprute, R.; Bethe, U.; Chen, S.C.A.; Cornely, O.A. EQUAL Trichosporon Score 2022: An ECMM Score to Measure QUALity of the Clinical Management of Invasive Trichosporon Infections. J. Antimicrob. Chemother. 2022, 77, 1779–1784. [Google Scholar] [CrossRef]
- Gaurav, V.; Grover, C.; Das, S.; Rai, G. White Piedra: An Uncommon Superficial Fungal Infection of Hair. Ski. Appendage Disord. 2022, 8, 34–37. [Google Scholar] [CrossRef]
| Strain Name | Source | Nº of Trichosporon Cells Adhered to 150 HBECs | Biofilm Formation (D.O.570nm) | Cell Surface Hydrophobicity (CSH) | Hemolytic Index (HI) | Phospholipase Zone (Pz) | DNAse |
|---|---|---|---|---|---|---|---|
| Trichosporon asahii | |||||||
| CBS2630 | 34.3 ± 1.7 | 0.40 ± 0.01 | 54.5 ± 0.1 | 0.74 ± 0.01 | 1 ± 0 | ||
| LMMM14 | White Piedra | 35 ± 0.82 | 0.87 ± 0.01 | 54.05 ± 0.65 | 0.68 ± 0.02 | 1 ± 0 | (++) |
| 68A | Urine | 25 ± 2 | 1.34 ± 0.04 | 64.3 ± 0.4 | 0.74 ± 0.01 | 1 ± 0 | (++) |
| LMMM451 | Blood | 42.7 ± 2.08 | 1.99 ± 0.08 | 85 ± 0 | 0.69 ± 0.02 | 1 ± 0 | (++) |
| LMMM452 | Blood | 46.3 ± 3.61 | 1.72 ± 0.06 | 63.6 ± 0 | 0.56 ± 0.01 | 1 ± 0 | (+++) |
| Trichosporon austroamericanum | |||||||
| LMMM01 | White Piedra | 44.7 ± 0.47 | 1.16 ± 0.05 | 46.75 ± 4.65 | 0.84 ± 0.01 | 1 ± 0 | Negative |
| LMMM03 | White Piedra | 28 ± 0.82 | 1.05 ± 0.08 | 43.15 ± 2.15 | 0.74 ± 0.01 | 1 ± 0 | (+) |
| LMMM06 | White Piedra | 36.7 ± 0.47 | 1.14 ± 0.06 | 60.65 ± 2.15 | 0.66 ± 0.01 | 1 ± 0 | (++) |
| LMMM09 | White Piedra | 63.7 ± 0.47 | 1.02 ± 0.06 | 54.5 ± 0 | 1 ± 0 | 1 ± 0 | (++) |
| LMMM11 | White Piedra | 66 ± 3.56 | 0.51 ± 0.01 | 62.3 ± 0.1 | 0.76 ± 0.01 | 1 ± 0 | (++) |
| LMMM12 | White Piedra | 43.7 ± 0.94 | 0.39 ± 0.01 | 66.7 ± 0.1 | 0.65 ± 0.02 | 1 ± 0 | (++) |
| LMMM17 | White Piedra | 45.3 ± 1.25 | 0.62 ± 0.02 | 38.8 ± 0.4 | 0.63 ± 0.04 | 0.91 ± 0.08 | (+) |
| LMMM19 | White Piedra | 33.3 ± 1.25 | 0.54 ± 0.03 | 42.05 ± 2.95 | 0.76 ± 0.04 | 1 ± 0 | (+++) |
| LMMM21 | White Piedra | 24.3 ± 0.47 | 1.49 ± 0.01 | 52.1 ± 0.3 | 0.76 ± 0.09 | 1 ± 0 | (++) |
| LMMM23 | White Piedra | 32.3 ± 1.25 | 0.47 ± 0.03 | 59.6 ± 2.2 | 0.61 ± 0.02 | 1 ± 0 | (++) |
| LMMM30 | White Piedra | 84.3 ± 1.25 | 0.52 ± 0.02 | 50.75 ± 3.55 | 0.73 ± 0.02 | 1 ± 0 | (++) |
| LMMM36 | White Piedra | 33.3 ± 1.7 | 1.43 ± 0.02 | 63.35 ± 3.85 | 0.67 ± 0.04 | 1 ± 0 | (++) |
| LMMM37 | White Piedra | 40 ± 1.63 | 0.60 ± 0.01 | 62.25 ± 2.15 | 0.78 ± 0.03 | 1 ± 0 | (++) |
| LMMM38 | White Piedra | 32 ± 1.63 | 0.67 ± 0.02 | 59.6 ± 0.2 | 0.64 ± 0.01 | 1 ± 0 | Negative |
| LMMM42 | White Piedra | 66.3 ± 2.49 | 0.47 ± 0.03 | 50.15 ± 2.15 | 0.70 ± 0.01 | 1 ± 0 | (++) |
| HGT914 | Liquor | 37.3 ± 1.53 | 1.05 ± 0.01 | 58.25 ± 0.15 | 0.56 ± 0.01 | 1 ± 0 | Negative |
| HGT198 | Liquor | 36.7 ± 1.53 | 0.77 ± 0.05 | 44.9 ± 0.5 | 0.68 ± 0.01 | 1 ± 0 | Negative |
| Trichosporon faecale | |||||||
| LMMM18 | White Piedra | 24 ± 0.82 | 1.56 ± 0.03 | 30.65 ± 8.45 | 0.67 ± 0.05 | 0.86 ± 0 | (++) |
| LMMM40 | White Piedra | 46 ± 0.82 | 1.47 ± 0.03 | 49.6 ± 4 | 0.62 ± 0.01 | 1 ± 0 | (+++) |
| Trichosporon asteroides | |||||||
| LMMM08 | White Piedra | 41 ± 0.82 | 1.51 ± 0.05 | 48.6 ± 3.2 | 0.69 ± 0.01 | 1 ± 0 | (+) |
| LMMM39 | White Piedra | 74.7 ± 1.7 | 1.67 ± 0.09 | 44.7 ± 0.1 | 0.87 ± 0.01 | 1 ± 0 | (+) |
| Antifungal Drug | Species | MIC 50 | MIC 90 | MIC 97.5 | MIC Geometric Mean (μg/mL) | Nº of Isolates Tested | Nº of Isolates with an MIC (μg/mL) of | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0313 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | >16 | |||||||
| Fluconazole | Trichosporon asahii | 1 | 2 | 2 | 1.41 | 4 | 2 | 2 | ||||||||
| Trichosporon austroamericanum | 2 | 2 | 2 | 1.39 | 17 | 1 | 7 | 9 | ||||||||
| Trichosporon faecale | 4 | 8 | 8 | 5.66 | 2 | 1 | 1 | |||||||||
| Trichosporon asteroides | 0.5 | 2 | 2 | 1 | 2 | 1 | 1 | |||||||||
| Ketoconazole | Trichosporon asahii | 0.0313 | 0.25 | 0.25 | 0.06 | 4 | 2 | 1 | 1 | |||||||
| Trichosporon austroamericanum | 0.0625 | 0.125 | 0.125 | 0.05 | 17 | 7 | 7 | 3 | ||||||||
| Trichosporon faecale | 0.125 | 0.5 | 0.5 | 0.25 | 2 | 1 | 1 | |||||||||
| Trichosporon asteroides | 0.0313 | 0.0313 | 0.0313 | 0.03 | 2 | 2 | ||||||||||
| Itraconazole | Trichosporon asahii | 0.125 | 0.125 | 0.125 | 0.11 | 4 | 1 | 3 | ||||||||
| Trichosporon austroamericanum | 0.0625 | 0.25 | 0.25 | 0.08 | 17 | 3 | 7 | 6 | 1 | |||||||
| Trichosporon faecale | 0.25 | 1 | 1 | 0.5 | 2 | 1 | 1 | |||||||||
| Trichosporon asteroides | 0.0313 | 0.125 | 0.125 | 0.06 | 2 | 1 | 1 | |||||||||
| Amphotericin B | Trichosporon asahii | 0.125 | >16 | >16 | 0.25 | 4 | 2 | 1 | 1 | |||||||
| Trichosporon austroamericanum | 0.5 | 2 | 2 | 2 | 17 | 1 | 2 | 8 | 5 | 1 | ||||||
| Trichosporon faecale | 0.5 | 2 | 2 | 1 | 2 | 1 | 1 | |||||||||
| Trichosporon asteroides | 0.25 | 1 | 1 | 0.5 | 2 | 1 | 1 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Jimenez, M.G.; de Azevedo, M.F.; Francisco, E.C.; de Andrade Oliveira Boczar, A.M.; Padovan, A.C.B.; Milan, E.P.; da Silva Rocha, W.P.; Chaves, G.M. Characterization of Virulence Factors, Cellular Stress Response, and Antifungal Susceptibility Testing of Trichosporon spp. Isolated from Northeast Brazilian Patients. J. Fungi 2025, 11, 255. https://doi.org/10.3390/jof11040255
de Souza Jimenez MG, de Azevedo MF, Francisco EC, de Andrade Oliveira Boczar AM, Padovan ACB, Milan EP, da Silva Rocha WP, Chaves GM. Characterization of Virulence Factors, Cellular Stress Response, and Antifungal Susceptibility Testing of Trichosporon spp. Isolated from Northeast Brazilian Patients. Journal of Fungi. 2025; 11(4):255. https://doi.org/10.3390/jof11040255
Chicago/Turabian Stylede Souza Jimenez, Márcia Gabriele, Matheus Firmino de Azevedo, Elaine Cristina Francisco, Ana Maria de Andrade Oliveira Boczar, Ana Carolina Barbosa Padovan, Eveline Pipolo Milan, Walicyranison Plinio da Silva Rocha, and Guilherme Maranhão Chaves. 2025. "Characterization of Virulence Factors, Cellular Stress Response, and Antifungal Susceptibility Testing of Trichosporon spp. Isolated from Northeast Brazilian Patients" Journal of Fungi 11, no. 4: 255. https://doi.org/10.3390/jof11040255
APA Stylede Souza Jimenez, M. G., de Azevedo, M. F., Francisco, E. C., de Andrade Oliveira Boczar, A. M., Padovan, A. C. B., Milan, E. P., da Silva Rocha, W. P., & Chaves, G. M. (2025). Characterization of Virulence Factors, Cellular Stress Response, and Antifungal Susceptibility Testing of Trichosporon spp. Isolated from Northeast Brazilian Patients. Journal of Fungi, 11(4), 255. https://doi.org/10.3390/jof11040255






