First Report of Trichophyton indotineae Infection in Hungary
Abstract
1. Introduction
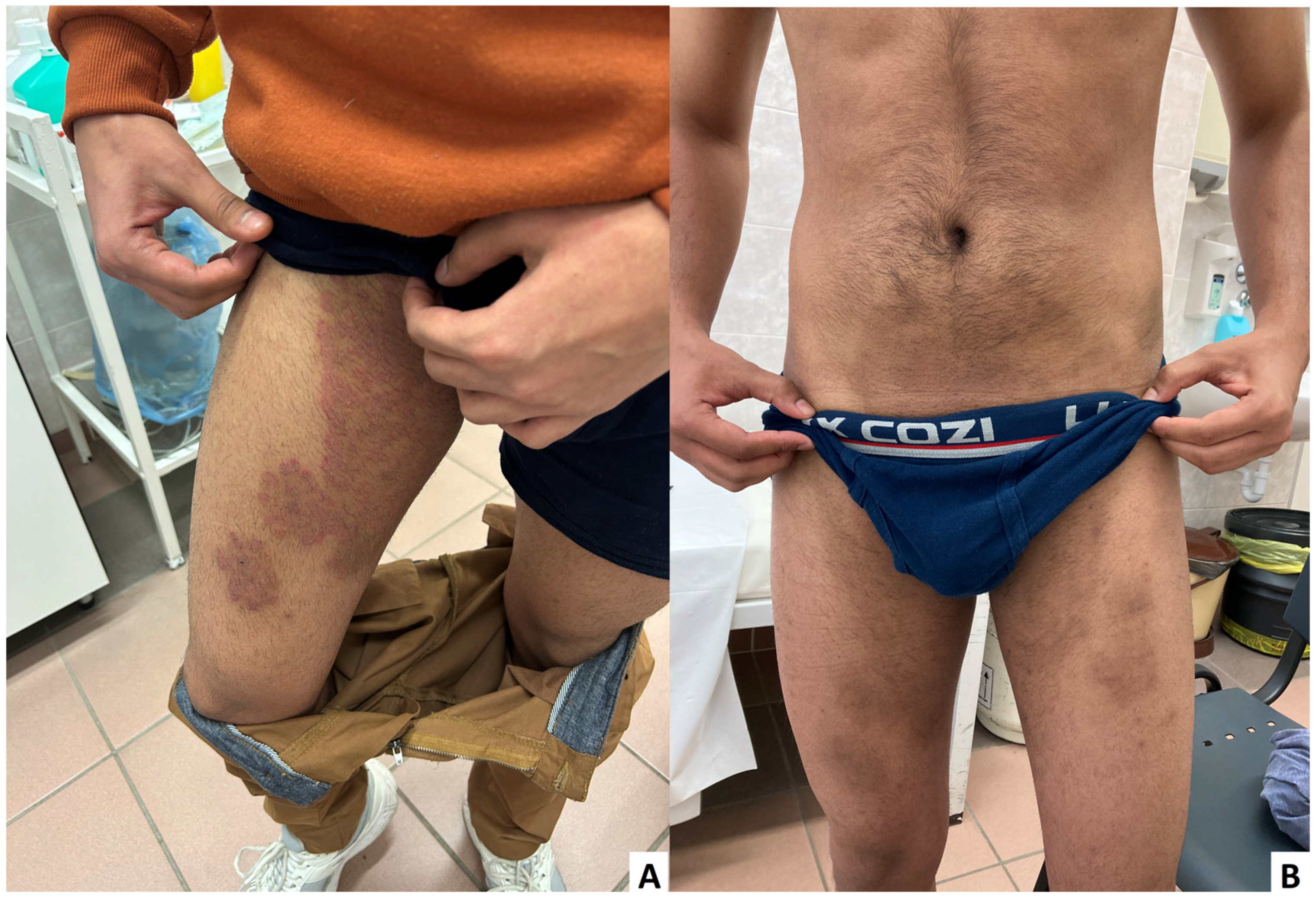
2. Case Presentation
3. Materials and Methods
3.1. Isolation and Culturing
3.2. Identification by MALDI-TOF MS
3.3. Whole-Genome Sequencing (WGS)
3.4. Whole-Genome Sequencing Data Analysis
3.5. Susceptibility Testing
4. Results
4.1. MALDI-TOF MS
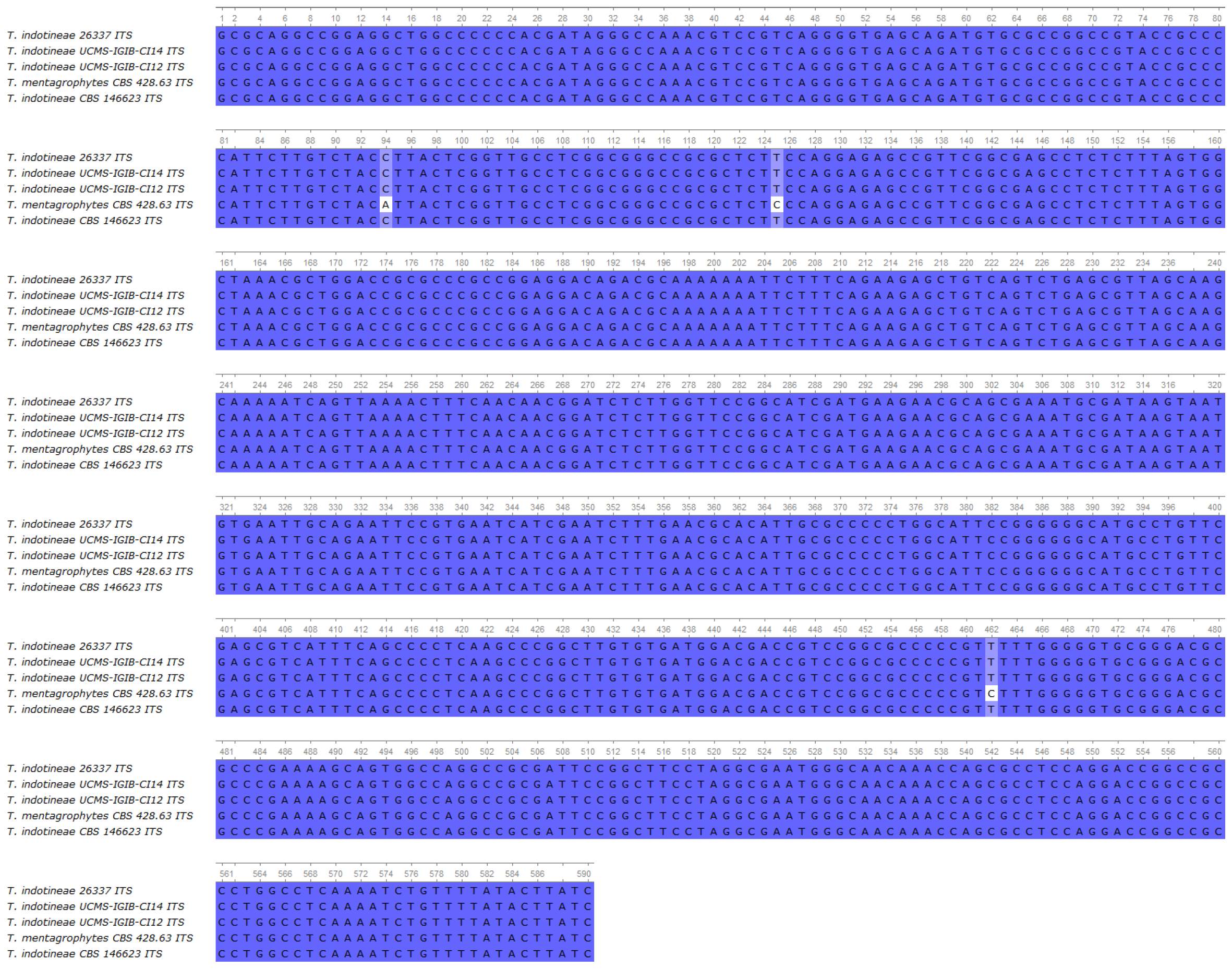
4.2. Whole-Genome Sequencing
4.3. Susceptibility Testing Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A New Highly Terbinafine-Resistant Anthropophilic Dermatophyte Species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Jabet, A.; Normand, A.C.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae, from epidemiology to therapeutic. J. Mycol. Med. 2023, 33, 101383. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Smith, D.J.; Gold, J.A.W. Trichophyton indotineae and other terbinafine-resistant dermatophytes in North America. J. Clin. Microbiol. 2023, 61, e0090323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Paepe, R.; Normand, A.C.; Uhrlaß, S.; Nenoff, P.; Piarroux, R.; Packeu, A. Resistance Profile, Terbinafine Resistance Screening and MALDI-TOF MS Identification of the Emerging Pathogen Trichophyton indotineae. Mycopathologia 2024, 189, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Cronin, K.; Ruscica, S.; Patel, S.N.; Kus, J.V. Emergence of terbinafine-resistant Trichophyton indotineae in Ontario, Canada, 2014–2023. J. Clin. Microbiol. 2025, 63, e01535-24. [Google Scholar]
- Bhuiyan, M.S.I.; Verma, S.B.; Illigner, G.M.; Uhrlaß, S.; Klonowski, E.; Burmester, A.; Noor, T.; Nenoff, P. Trichophyton mentagrophytes ITS Genotype VIII/Trichophyton indotineae Infection and Antifungal Resistance in Bangladesh. J. Fungi 2024, 10, 768. [Google Scholar] [CrossRef]
- Crotti, S.; Cruciani, D.; Spina, S.; Piscioneri, V.; Natalini, Y.; Pezzotti, G.; Sabbatucci, M.; Papini, M. A Terbinafine Sensitive Trichophyton indotineae Strain in Italy: The First Clinical Case of tinea corporis and onychomycosis. J. Fungi 2023, 9, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jabet, A.; Brun, S.; Normand, A.C.; Imbert, S.; Akhoundi, M.; Dannaoui, E.; Audiffred, L.; Chasset, F.; Izri, A.; Laroche, L.; et al. Extensive Dermatophytosis Caused by Terbinafine-Resistant Trichophyton indotineae, France. Emerg. Infect. Dis. 2022, 28, 229–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caplan, A.S.; Chaturvedi, S.; Zhu, Y.; Todd, G.C.; Yin, L.; Lopez, A.; Travis, L.; Smith, D.J.; Chiller, T.; Lockhart, S.R.; et al. Notes from the Field: First Reported, U.S. Cases of Tinea Caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 536–537. [Google Scholar] [CrossRef]
- Abdolrasouli, A.; Barton, R.C.; Borman, A.M. Spread of Antifungal-Resistant Trichophyton indotineae, United Kingdom, 2017–2024. Emerg. Infect. Dis. 2025, 31, 192–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brasch, J.; Gräser, Y.; Beck-Jendroscheck, V.; Voss, K.; Torz, K.; Walther, G.; Schwarz, T. “Indian” strains of Trichophyton mentagrophytes with reduced itraconazole susceptibility in Germany. J. Dtsch. Dermatol. Ges. 2021, 19, 1723–1727. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Lisboa, C. A Systematic Review on the Emergence of Terbinafine-Resistant Trichophyton indotineae in Europe: Time to Act? J. Eur. Acad. Dermatol. Venereol. 2025, 39, 364–376. [Google Scholar] [CrossRef]
- Chowdhary, A.; Singh, A.; Kaur, A.; Khurana, A. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: A new challenge in the management of dermatophytosis. PLoS Pathog. 2022, 18, e1010795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, C.; Ahmed, S.A.; Deng, S.; Zhang, L.; Zoll, J.; Al-Hatmi, A.M.; Meis, J.F.; Thakur, R.; Kang, Y.; De Hoog, G.S. Detection of emerging genotypes in Trichophyton mentagrophytes species complex causing dermatophytosis in India: A molecular study. Front. Microbiol. 2022, 13, 960190. [Google Scholar] [CrossRef]
- Normand, A.C.; Moreno-Sabater, A.; Jabet, A.; Hamane, S.; Cremer, G.; Foulet, F.; Blaize, M.; Dellière, S.; Bonnal, C.; Imbert, S.; et al. MALDI-TOF Mass Spectrometry Online Identification of Trichophyton indotineae Using the MSI-2 Application. J. Fungi 2022, 8, 1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lau, A.F.; Walchak, R.C.; Miller, H.B.; Slechta, E.S.; Kamboj, K.; Riebe, K.; Robertson, A.E.; Gilbreath, J.J.; Mitchell, K.F.; Wallace, M.A.; et al. Multicenter Study Demonstrates Standardization Requirements for Mold Identification by MALDI-TOF MS. Front. Microbiol. 2019, 10, 2098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Normand, A.C.; Blaize, M.; Imbert, S.; Packeu, A.; Becker, P.; Fekkar, A.; Stubbe, D.; Piarroux, R. Identification of Molds with Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry: Performance of the Newly Developed MSI-2 Application in Comparison with the Bruker Filamentous Fungi Database and MSI-1. J. Clin. Microbiol. 2021, 59, e0129921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, P.; Das, S.; Tigga, R.; Pandey, R.; Bhattacharya, S.N.; Taneja, B. Whole genome sequences of two Trichophyton indotineae clinical isolates from India emerging as threats during therapeutic treatment of dermatophytosis. 3 Biotech 2021, 11, 402, Erratum in 3 Biotech 2021, 11, 482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, X.; Tang, C.; Singh, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Chowdhary, A.; Nenoff, P.; Gräser, Y.; Hainsworth, S.; Zhan, P.; et al. Antifungal Susceptibility and Mutations in the Squalene Epoxidase Gene in Dermatophytes of the Trichophyton mentagrophytes Species Complex. Antimicrob. Agents Chemother. 2021, 65, e0005621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berstecher, N.; Burmester, A.; Gregersen, D.M.; Tittelbach, J.; Wiegand, C. Trichophyton indotineae Erg1Ala448Thr Strain Expressed Constitutively High Levels of Sterol 14-α Demethylase Erg11B mRNA, While Transporter MDR3 and Erg11A mRNA Expression Was Induced After Addition of Short Chain Azoles. J. Fungi 2024, 10, 731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Infect. 2021, 27, 55–60. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.4, E.Def 9.4 and E.Def 11.0 Procedures. Version 5.0. 2024. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/BP_ECOFF_v5.0.pdf (accessed on 11 December 2024).
- The European Committee on Antimicrobial Susceptibility Testing Routine Extended Internal Quality Control for MIC Determination Agar Dilution for Yeasts Moulds Dermatophytes as Recommended by, E.U.C.A.S.T. Version 7.0. 2023. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/QC/EUCAST_AFST_QC_v_7.0.pdf (accessed on 11 June 2024).
- Kong, X.; Song, G.; Mei, H.; Zheng, H.; Tang, C.; de Hoog, S.; Li, X.; She, X.; Liu, W.; Liang, G. The Domestic Isolation of Terbinafine- and Itraconazole-Resistant Trichophyton indotineae in Chinese Mainland. Mycopathologia 2023, 188, 383–393, Erratum in Mycopathologia 2023, 188, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae, an Emerging Drug-Resistant Dermatophyte: A Review of the Treatment Options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khurana, A.; Sharath, S.; Sardana, K.; Chowdhary, A. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J. Am. Acad. Dermatol. 2024, 91, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.T.; Hare, R.K.; Jørgensen, K.M.; Saunte, D.M.L.; Thomsen, P.K.; Arendrup, M.C. Increasing Terbinafine Resistance in Danish Trichophyton Isolates 2019–2020. J. Fungi 2022, 8, 150. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mann, A.; Polla Ravi, S.; Wang, T. An update on antifungal resistance in dermatophytosis. Expert. Opin. Pharmacother. 2024, 25, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Favre, B.; Hofbauer, B.; Hildering, K.S.; Ryder, N.S. Comparison of in vitro activities of 17 antifungal drugs against a panel of 20 dermatophytes by using a microdilution assay. J. Clin. Microbiol. 2003, 41, 4817–4819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zinn, M.K.; Bockmühl, D. Did granny know best? Evaluating the antibacterial, antifungal and antiviral efficacy of acetic acid for home care procedures. BMC Microbiol. 2020, 20, 265. [Google Scholar] [CrossRef]
- Elhage, K.G.; St Claire, K.; Daveluy, S. Acetic acid and the skin: A review of vinegar in dermatology. Int. J. Dermatol. 2022, 61, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Liu, D.; Wang, T.; Rajpara, A.; Franano, C.; Aires, D. Vinegar sock soak for tinea pedis or onychomycosis. J. Am. Acad. Dermatol. 2023, 89, e23. [Google Scholar] [CrossRef] [PubMed]
- Petranyi, G.; Meingassner, J.G.; Mieth, H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob. Agents Chemother. 1987, 31, 1365–1368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgopoulos, A.; Petranyi, G.; Mieth, H.; Drews, J. In vitro activity of naftifine, a new antifungal agent. Antimicrob. Agents Chemother. 1981, 19, 386–389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veraldi, S. Isoconazole Nitrate: A Unique Broad-Spectrum Antimicrobial Azole Effective in the Treatment of Dermatomycoses, Both as Monotherapy and in Combination with Corticosteroids. Mycoses 2013, 56 (Suppl. S1), 3–15. [Google Scholar] [CrossRef]
- Oyeka, C.A.; Gugnani, H.C. In Vitro Activity of Seven Azole Compounds against Some Clinical Isolates of Non-Dermatophytic Filamentous Fungi and Some Dermatophytes. Mycopathologia 1990, 110, 157–161. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Nagai, H.; Salamin, K.; Chang, Y.-T.; Guenova, E.; Feuermann, M.; Monod, M. Two Different Types of Tandem Sequences Mediate the Overexpression of TinCYP51B in Azole-Resistant Trichophyton indotineae. Antimicrob. Agents Chemother. 2023, 67, e0093323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene Amplification of CYP51B: A New Mechanism of Resistance to Azole Compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, e0005922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khurana, A.; Sharath, S.; Sardana, K.; Chowdhary, A.; Panesar, S. Therapeutic Updates on the Management of Tinea Corporis or Cruris in the Era of Trichophyton indotineae: Separating Evidence from Hype—A Narrative Review. Indian. J. Dermatol. 2023, 68, 525–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gräser, Y.; Saunte, D.M.L. A Hundred Years of Diagnosing Superficial Fungal Infections: Where Do We Come From, Where Are We Now and Where Would We Like To Go? Acta Derm. Venereol. 2020, 100, adv00111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pospischil, I.; Reinhardt, C.; Bontems, O.; Salamin, K.; Fratti, M.; Blanchard, G.; Chang, Y.-T.; Wagner, H.; Hermann, P.; Monod, M.; et al. Identification of Dermatophyte and Non-Dermatophyte Agents in Onychomycosis by PCR and DNA Sequencing—A Retrospective Comparison of Diagnostic Tools. J. Fungi 2022, 8, 1019. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Sacheli, R.; Diongue, K.; Adjetey, C.; Darfouf, R.; Seck, M.C.; Badiane, A.S.; Diallo, M.A.; Dieng, T.; Hayette, M.-P.; et al. Evaluation of the Multiplex Real-Time PCR DermaGenius® Assay for the Detection of Dermatophytes in Hair Samples from Senegal. J. Fungi 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]

| Antifungal | T. indotineae 26337 | C. krusei ATCC6258 | C. parapsilosis ATCC22019 |
|---|---|---|---|
| Fluconazole | 16 | 16–32 | 0.25 |
| Voriconazole | 0.06–0.12 | 0.06 | 0.016 |
| Posaconazole | 0.008 | 0.03 | 0.016 |
| Itraconazole | 0.008 | 0.03 | 0.03 |
| Terbinafine | 0.03–0.06 | 4 | 0.25–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, Z.; Ványai, B.; Kovács, R.; Jakab, Á.; Szegedi, A.; Balázs, B.; Majoros, L. First Report of Trichophyton indotineae Infection in Hungary. J. Fungi 2025, 11, 248. https://doi.org/10.3390/jof11040248
Tóth Z, Ványai B, Kovács R, Jakab Á, Szegedi A, Balázs B, Majoros L. First Report of Trichophyton indotineae Infection in Hungary. Journal of Fungi. 2025; 11(4):248. https://doi.org/10.3390/jof11040248
Chicago/Turabian StyleTóth, Zoltán, Beatrix Ványai, Renátó Kovács, Ágnes Jakab, Andrea Szegedi, Bence Balázs, and László Majoros. 2025. "First Report of Trichophyton indotineae Infection in Hungary" Journal of Fungi 11, no. 4: 248. https://doi.org/10.3390/jof11040248
APA StyleTóth, Z., Ványai, B., Kovács, R., Jakab, Á., Szegedi, A., Balázs, B., & Majoros, L. (2025). First Report of Trichophyton indotineae Infection in Hungary. Journal of Fungi, 11(4), 248. https://doi.org/10.3390/jof11040248









