Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Setting and Patient Cohort
2.2. Invasive Candidiasis (CAGTA) IgG VirClia Monotest
2.3. Wako β-D-Glucan Test
2.4. CandId Real-Time PCR
2.5. Statistical Analysis
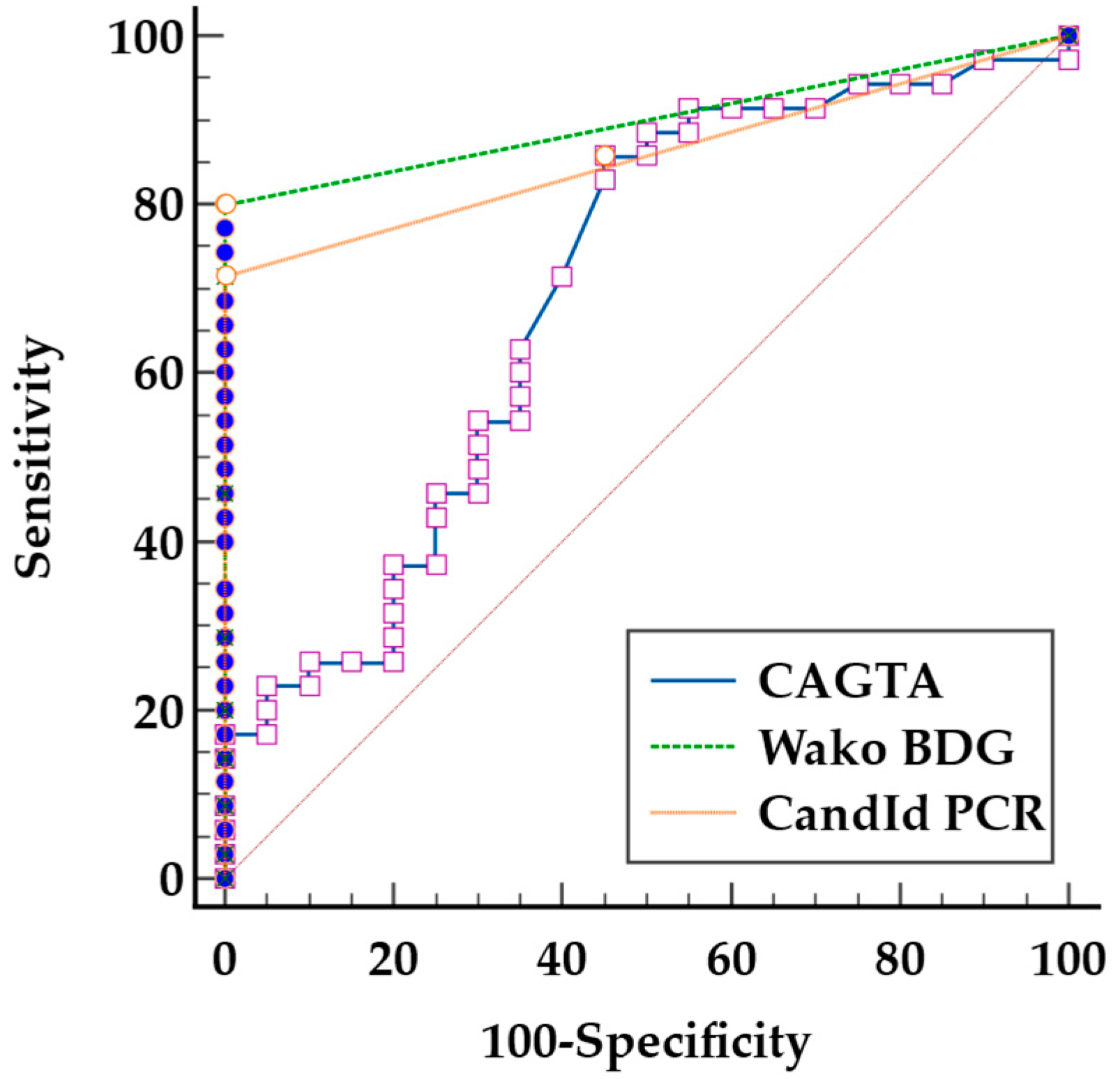
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taieb, F.; Méchaï, F.; Lefort, A.; Lanternier, F.; Bougnoux, M.E.; Lortholary, O. Management of candidemia and invasive candidiasis. Rev. Med. Interne 2011, 32, 173–180. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Marelli, C.; Mora, S.; Guastavino, S.; Russo, C.; Brucci, G.; Limongelli, A.; Vena, A.; Mikulska, M.; Tayefi, M.; et al. Early diagnosis of candidemia with explainable machine learning on automatically extracted laboratory and microbiological data: Results of the AUTO-CAND project. Ann. Med. 2023, 55, 2285454. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sánchez, M.; Zakariya-Yousef Breval, I.; Castro Méndez, C.; García-Rey, S.; Loza Vazquez, A.; Úbeda Iglesias, A.; Macías Guerrero, D.; Romero Mejías, A.; León Gil, C.; Martín-Mazuelos, E.; et al. Candida albicans Germ-Tube Antibody: Evaluation of a New Automatic Assay for Diagnosing Invasive Candidiasis in ICU Patients. Mycopathologia 2017, 182, 645–652. [Google Scholar] [CrossRef]
- Eades, C.P.; Bakri, A.R.B.A.; Lau, J.C.Y.; Moore, C.B.; Novak-Frazer, L.; Richardson, M.D.; Rautemaa-Richardson, R. Comparison of β-1-3-D-Glucan and Candida Mannan Biomarker Assays with Serological Tests for the Diagnosis of Candidemia. J. Fungi 2023, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Balletto, E.; Castagnola, E.; Mularoni, A. Beta-D-Glucan in Patients with Haematological Malignancies. J. Fungi 2021, 7, 1046. [Google Scholar] [CrossRef]
- Finkelman, M.A. Specificity Influences in (1→3)-β-d-Glucan-Supported Diagnosis of Invasive Fungal Disease. J. Fungi 2020, 7, 14. [Google Scholar] [CrossRef]
- Azim, A.; Ahmed, A. Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients, with focus on candidiasis and aspergillosis: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1256158. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Y.; Zhang, W.; Xie, S.; Yan, P.; Liu, Y.; Li, Y.; Ma, X.; Xiao, K.; Fu, H.; et al. Diagnostic value of Candida mannan antigen and anti-mannan IgG and IgM antibodies for Candida infection. Mycoses 2020, 63, 181–188. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.C.; Muñoz, P.; Guinea, J.; Valerio, M.; Alonso, R.; Escribano, P.; Bouza, E. Potential role of Candida albicans germ tube antibody in the diagnosis of deep-seated candidemia. Med. Mycol. 2014, 52, 270–275. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Non-Culture Diagnostics for Invasive Candidiasis: Promise and Unintended Consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef]
- Wei, S.; Wu, T.; Wu, Y.; Ming, D.; Zhu, X. Diagnostic accuracy of Candida albicans germ tube antibody for invasive candidiasis: Systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2019, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Pini, P.; Colombari, B.; Marchi, E.; Castagnoli, A.; Venturelli, C.; Sarti, M.; Blasi, E. Performance of Candida albicans germ tube antibodies (CAGTA) and its association with (1 → 3)-β-D-glucan (BDG) for diagnosis of invasive candidiasis (IC). Diagn. Microbiol. Infect. Dis. 2019, 93, 39–43. [Google Scholar] [CrossRef]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Castro, C.; Loza, A.; Zakariya, I.; Úbeda, A.; Parra, M.; Macías, D.; Tomás, J.I.; et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit. Care 2016, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Arikan-Akdagli, S.; Jørgensen, K.M.; Barac, A.; Steinmann, J.; Toscano, C.; Arsenijevic, V.A.; Sartor, A.; Lass-Flörl, C.; Hamprecht, A.; et al. European candidaemia is characterised by notable differential epidemiology and susceptibility pattern: Results from the ECMM Candida III study. J. Infect. 2023, 87, 428–437. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. β-D-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17. [Google Scholar] [CrossRef]
- Morjaria, S.; Frame, J.; Franco-Garcia, A.; Geyer, A.; Kamboj, M.; Babady, N.E. Clinical Performance of (1,3) Beta-D Glucan for the Diagnosis of Pneumocystis Pneumonia (PCP) in Cancer Patients Tested with PCP Polymerase Chain Reaction. Clin. Infect. Dis. 2019, 69, 1303–1309. [Google Scholar] [CrossRef]
- Friedrich, R.; Rappold, E.; Bogdan, C.; Held, J. Comparative Analysis of the Wako β-Glucan Test and the Fungitell Assay for Diagnosis of Candidemia and Pneumocystis jirovecii Pneumonia. J. Clin. Microbiol. 2018, 56, e00464-18. [Google Scholar] [CrossRef]
- Dichtl, K.; Seybold, U.; Wagener, J. Serological biomarkers of candidemia: A retrospective evaluation of three assays. Infection 2019, 47, 217–224. [Google Scholar] [CrossRef]
- De Carolis, E.; Marchionni, F.; Torelli, R.; Angela, M.G.; Pagano, L.; Murri, R.; De Pascale, G.; De Angelis, G.; Sanguinetti, M.; Posteraro, B. Comparative performance evaluation of Wako β-glucan test and Fungitell assay for the diagnosis of invasive fungal diseases. PLoS ONE 2020, 15, e0236095. [Google Scholar] [CrossRef] [PubMed]
- Monday, L.M.; Parraga Acosta, T.; Alangaden, G. T2Candida for the Diagnosis and Management of Invasive Candida Infections. J. Fungi 2021, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Camp, I.; Spettel, K.; Willinger, B. Molecular Methods for the Diagnosis of Invasive Candidiasis. J. Fungi 2020, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Price, J.S.; Fallon, M.; Posso, R.; Backx, M.; White, P.L. An Evaluation of the OLM CandID Real-Time PCR to Aid in the Diagnosis of Invasive Candidiasis When Testing Serum Samples. J. Fungi 2022, 8, 935. [Google Scholar] [CrossRef]
- Deckers, C.; Bélik, F.; Khourssaji, M.; Plum, P.E.; Ausselet, N.; Bulpa, P.; Sonet, A.; Bihin, B.; Huang, T.D.; Denis, O.; et al. A decade of candidaemia: A comprehensive analysis of prognosis and risk factors at a Belgian tertiary hospital. Diagn. Microbiol. Infect. Dis. 2024, 110, 116493. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Westerneng, T.J.; Haynes, K.A.; Bennett, D.E.; Coleman, D.C. Candida dubliniensis sp. nov.: Phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 1995, 141 Pt 7, 1507–1521. [Google Scholar] [CrossRef]
| Positive BD 1 (n = 35) | Negative BD 1 (n = 20) | |
|---|---|---|
| Demographics | ||
| Age (median years, range) Gender (male/female) | 69 (21–90) | 68 (23–90) |
| 21/14 | 14/6 | |
| Hospitalization Units 2 | ||
| ICU 3 (%) Onco-Hematology (%) Digestive Surgery (%) Internal Medicine (%) Urology (%) Geriatrics (%) Others (%) | 14 (40%) | 1 (5%) |
| 5 (14%) | 4 (20%) | |
| 4 (11%) | 1 (5%) | |
| 4 (11%) | 9 (45%) | |
| 3 (9%) | - | |
| 3 (9%) | 4 (20%) | |
| 2 (6%) | 1 (5%) | |
| Hospital admission reasons | ||
| Surgery (%) Severe bacterial infection (%) Febrile neutropenia (%) Gastrointestinal obstruction (%) Neoplastic recurrence (%) Viral infection (%) Others (%) | 12 (34%) | 5 (25%) |
| 8 (23%) | 5 (25%) | |
| 4 (11%) | 1 (5%) | |
| 4 (11%) | - | |
| 3 (9%) | 1 (5%) | |
| 2 (6%) | 4 (20%) | |
| 2 (6%) | 4 (20%) | |
| Antifungal exposure 4 | 3 (9%) | - |
| One-month mortality | 15 (43%) | 3 (15%) |
| Probable deep-seated candidiasis | 12 (34%) | - |
| Candida spp distribution 5 | ||
| Candida albicans Candida glabrata 6 Candida tropicalis Candida krusei Candida guillermondii Candida dubliniensis | 18 (51%) | - |
| 9 (26%) | - | |
| 5 (14%) | - | |
| 1 (3%) | - | |
| 1 (3%) | - | |
| 1 (3%) | - |
| Biomarker | Sens 1 (%) (95% CI 4) | Spec 2 (%) (95% CI) | AUC 3 (95% CI) |
|---|---|---|---|
| CAGTA 5 | 46 (29–63) | 75 (51–91) | 0.6 (0.4–0.7) |
| BDG 6 | 74 (57–87) | 100 (83–100) | 0.9 (0.7–0.9) |
| PCR 7 | 71 (54–85) | 100 (83–100) | 0.9 (0.7–0.9) |
| CAGTA and/or BDG | 86 (70–95) | 75 (51–91) | 0.8 (0.7–0.9) |
| CAGTA and/orPCR | 83 (66–93) | 75 (51–91) | 0.8 (0.7–0.9) |
| BDG and/orPCR | 91 (77–98) | 100 (83–100) | 0.9 (0.8–1) |
| CAGTA and/or BDG and/or PCR | 94 (81–99) | 75 (51–91) | 0.8 (0.7–0.9) |
| Candida spp. Candidemia | CAGTA 1 | Wako BDG 2 | CandId PCR 3 |
|---|---|---|---|
| Candida albicans (n = 18) | 10 (55%) | 14 (78%) | 15 (83%) |
| Candida glabrata (n = 9) | 4 (44%) | 8 (89%) | 4 (44%) |
| Candida tropicalis (n = 5) | 2 (40%) | 3 (60%) | 4 (80%) |
| Candida krusei (n = 1) | 0 (0%) | 0 (0%) | 1 (100%) |
| Candida guillermondii (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) |
| Candida dubliniensis (n = 1) | 0 (0%) | 1 (100%) | 1 (100%) 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinet-Poleur, A.; Deckers, C.; Saad Albichr, I.; Bogaerts, P.; Honoré, P.M.; Bulpa, P.; Ausselet, N.; Foret, F.; Kidd, F.; Huang, T.-D.; et al. Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia. J. Fungi 2025, 11, 224. https://doi.org/10.3390/jof11030224
Kinet-Poleur A, Deckers C, Saad Albichr I, Bogaerts P, Honoré PM, Bulpa P, Ausselet N, Foret F, Kidd F, Huang T-D, et al. Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia. Journal of Fungi. 2025; 11(3):224. https://doi.org/10.3390/jof11030224
Chicago/Turabian StyleKinet-Poleur, Amélie, Corentin Deckers, Imane Saad Albichr, Pierre Bogaerts, Patrick M. Honoré, Pierre Bulpa, Nathalie Ausselet, Frederic Foret, François Kidd, Te-Din Huang, and et al. 2025. "Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia" Journal of Fungi 11, no. 3: 224. https://doi.org/10.3390/jof11030224
APA StyleKinet-Poleur, A., Deckers, C., Saad Albichr, I., Bogaerts, P., Honoré, P. M., Bulpa, P., Ausselet, N., Foret, F., Kidd, F., Huang, T.-D., & Montesinos, I. (2025). Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia. Journal of Fungi, 11(3), 224. https://doi.org/10.3390/jof11030224







