Occurrence and Diversity of Fungi and Their Mycotoxin Production in Common Edible and Medicinal Substances from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Edible and Medicinal Substances
2.2. Fungal Isolation and Enumeration
2.3. Morphological Identification
2.4. Molecular Identification
2.5. Mycotoxin-Producing Abilities of Aspergillus and Penicillium Strains
2.6. Mycotoxin Determination in Edible and Medicinal Substances
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fungal Contamination in Edible and Medicinal Substances
3.2. Fungal Genera and Species Diversity
3.3. Mycotoxin-Producing Abilities of Aspergillus and Penicillium Isolates
3.4. Mycotoxin Content in Edible and Medicinal Substances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | aflatoxin |
| CTN | citrinin |
| CYA | Czapek yeast extract agar |
| F | fumonisin |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MEA | malt extract agar |
| OTA | ochratoxin A |
| PAT | patulin |
| TYMC | total combined yeast and mold count |
| UPLC–MS/MS | ultra-performance liquid chromatography coupled with tandem mass spectrometry |
| ZEN | zearalenone |
References
- Giblette, J. Production of Chinese medicinal herbs in North America: Challenge and reconciliation. China Med. 2022, 5, 234–240. [Google Scholar] [CrossRef]
- Liu, H.P.; Tang, J.F.; Chen, T.J.; Zhu, P.P.; Sun, D.D.; Wang, W.Y. Assessment of heavy metals contamination and human health risk assessment of the commonly consumed medicinal herbs in China. Environ. Sci. Pollut. Res. Int. 2023, 30, 7345–7357. [Google Scholar] [CrossRef]
- Nakhaee, S.; Kooshki, A.; Hormozi, A.; Akbari, A.; Mehrpour, O.; Farrokhfall, K. Cinnamon and cognitive function: A systematic review of preclinical and clinical studies. Nutr. Neurosci. 2024, 27, 132–146. [Google Scholar] [CrossRef]
- Global Industry Analysts Inc. Herbal Supplements and Remedies: A Global Strategic Business Report MCP-1081. 2024. Available online: http://www.strategyr.com/Herbal_Supplements_and_Remedies_Market_Report.asp (accessed on 1 February 2025).
- National Health Commission of the People’s Republic of China; State Administration for Market Regulation. Announcement on Rehmannia glutinosa Libosch. and Other Four Substances Listed in Edible and Medicinal Substances. 2024. Available online: http://www.nhc.gov.cn/sps/s7890/202408/01f4b82eff294f4182102cf43e2e898e.shtml (accessed on 26 August 2024).
- Xue, H.K.; Wang, W.L.; Bian, J.Y.; Gao, Y.C.; Hao, Z.T.; Tan, J.Q. Recent advances in medicinal and edible homologous polysaccharides: Extraction, purification, structure, modification, and biological activities. Int. J. Biol. Macromol. 2022, 222, 1110–1126. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Zhang, J.; Xie, Y.; Cai, W.; Tan, J. Antidiabetic activity of Ganoderma lucidum polysaccharides f31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol. 2017, 196, 47–57. [Google Scholar] [CrossRef]
- Oztekin, S.; Dikmetas, D.N.; Devecioglu, D.; Acar, E.G.; Karbancioglu-Guler, F. Recent insights into the use of antagonistic yeasts for sustainable biomanagement of postharvest pathogenic and mycotoxigenic fungi in fruits with their prevention strategies against mycotoxins. J. Agric. Food Chem. 2023, 71, 9923–9950. [Google Scholar] [CrossRef]
- Rocha-Miranda, F.; Venâncio, A. Mycotoxigenic fungi in plant-based supplements and medicines. Curr. Opin. Food Sci. 2019, 30, 27–31. [Google Scholar] [CrossRef]
- Chen, L.; Guo, W.P.; Zheng, Y.Q.; Zhou, J.Z.; Liu, T.T.; Chen, W.; Liang, D.Q.; Zhao, M.P.; Zhu, Y.D.; Wu, Q.P.; et al. Occurrence and characterization of fungi and mycotoxins in contaminated medicinal herbs. Toxins 2020, 12, 30. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tie, X.Y.; Yang, X.C.; Cheng, F.; Li, Y. Study on the quality change and validity of Chinese herbal medicine and Chinese herbal decoction pieces. J. Trad. Chin. Vet. 2021, 40, 44–48. [Google Scholar]
- European Pharmacopoeia Commission. European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2019; pp. 68–97. [Google Scholar]
- Chen, A.J.; Huang, L.F.; Wang, L.Z.; Tang, D.; Cai, F.; Gao, W.W. Occurrence of toxigenic fungi in ochratoxin A contaminated liquorice root. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1091–1097. [Google Scholar] [CrossRef]
- Han, Z.; Ren, Y.P.; Liu, X.S.; Luan, L.J.; Wu, Y.J. A reliable isotope dilution method for simultaneous determination of fumonisins B1, B2 and B3 in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2010, 33, 2723–2733. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.; Yu, J.; Fan, C.; Yang, M.; Pang, X. Variations of the fungal microbiome in Corydalis rhizoma with different collection areas, processing methods, and storage conditions. Food Res. Int. 2024, 180, 114045. [Google Scholar] [CrossRef]
- Massi, F.P.; Sartori, D.; de Souza Ferranti, L.D.S.; Iamanaka, B.T.; Taniwaki, M.H.; Vieira, M.L.C.; Fungaro, M.H.P. Prospecting for the incidence of genes involved in ochratoxin and fumonisin biosynthesis in Brazilian strains of Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2016, 221, 19–28. [Google Scholar] [CrossRef]
- Susca, A.; Moretti, A.; Stea, G.; Villani, A.; Haidukowski, M.; Logrieco, A.; Munkvold, G. Comparison of species composition and fumonisin production in Aspergillus section Nigri populations in maize kernels from USA and Italy. Int. J. Food Microbiol. 2014, 188, 75–82. [Google Scholar] [CrossRef]
- Wei, G.F.; Guo, X.T.; Liang, Y.C.; Liu, C.S.; Zhang, G.Z.; Liang, C.L.; Huang, Z.X.; Zheng, Y.Q.; Chen, S.L.; Dong, L.L. Occurrence of fungi and mycotoxins in herbal medicines and rapid detection of toxin-producing fungi. Environ. Pollut. 2023, 333, 122082. [Google Scholar] [CrossRef]
- Zhao, X.S.; Ying, G.Y.; Wei, J.H.; Sun, H.; Yang, M.H. Overview of aflatoxin B1 contamination in traditional Chinese medicine. Chin. J. Pharmacovigilance 2018, 15, 608–616, 622. [Google Scholar]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins, and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Schulz, M.C.; Schumann, L.; Rottkord, U.; Humpf, H.U.; Gekle, M.; Schwerdt, G. Synergistic action of the nephrotoxic mycotoxins ochratoxin A and citrinin at nanomolar concentrations in human proximal tubule-derived cells. Toxicol. Lett. 2018, 291, 149–157. [Google Scholar] [CrossRef]
- Wang, J.; Gan, C.; Qi, X.; Lebre, M.C.; Schinkel, A.H. Human organic anion transporting polypeptide (OATP) 1B3 and mouse OATP1A/1B affect liver accumulation of ochratoxin A in mice. Toxicol. Appl. Pharmacol. 2020, 401, 115072. [Google Scholar] [CrossRef]
- Ehsanifar, M.; Rajati, R.; Gholami, A.; Reiss, J.P. Mold and mycotoxin exposure and brain disorders. J. Integr. Neurosci. 2023, 22, 137. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances in nanomaterial-mediated bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017, 87, 112–128. [Google Scholar] [CrossRef]
- Lach, M.; Kotarska, K. Negative effects of occurrence of mycotoxins in animal feed and biological methods of their detoxification: A review. Molecules 2024, 29, 4563. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Pharmacopeia Commission: Beijing, China, 2020. [Google Scholar]
- US Food and Drug Administration. Bacteriological Analytical Manual (BAM). Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam (accessed on 30 December 2024).
- Kirk, P.; Cannon, P.; Minter, D.; Stalpers, J. Ainsworth and Bisby’s Dictionary of the Fungi, 10th ed.; CABI Publishing: Wallingford, UK, 2008. [Google Scholar]
- Kong, H.Z. Flora fungorum sinicorum. In Penicillium et Teleomorphi Cognati; Science Press: Beijing, China, 2007; Volume 35. [Google Scholar]
- Qi, Z.T. Flora fungorum sinicorum. In Aspergillus et Teleomorphi Cognati; Science Press: Beijing, China, 1997; Volume 5. [Google Scholar]
- Huang, T.S.; Wang, K.V.; Ye, X.Y.; Chen, C.S.; Chang, F.C. Attention-guided transfer learning for identification of filamentous fungi encountered in the clinical laboratory. Microbiol. Spectr. 2023, 11, e0461122. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus, Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Silva, J.J.; Puel, O.; Lorber, S.; Ferranti, L.S.; Ortiz, L.F.; Taniwaki, M.H.; Iamanaka, B.T.; Fungaro, M.H.P. Occurrence and diversity of Aspergillus in commercial yerba mate elaborated for the Brazilian beverage, “chimarrão”. Food Res. Int. 2019, 121, 940–946. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China; National Food and Medical Products Administration. GB 5009.22-2016; Determination of Aflatoxin B and G in Foods of National Food Safety Standards; China Standard Press: Beijing, China, 2016; pp. 11–17. [Google Scholar]
- National Health Commission of the People’s Republic of China; National Food and Medical Products Administration. GB 5009.96-2016; Determination of Ochratoxin A in Foods of National Food Safety Standards; China Standard Press: Beijing, China, 2016; pp. 1–6. [Google Scholar]
- National Health Commission of the People’s Republic of China; National Food and Medical Products Administration. GB 5009.209-2016; Determination of Zearalenone in Foods of National Food Safety Standards; China Standard Press: Beijing, China, 2016; pp. 2–5. [Google Scholar]
- Lu, Q.; Ruan, H.N.; Sun, X.Q.; Luo, J.Y.; Yang, M.H. Contamination status and health risk assessment of 31 mycotoxins in six edible and medicinal plants using a novel green defatting and depigmenting pretreatment coupled with LC–MS/MS. LWT 2022, 161, 113401. [Google Scholar] [CrossRef]
- Huang, X.J.; Feng, R.; Hu, Q.; Mao, X.H.; Zhou, H. Contamination status and health risk assessment of 73 mycotoxins in four edible and medicinal plants using an optimized QuEChERS pretreatment coupled with LC–MS/MS. Toxins 2025, 17, 52. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Fan, Y.L.; Li, Q.Q.; Fang, L.; Yang, M.C. A survey of microbial contamination for ten processed pieces of Chinese materia medica in Shanghai. Chin. Trad. Herb. Drugs 2015, 46, 1908–1913. [Google Scholar]
- Zhang, G.H.; Wang, S.J.; Jiang, Z.J.; Gao, C. Investigation of microbial contamination for 10 kinds of TCM decoction pieces in Beijing area. China Pharm. 2018, 29, 1940–1944. [Google Scholar]
- Deng, H.Y.; Gong, Y.X.; Li, L.F.; Deng, X.D.; Tang, S.; Kang, S.H.; Jiang, Z.Y. Research progress on microbial contamination of decoction pieces and identification of typical bacteria. Chin. Trad. Herb. Drugs 2019, 50, 2242–2250. [Google Scholar]
- Gao, X.Z.; Liu, Z.; Wang, Y.; Wang, Y.H. Investigation and research on current situation of microbial contamination of medicinal and edible Chinese medicine prepared pieces. Food Drug 2019, 21, 70–74. [Google Scholar]
- Lin, T.H.; Zhang, F.; Zhu, H.M.; Hong, J.W. Investigation and risk analysis of microbial contamination in the Chinese herbal medicine Angelicae sinensis radix in Guangdong Province. Chin. J. Pharm. Anal. 2024, 44, 859–865. [Google Scholar]
- Li, K.; Wang, H.Y.; Guo, X.Z.; Zhang, C.C.; Wang, Y.F.; Guo, L.P. Advances in research and application of Trichoderma for inducing resistance against root rot diseases in root and rhizome of Chinese medicinal materials. Zhongguo Zhong Yao Za Zhi 2023, 48, 4942–4949. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Y.; Wang, J.; Cai, M.; Ye, B.; Yang, K.; Sun, P. Insoluble dietary fibers from by-products of edible fungi industry: Basic structure, physicochemical properties, and their effects on energy intake. Front. Nutr. 2022, 9, 851228. [Google Scholar] [CrossRef]
- Xu, X.X.; Li, X.J.; Pan, K.W.; Deng, L.; Xia, S.B.; Dong, J.W. Microbial transformation of geniposide in Gardeniae fructus under the fermentation with Aspergillus niger DQWM-G11. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Gupta, A.; Updahyay, H.; Pandey, S. Evaluation of Microbial Contamination in Herbal Drugs. IJSR 2023, 12, 2333–2336. [Google Scholar] [CrossRef]
- Li, Q.C.; Jiang, Y.F.; Lai, S.; Li, W.J.; Lin, T.H. Study on the whole chain microbial load in the production of one traditional Chinese medicine pills. Chin. J. Pharm. Anal. 2024, 44, 1394–1399. Available online: https://ywfx.nifdc.org.cn/EN/10.16155/j.0254-1793.2024-0192 (accessed on 1 February 2025).
- Yu, J.S. Fungal Identification on the Surface of Chinese Herbal Materials Through High Throughput Sequencing—Case Studies of Six Herbal Materials; Peking Union Medical College: Beijing, China, 2022. [Google Scholar]
- Moorthy, K.; Prasanna, I.; Thajuddin, N.; Arjunan, S.; Gnanendra, T.S.; Zahir Hussain, M.I. Occurrence of myco population in spices and herbal drugs. Int. J. Biosci. Technol. 2010, 1, 6–14. [Google Scholar]
- Brito, V.D.; Achimón, F.; Zunino, M.P.; Zygadlo, J.A.; Pizzolitto, R.P. Fungal diversity and mycotoxins detected in maize stored in silo-bags: A review. J. Sci. Food Agric. 2022, 102, 2640–2650. [Google Scholar] [CrossRef]
- Nikolic, M.; Savić, I.; Nikolic, A.; Stevanović, M.; Kandić, V.; Stanković, G.; Stankovic, S. First report of Aspergillus welwitschiae causing maize ear rot in Serbia. Plant Dis. 2023, 108, 209. [Google Scholar] [CrossRef]
- Quintanilha-Peixoto, G.; Marone, M.P.; Raya, F.T.; José, J.; Oliveira, A.; Fonseca, P.L.C.; Tomé, L.M.R.; Bortolini, D.E.; Kato, R.B.; Araújo, D.S.; et al. Phylogenomics and gene selection in Aspergillus welwitschiae: Possible implications in the pathogenicity in Agave sisalana. Genomics 2022, 114, 110517. [Google Scholar] [CrossRef]
- Halvaeezadeh, M.; Jalaee, G.A.; Fatahinia, M.; Mahmoudabadi, A.Z. Aspergillus welwitschiae; an otomycosis predominant agent, new epidemiological and antifungal susceptibility data from Iran. Microb. Pathog. 2023, 181, 106180. [Google Scholar] [CrossRef]
- Li, H.F.; Zhao, L.N.; Zheng, X.F.; Wang, Y.; Diao, J.W.; Zhang, X.Y.; Zhang, H.Y. Identification of a Penicillium expansum strain producing citrinin and optimization of culture conditions for citrinin production. Food Sci. 2018, 39, 162–167. [Google Scholar]
- Pillay, Y.; Nagiah, S.; Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. mir-27b inhibition contributes to cytotoxicity in patulin-exposed HEK293 cells. Toxicon 2022, 210, 58–65. [Google Scholar] [CrossRef]
- Mallela, V.J.; Rudrapal, M.; Prasanth, D.S.N.B.K.; Pasala, P.K.; Bendale, A.R.; Bhattacharya, S.; Aldosari, S.M.; Khan, J. Lotus seed (Nelumbinis semen) extract: Anticancer potential and chemoprofiling by in vitro, in silico, and GC–MS studies. Front. Chem. 2024, 12, 1505272. [Google Scholar] [CrossRef]
- Arooj, M.; Imran, S.; Inam-Ur-Raheem, M.; Rajoka, M.S.R.; Sameen, A.; Siddique, R.; Sahar, A.; Tariq, S.; Riaz, A.; Hussain, A.; et al. Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: A comprehensive review. Food Sci. Nutr. 2021, 9, 3971–3987. [Google Scholar] [CrossRef]
- Jo, S.A.; Lee, S.D.; Kim, D.G.; Lee, H.K.; Jung, K. A survey of aflatoxin contamination in medicinal herbs for food and medicine. Korean J. Pharmacogn. 2014, 45, 154–160. [Google Scholar]
- Liu, L.N.; Jin, H.Y.; Sun, L.; Ma, S.C. General investigation and preliminary risk assessment of aflatoxins in traditional Chinese medicines and their preparations. Chin. Pharm. J. 2015, 50, 1541–1546. [Google Scholar]
- Adelusi, O.A.; Gbashi, S.; Adebo, J.A.; Aasa, A.O.; Oladeji, O.M.; Kah, G.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Seasonal and geographical impact on the mycotoxigenicity of Aspergillus and Fusarium species isolated from smallholder dairy cattle feeds and feedstuffs in Free State and Limpopo provinces of South Africa. Toxins 2023, 15, 128. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in Fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef]
- Houbraken, J.A.M.P.; Frisvad, J.C.; Samson, R.A. Taxonomy of Penicillium citrinum and related species. Fungal Divers. 2010, 44, 117–133. [Google Scholar] [CrossRef]
- Xu, W.; Xiang, L.; Chen, Y.; Zhao, Y.; Liao, S.; Li, Y.; Li, Y.; Tunyaluk, B.; Lin, L. Risk assessment of citrinin in Chinese dark tea and inhibitory effects of tea polyphenols on citrinin production. LWT Food Sci. Technol. 2024, 205, 116527. [Google Scholar] [CrossRef]
- Chhaya, R.S.; Nag, R.; Cummins, E. Human health risk from co-occurring mycotoxins in dairy: A feed-to-fork approach. Food Control 2025, 168, 110954. [Google Scholar] [CrossRef]
- Abdullah, R.; Kamarozaman, N.S.; Ab Dullah, S.S.A.; Aziz, M.Y.; Aziza, H.B.A. Health risks evaluation of mycotoxins in plant-based supplements marketed in Malaysia. Sci. Rep. 2025, 15, 1244. [Google Scholar] [CrossRef]
- Liu, L.N.; Jin, H.Y.; Sun, L.; Ma, S.C.; Lin, R.C. Determination of aflatoxins in medicinal herbs by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 2012, 23, 469–476. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Zhang, X.M.; Jiao, X.L.; Su, C.Y.; Luo, Y.; Zhang, L.; Gao, W.W. Research progresses in and management strategies for aflatoxin contamination of fruits and seeds medicinal materials in China. Plant Prot. 2023, 49, 118–126. [Google Scholar]
- Vinacour, M.; Moiana, M.; Forné, I.; Jung, K.; Bertea, M.; Valdayo, C.P.M.; Nikel, P.I.; Imhof, A.; Palumbo, M.C.; Do Porto, F.D.; et al. Genetic dissection of the degradation pathways for the mycotoxin fusaric acid in Burkholderia ambifaria T16. Appl. Environ. Microbiol. 2023, 89, e0063023. [Google Scholar] [CrossRef]
- United States Pharmacopeia Commission. United States Pharmacopeia. 2017, 38, 43. Available online: https://www.uspnf.com (accessed on 1 February 2025).
- Mafe, A.N.; Büsselberg, D. Mycotoxins in food: Cancer risks and strategies for control. Foods 2024, 13, 3502. [Google Scholar] [CrossRef] [PubMed]
- Daba, H.G.; Delele, M.A.; Fanta, S.W.; Habtu, N.G.; Abera, M.K. Mycotoxigenic fungal growth within stored maize (Zea mays L.) at different storage altitudes and durations. J. Stored Prod. Res. 2024, 108, 102383. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- National Health Commission of the People’s Republic of China; National Food and Medical Products Administration. National Food Safety Standard-Limits of Mycotoxins in Foods: GB 2761–2017; Standards Press of China: Beijing, China, 2017. [Google Scholar]
- Stakheev, A.A.; Taliansky, M.; Kalinina, N.O.; Zavriev, S.K. RNAi-based approaches to control mycotoxin producers: Challenges and perspectives. J. Fungi 2024, 10, 682. [Google Scholar] [CrossRef]

| Scientific Name * | Type of Edible and Medicinal Substances | Number of Samples | Sample Name | Producing Regions |
|---|---|---|---|---|
| Acanthopanacis senticosi radix et rhizoma seu caulis | radix et rhizoma | 4 | 01-1; 01-2; 01-3; 01-4 | Heilongjiang |
| Alismatis rhizoma | radix et rhizoma | 3 | 02-1; 02-2; 02-3 | Sichuan |
| Angelicae dahuricae radix | radix et rhizoma | 3 | 03-1; 03-2; 03-3 | Sichuan |
| Angelicae sinensis radix | radix et rhizoma | 4 | 04-1; 04-2; 04-3; 04-4 | Gansu |
| Asini corii colla | animal | 3 | 05-1; 05-2; 05-3 | Shandong |
| Atractylodis macrocephalae rhizoma | radix et rhizoma | 5 | 06-1; 06-3; 06-4; 06-5 | Zhejiang |
| 06-2 | Sichuan | |||
| Auricularia cornea | edible fungi | 5 | 07-1; 07-5 | Jiangsu |
| 07-2; 07-3; 07-4 | Zhejiang | |||
| Cassiae semen | semen | 5 | 08-1 | Hebei |
| 08-2; 08-3; 08-4; 08-5 | Anhui | |||
| Chrysanthemi flos | flos et folium | 5 | 09-1; 09-2; 09-3; 09-4; 09-5 | Zhejiang |
| Crataegi fructus | fructus | 8 | 10-1; 10-2; 10-3; 10-4; 10-5; 10-6; 10-7;10-8 | Shandong |
| Cuscutae semen | semen | 4 | 11-1; 11-2; 11-3; 11-4 | Inner Mongolia |
| Dictyophora inausiata | edible fungi | 6 | 12-1; 12-2; 12-3; 12-4; 12-5; 12-6 | Zhejiang |
| Dioscoreae rhizoma | radix et rhizoma | 3 | 13-1; 13-2; 13-3 | Henan |
| Euryales semen | semen | 6 | 14-1; 14-3; 14-4; 14-5; 14-6 | Jiangxi |
| 14-2 | Guangdong | |||
| Flammulina velutipes | edible fungi | 4 | 15-1; 15-2; 15-3; 15-4 | Zhejiang |
| Ganoderma lingzhi | edible fungi | 4 | 16-1; 16-3 | Shaanxi |
| 16-2 | Zhejiang | |||
| 16-4 | Sichuan | |||
| Gardeniae fructus | fructus | 3 | 17-1 | Zhejiang |
| 17-2; 17-3 | Jiangxi | |||
| Ginseng radix et rhizoma | radix et rhizoma | 5 | 18-1; 18-2; 18-3; 18-4; 18-5 | Jilin |
| Glycyrrhizae radix et rhizoma | radix et rhizoma | 5 | 19-1; 19-2; 19-3; 19-4; 19-5 | Inner Mongolia |
| Juglandis semen | semen | 3 | 20-1 | Yunnan |
| 20-2; 20-3 | Hebei | |||
| Lablab semen album | semen | 4 | 21-1; 21-2; 21-3; 21-4 | Yunnan |
| Leonuri herba | flos et folium | 4 | 22-1; 22-4 | Guangdong |
| 22-2 | Hubei | |||
| 22-3 | Hebei | |||
| Mori folium | flos et folium | 8 | 23-1; 23-2; 23-3; 23-6; 23-7; 23-8 | Guangdong |
| 23-4; 23-5 | Henan | |||
| Mori fructus | fructus | 4 | 24-1 | Guangdong |
| 24-2; 24-3 | Zhejiang | |||
| 24-4 | Guangxi | |||
| Mume fructus | fructus | 3 | 25-1; 25-2; 25-3 | Sichuan |
| Nelumbinis semen | semen | 5 | 26-1; 26-2; 26-3; 26-4; 26-5 | Zhejiang |
| Ophiopogonis radix | radix et rhizoma | 3 | 27-1; 27-2; 27-3 | Sichuan |
| Ostreae concha | animal | 3 | 28-1 | Guangdong |
| 28-2; 28-3 | Shandong | |||
| Panacis quinquefolii radix | radix et rhizoma | 3 | 29-1; 29-2 | Liaoning |
| 29-3 | Beijing | |||
| Platycodonis radix | radix et rhizoma | 3 | 30-1; 30-2 | Shaanxi |
| 30-3 | Inner Mongolia | |||
| Polygonati rhizoma | radix et rhizoma | 4 | 31-1; 31-2; 31-3; 31-4 | Zhejiang |
| Polygonati rodorati rhizoma | radix et rhizoma | 4 | 32-1; 32-2; 32-3; 32-4 | Hunan |
| Puerariae lobatae radix | radix et rhizoma | 3 | 33-1; 33-2; 33-3 | Anhui |
| Raisin tree semen | semen | 3 | 34-1 | Zhejiang |
| 34-2; 34-3 | Shaanxi | |||
| Rhodiolae crenulatae radix et rhizoma | radix et rhizoma | 4 | 35-1; 35-2; 35-3; 35-4 | Tibet |
| Rosae laevigatae fructus | fructus | 3 | 36-1 | Anhui |
| 36-2; 36-3 | Jiangxi | |||
| Salviae miltiorrhizae radix et rhizoma | radix et rhizoma | 4 | 37-1; 37-2; 37-3; 37-4 | Shandong |
| Schisandrae chinensis fructus | fructus | 3 | 38-1; 38-2; 38-3 | Jilin |
| Scrophulariae radix | radix et rhizoma | 4 | 39-1; 39-2; 39-3 | Hebei |
| 39-4 | Zhejiang | |||
| Sennae folium | flos et folium | 3 | 40-1; 40-2; 40-3 | Yunnan |
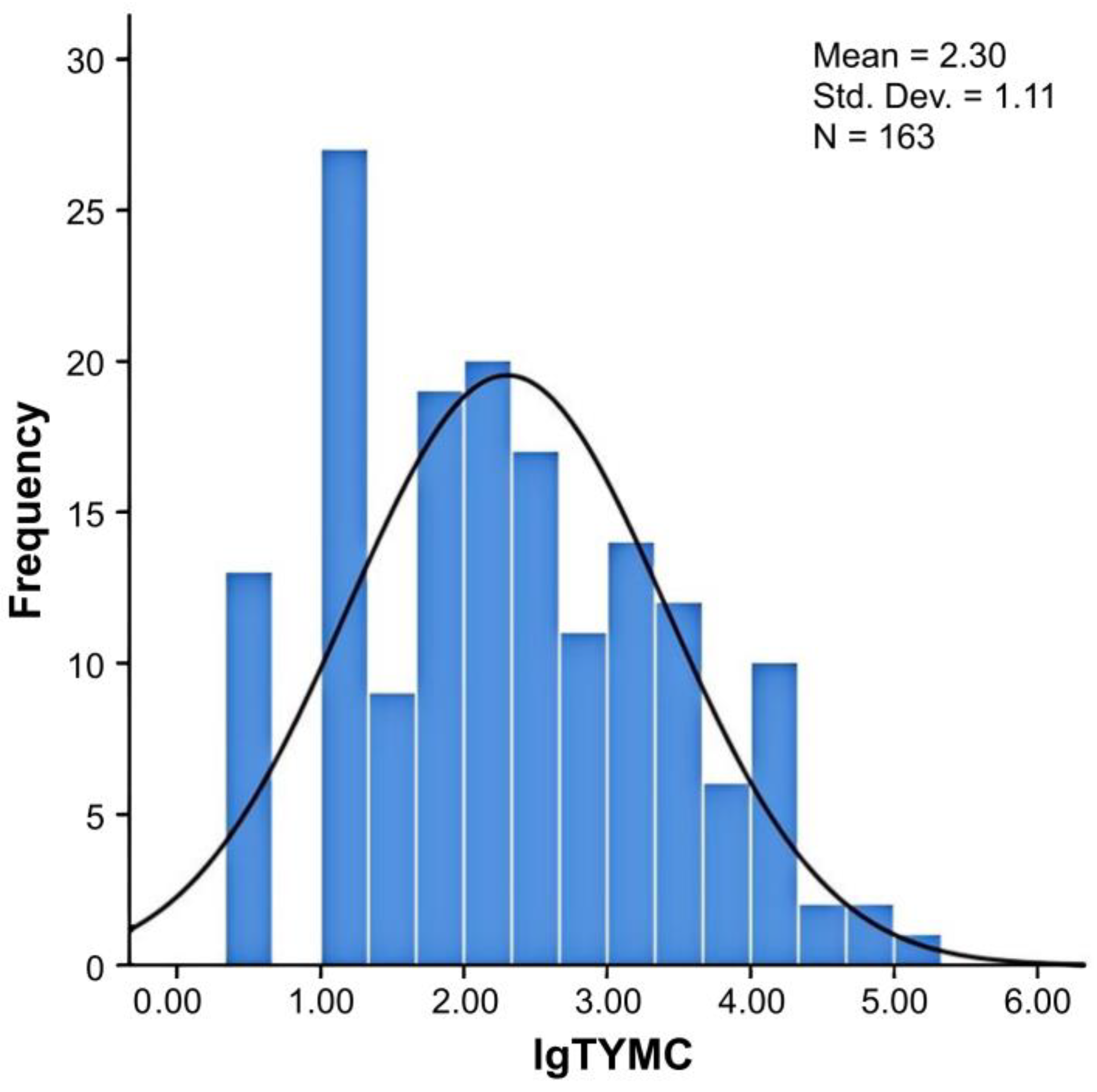
| Type of Edible and Medicinal Substance | Number of Samples | Log TYMC | Mean of Log TYMC | Median of Log TYMC |
|---|---|---|---|---|
| Animal | 6 | 0.50~4.00 | 2.18 | 2.15 |
| Edible fungi | 19 | 0.50~4.28 | 2.71 | 3.00 |
| Flos et folium | 20 | 1.85~5.28 | 2.98 | 2.83 |
| Fructus | 24 | 0.50~3.78 | 1.58 | 1.30 |
| Radix et rhizoma | 64 | 0.50~4.80 | 2.21 | 2.21 |
| Semen | 30 | 0.50~4.08 | 2.40 | 2.45 |
| Number of Isolates | Number of Mycotoxigenic Fungi | ||||
|---|---|---|---|---|---|
| Aflatoxins B1 and B2 | Ochratoxin A | Fumonisin B2 | Citrinin | ||
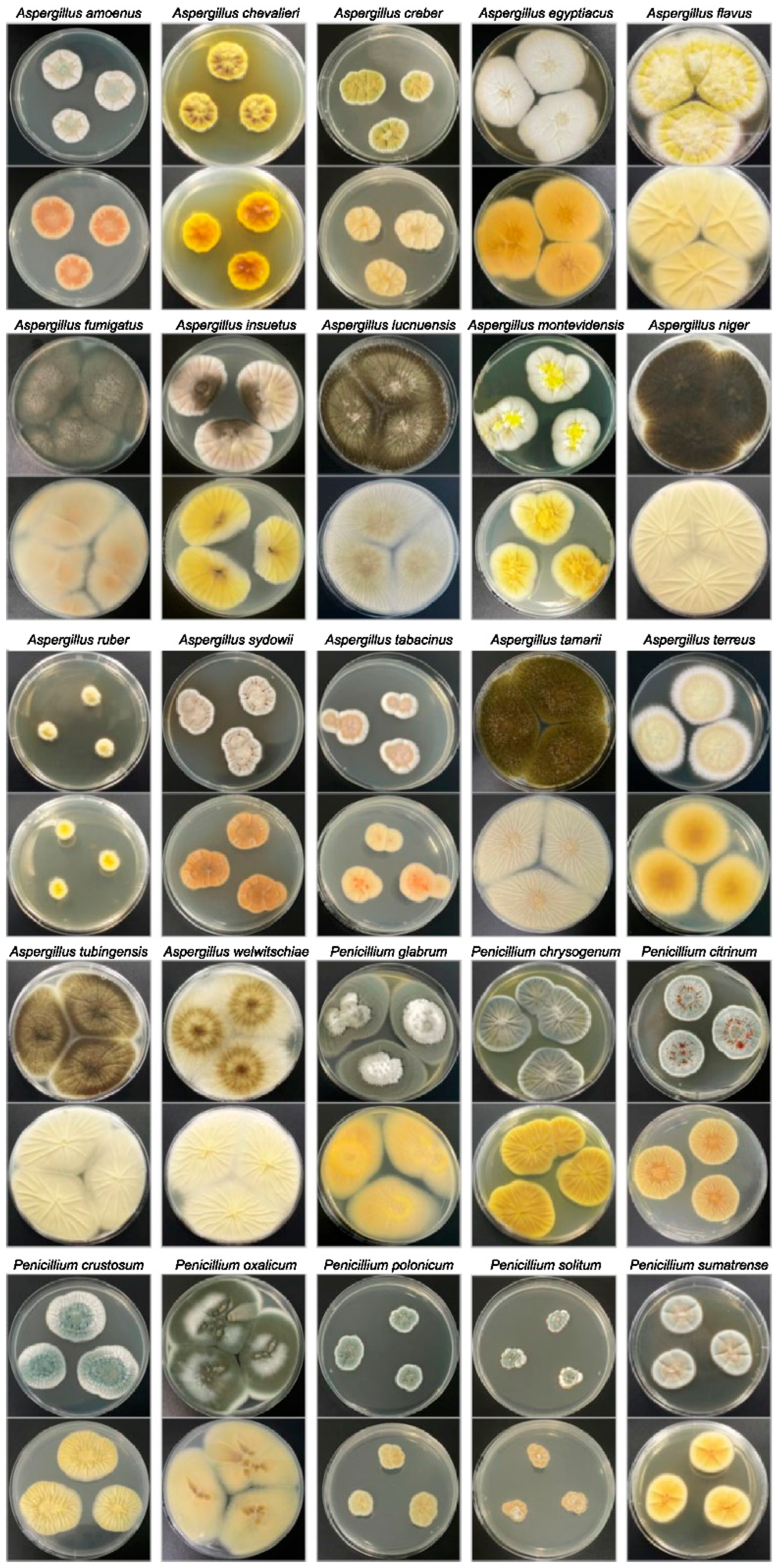
| Aspergillus amoenus | 1 | - | - | - | - |
| Aspergillus chevalieri | 3 | - | - | - | - |
| Aspergillus creber | 1 | - | - | - | - |
| Aspergillus egyptiacus | 1 | - | - | - | - |
| Aspergillus flavus | 17 | 3 | - | - | - |
| Aspergillus fumigatus | 2 | - | - | - | - |
| Aspergillus insuetus | 1 | - | - | - | - |
| Aspergillus luchuensis | 3 | - | - | - | - |
| Aspergillus montevidensis | 3 | - | - | - | - |
| Aspergillus niger | 33 | - | 3 a | 10 | - |
| Aspergillus ruber | 2 | - | - | - | - |
| Aspergillus sydowii | 5 | - | - | - | - |
| Aspergillus tabacinus | 3 | - | - | - | - |
| Aspergillus tamarii | 2 | - | - | - | - |
| Aspergillus terreus | 1 | - | - | - | - |
| Aspergillus tubingensis | 5 | - | - | - | - |
| Aspergillus welwitschiae | 7 | - | - | - | - |
| Penicillium glabrum | 1 | - | - | - | - |
| Penicillium chrysogenum | 10 | - | - | - | - |
| Penicillium citrinum | 6 | - | - | - | 3 |
| Penicillium crustosum | 2 | - | - | - | - |
| Penicillium oxalicum | 1 | - | - | - | - |
| Penicillium polonicum | 2 | - | - | - | - |
| Penicillium solitum | 1 | - | - | - | - |
| Penicillium sumatrense | 1 | - | - | - | - |
| unclassified Penicillium | 3 | - | - | - | - |
| Total | 117 | 3 | 3 a | 10 | 3 |
| Number of Sample | Mycotoxin Content (μg·kg−1) | ||||
|---|---|---|---|---|---|
| Aflatoxins B1 | Aflatoxins B2 | Ochratoxin A | Zearalenone | ||
| Angelicae dahuricae radix | 03–1 | 0.16 ± 0.00 | - | - | - |
| 03–2 | 1.35 ± 0.01 | 0.07 ± 0.01 | - | - | |
| Chrysanthemi flos | 09–1 | 0.76 ± 0.01 | - | 1.29 ± 0.01 | - |
| 09–3 | - | - | 3.48 ± 0.02 | - | |
| Glycyrrhizae radix et rhizoma | 19–1 | - | - | 2.54 ± 0.07 | - |
| 19–4 | - | - | 2.84 ± 0.01 | - | |
| 19–5 | - | - | 8.82 ± 0.05 | - | |
| Mori fructus | 24–2 | - | - | 3.33 ± 0.02 | - |
| 24–4 | - | - | 0.98 ± 0.02 | - | |
| Nelumbinis semen | 26–1 | 10.75 ± 0.16 a | 1.31 ± 0.04 | - | - |
| Raisin tree semen | 34–1 | - | - | - | 484.30 ± 6.18 b |
| 34–2 | - | - | - | 38.23 ± 2.48 | |
| 34–3 | - | - | - | 35.05 ± 10.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wu, J.; Zhang, S.; Liu, X.; Zhao, M.; Guo, W.; Zhang, J.; Chen, W.; Liu, Z.; Deng, M.; et al. Occurrence and Diversity of Fungi and Their Mycotoxin Production in Common Edible and Medicinal Substances from China. J. Fungi 2025, 11, 212. https://doi.org/10.3390/jof11030212
Chen L, Wu J, Zhang S, Liu X, Zhao M, Guo W, Zhang J, Chen W, Liu Z, Deng M, et al. Occurrence and Diversity of Fungi and Their Mycotoxin Production in Common Edible and Medicinal Substances from China. Journal of Fungi. 2025; 11(3):212. https://doi.org/10.3390/jof11030212
Chicago/Turabian StyleChen, Ling, Junhui Wu, Shuhong Zhang, Xinqi Liu, Meiping Zhao, Weipeng Guo, Jumei Zhang, Wei Chen, Zhenjie Liu, Meiqing Deng, and et al. 2025. "Occurrence and Diversity of Fungi and Their Mycotoxin Production in Common Edible and Medicinal Substances from China" Journal of Fungi 11, no. 3: 212. https://doi.org/10.3390/jof11030212
APA StyleChen, L., Wu, J., Zhang, S., Liu, X., Zhao, M., Guo, W., Zhang, J., Chen, W., Liu, Z., Deng, M., & Wu, Q. (2025). Occurrence and Diversity of Fungi and Their Mycotoxin Production in Common Edible and Medicinal Substances from China. Journal of Fungi, 11(3), 212. https://doi.org/10.3390/jof11030212






