Fungal Stress Responses and the Importance of GPCRs
Abstract
1. Introduction
2. G-Protein Coupled Receptors (GPCRs)
3. GPCR Activation and Signaling
3.1. Gα and Gβγ Subunit Functions
| Organism | G-protein Subunit | Function | Ref. |
|---|---|---|---|
| Aspergillus flavus | Gα: Unknown | RGS proteins, functioning as negative regulators of Gα subunits, play significant roles in governing conidia, sclerotia, and aflatoxin formation, indicating involvement of Gα subunit in fungal development and secondary metabolite production. | [25] |
| Metarhizium robertsii | Gα: MrGPA2, MrGPA4 | Gα subunits MrGPA2 and MrGPA4 contribute to vegetative growth, stress tolerance, and pest control potential, emphasizing their role in fungal adaptation and virulence. | [31] |
| Botrytis cinerea | Gα: Bcg1, Bcg2, Bcg3; Gβ: Bcgb1 | Deletion of the Gβ subunit resulted in alterations in cAMP signaling and MAPK pathway activation, suggesting the critical role of Gα subunit-mediated signaling cascades in fungal development and virulence. | [33,34] |
| Aspergillus fumigatus | Gα: Unknown | Gα subunits regulate growth, germination, asexual development, and resistance to oxidative stress via PKA or PKC signaling pathways. | [31] |
| Fusarium verticillioides | Gβ: FvGbb2 | Gβ-like protein, FvGbb2, regulates fumonisin biosynthesis, vegetative growth, conidiation, and stress response, highlighting diverse functions of Gβγ subunits in fungal physiology and pathogenicity. | [35] |
| Magnaphorte oryzae | Gα: magA, magB, magC; Gβ: mgb1; Gγ: MGG1 | All three Gα subunit genes are involved in mating, but only magB, Gβ, and Gγ are required for appressoria formation and virulence. | [23] |
3.2. Fungal GPCR Classification
3.3. GPCRs and Signaling Pathways
4. Pal/Rim Pathway
5. MAPK Pathway
6. cAMP/PKA Pathway
7. Adaptation Mechanisms of Fungi
7.1. GPCRs and Their Association with Fungal Adaptation
7.1.1. Osmotic Stress
| Organism | Response to Osmotic Stress | Mechanisms and Pathways Involved | Key Findings | Ref. |
|---|---|---|---|---|
| Setosphaeria turcica | Alters mycelial growth and boosts conidia germination and yield. | Activation of HOG-MAPK pathway, up-regulation of aquaglyceroporin gene StFPS1. | Crucial for appressorium formation and penetration. | [105] |
| Beauveria bassiana | Influences carbohydrate utilization and stress sensitivity. | G-protein coupled receptor BbGPCR3, major MAP kinase pathways. | Integral to broad developmental and genetic networks. | [106] |
| Saccharomyces cerevisiae | Utilizes general stress response (GSR) and Unfolded Protein Response (UPR). | Involvement of Hac1p, Rpd3p, activation of GSR genes. | Protection against hyperosmotic stress. | [107] |
| Saccharomyces cerevisiae | Crosstalk between osmotic stress and GPCR signaling pathways. | Mediated by branched-chain amino acid metabolites and involvement of MAPKs and GPCRs. | Significance in stress adaptation. | [108] |
| Saccharomyces cerevisiae | Activates HOG pathway and glycerol synthesis for osmolarity adjustment. | Involvement of the Sho1 and Sln1 pathways. | Crucial for intracellular osmotic balance. | [109] |
| Pichia pastoris | Emphasizes stress-induced damage repair over glycerol accumulation. | Unique regulatory mechanism of the MAPK/HOG pathway. | Essential for survival under hyperosmotic stress. | [110] |
| Aspergillus fumigatus | Relies on glycerol biosynthesis, particularly via the G3PDH gene. | Crucial for adaptation to various stressors. | Importance in stress adaptation. | [89] |
| Candida albicans | Influences glycerol accumulation and osmotic stress response gene expression. | Mediated by SPT20 gene via Hog1-MAPK pathway. | Crucial for osmotic stress adaptation. | [78] |
| Candida glabrata | Importance of glycerophospholipid metabolism and membrane integrity | Interaction between Hog1 and transcription factor CgRds2. | Vital for cell survival under osmotic stress. | [103] |
| Aspergillus flavus and Aspergillus ochraceus | Regulates growth, development, and mycotoxin production under stress. | HOG-MAPK pathway, SLN1 and SHO1 branches, genes such as sln1, sho1, ste11, ssk2, pbs2, and hog1. | Controls aflatoxins and ochratoxins in agricultural products. | [111] |
| Hortaea werneckii and Wallemia ichthyophaga | Senses and responds to hypersaline conditions with unique adaptations. | HOG pathway, proteins involved in sensing high osmolarity, and MAP kinase module. | Structural differences in salt tolerance. | [112] |
| Scedosporium apiospermum | Activated by various stressors, including osmotic agents. | HOG pathway. | Acts as a general stress hub. | [113] |
| Debaryomyces hansenii | Essential for survival under high osmolarity, regulates glycerol and stress responses. | HOG1 pathway, mitogen-activated protein kinase Hog1. | Enhances halotolerance by regulating stress responses. | [114] |
7.1.2. Oxidative Stress
7.1.3. Heat Shock Stress
7.1.4. pH
8. Dimorphism
9. Perspectives
- (I)
- Identification of new GPCRs: Despite the progress made in identifying GPCRs in fungi, there are likely other receptors yet to be discovered. Searching for and characterizing new GPCRs would provide a more comprehensive view of the signaling pathways and biological functions regulated by these receptors;
- (II)
- Signaling pathway specificity: A deeper understanding is needed of how different GPCRs in fungi activate specific signaling pathways. This is crucial to understanding how these receptors coordinate diverse biological and adaptive responses to different stresses;
- (III)
- Interaction with other proteins: Studying the interactions between GPCRs and other intracellular proteins would provide a better understanding of how cellular responses are regulated and how these interactions may vary depending on the biological context;
- (IV)
- Specific biological functions: It is essential to determine the specific biological functions of GPCRs in different fungal species. Understanding how these receptors are involved in growth, reproduction, pathogenicity, and other physiological functions is essential to understanding their impact on fungal biology;
- (V)
- Therapeutic Potential: Research on GPCRs in fungi may have therapeutic implications, as some of these receptors may be targets for the development of new antifungal agents or treatments for fungal diseases.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucena, D. Percepción del ambiente alcalino por Aspergillus nidulans. Doctoral Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=98324 (accessed on 5 February 2024).
- Xue, C.; Hsueh, Y.P.; Heitman, J. Magnificent seven: Roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008, 32, 1010–1032. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Stress response signaling and virulence: Insights from entomopathogenic fungi. Curr. Genet. 2015, 61, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.P.; Cowen, L.E.; Di Pietro, A.; Quinn, J. Stress adaptation. In The Fungal Kingdom; ASM Press: Washington, DC, USA, 2017; pp. 463–485. [Google Scholar] [CrossRef]
- Braunsdorf, C.; Mailänder-Sánchez, D.; Schaller, M. Fungal sensing of host environment. Cell. Microbiol. 2016, 18, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Signaling through Enzyme-Linked Cell-Surface Receptors; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Evans, P.D.; Levitan, I.B. Receptors and ion channels. J. Exp. Biol. 1986, 124, 1–4. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Velazhahan, V.; McCann, B.L.; Bignell, E.; Tate, C.G. Developing novel antifungals: Lessons from G protein-coupled receptors. Trends Pharmacol. Sci. 2023, 44, 162–174. [Google Scholar] [CrossRef]
- Versele, M.; Lemaire, K.; Thevelein, J.M. Sex and sugar in yeast: Two distinct GPCR systems. EMBO Rep. 2001, 2, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Hernández, R.; Hernández-Espinosa, D.A.; Medina, L.D.C.; García-Sáinz, J.A. G protein-coupled receptors as therapeutic target. Gac. Med. Mex. 2022, 158, 101–107. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.; Cardenas, M.E. Sensing the environment: Lessons from fungi. Nat. Rev. Microbiol. 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Brown, N.A.; Schrevens, S.; Van Dijck, P.; Goldman, G.H. Fungal G-protein-coupled receptors: Mediators of pathogenesis and targets for disease control. Nat. Microbiol. 2018, 3, 402–414. [Google Scholar] [CrossRef]
- Van Dijck, P. Nutrient sensing G protein-coupled receptors: Interesting targets for antifungals? Med. Mycol. 2009, 47, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, X.; Huang, K.; Liang, Z. Fungal G-Protein-Coupled Receptors: A Promising Mediator of the Impact of Extracellular Signals on Biosynthesis of Ochratoxin A. Front. Microbiol. 2021, 12, 631392. [Google Scholar] [CrossRef] [PubMed]
- Kochman, K. Superfamily of G-protein coupled receptors (GPCRs)—Extraordinary and outstanding success of evolution. Postepy Hig. Med. Dosw. 2014, 68, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef]
- dos Reis, T.F.; Mellado, L.; Lohmar, J.M.; Silva, L.P.; Zhou, J.J.; Calvo, A.M.; Goldman, G.H.; Brown, N.A. GPCR-mediated glucose sensing system regulates light-dependent fungal development and mycotoxin production. PLoS Genet. 2019, 15, e1008419. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Coronado, C.G.; Guzmán, A.; González-Aretia, D.; Medina-Moctezuma, Z.B.; Gutiérrez, C.G.; Rosales-Torres, A.M. Receptores acoplados a proteínas G (GPCR): Activación, señalización y regulación. Rev. Mex. Endocrinol. Metab. Nutr. 2022, 9, 137–151. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Lefkowitz, R.J. Signaling at the endosome: Cryo-EM structure of a GPCR–G protein–beta-arrestin megacomplex. FEBS J. 2021, 288, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Eichel, K.; von Zastrow, M. Subcellular organization of GPCR signaling. Trends Pharmacol. Sci. 2018, 39, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lee, N.Y.; Kim, S.S.; Park, H.S.; Shin, K.S. Comparative Characterization of G Protein α Subunits in Aspergillus fumigatus. Pathogens 2020, 9, 272. [Google Scholar] [CrossRef]
- Tang, J.; Wu, M.; Zhang, J.; Li, G.; Yang, L. Botrytis cinerea g protein β subunit bcgb1 controls growth, development and virulence by regulating camp signaling and mapk signaling. J. Fungi 2021, 7, 431. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, H.; Liu, Z.; Wang, Z.; Huang, B. G-Protein Subunit Gαi in Mitochondria MrGPA1 Affects Conidiation, Stress Resistance and Virulence of Entomopathogenic Fungus Metarhizium robertsii. Front. Microbiol. 2020, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, D.; Zheng, L.; Chen, L.; Ma, A. Characterization of a G protein α subunit encoded gene from the dimorphic fungus-Tremella fuciformis. Antonie Van Leeuwenhoek 2021, 114, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Kankanamge, D.; Tennakoon, M.; Karunarathne, A.; Gautam, N. G protein gamma subunit a hidden master regulator of GPCR signaling. J. Biol. Chem. 2022, 298, 102618. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.L.; Hall, R.A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 2009, 10, 819–830. [Google Scholar] [CrossRef]
- Yan, H.; Shim, W.B. Characterization of non-canonical G beta-like protein FvGbb2 and its relationship with heterotrimeric G proteins in Fusarium verticillioides. Environ. Microbiol. 2020, 22, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.M.; Tian, D.; Zhang, T.; Liao, L.S.; Li, C.X.; Luo, X.M.; Feng, J.X.; Zhao, S. G protein γ subunit modulates expression of plant-biomass-degrading enzyme genes and mycelial-development-related genes in Penicillium oxalicum. Appl. Microbiol. Biotechnol. 2021, 105, 4675–4691. [Google Scholar] [CrossRef] [PubMed]
- Fisher, I.J.; Outlaw, K.; Muralidharan, K.; Lyon, A. Molecular mechanisms of Gβγ-stimulated activation of PLCβ. J. Pharmacol. Exp. Ther. 2023, 385, 164. [Google Scholar]
- Tang, D.; Tang, X.; Fang, W. New downstream signaling branches of the Mitogen-Activated Protein Kinase cascades identified in the insect pathogenic and plant symbiotic fungus Metarhizium robertsii. Front. Fungal Biol. 2022, 3, 911366. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef]
- Kim, H.R.; Ahn, D.; Jo, J.B.; Chung, K.Y. Effect of α-helical domain of Gi/o α subunit on GDP/GTP turnover. Biochem. J. 2022, 479, 1843–1855. [Google Scholar] [CrossRef]
- Cabrera, I.E.; Oza, Y.; Carrillo, A.J.; Collier, L.A.; Wright, S.J.; Li, L.; Borkovich, K.A. Regulator of G protein signaling proteins control growth, development, and cellulase production in Neurospora crassa. J. Fungi 2022, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wu, H.; He, L.; Qu, J.; Liu, Z.; Wang, Y.; Chen, M.; Huang, B. Role of two G-protein α subunits in vegetative growth, cell wall integrity, and virulence of the entomopathogenic fungus Metarhizium robertsii. J. Fungi 2022, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Pang, Y.T.; Li, W.; Gumbart, J.C.; Kelley, J.; Torres, M. N-terminal intrinsic disorder is an ancestral feature of Gγ subunits that influences the balance between different Gβγ signaling axes in yeast. J. Biol. Chem. 2023, 299, 104947. [Google Scholar] [CrossRef]
- Yun, C.W.; Tamaki, H.; Nakayama, R.; Yamamoto, K.; Kumagai, H. G-Protein Coupled Receptor from Yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1997, 240, 287–292. [Google Scholar] [CrossRef]
- Grice, C.M.; Bertuzzi, M.; Bignell, E.M. Receptor-mediated signaling in Aspergillus fumigatus. Front. Microbiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Gehrke, A.; Heinekamp, T.; Jacobsen, I.D.; Brakhage, A.A. Heptahelical receptors GprC and GprD of Aspergillus fumigatus are essential regulators of colony growth, hyphal morphogenesis, and virulence. Appl. Environ. Microbiol. 2010, 76, 3989–3998. [Google Scholar] [CrossRef]
- Chung, K.S.; Won, M.; Lee, S.B.; Jang, Y.J.; Hoe, K.L.; Kim, D.U.; Lee, J.W.; Kim, K.W.; Yoo, H.S. Isolation of a Novel Gene from Schizosaccharomyces pombe: Stm1 Encoding a Seven-transmembrane Loop Protein that May Couple with the Heterotrimeric Gα2 Protein Gpa2. J. Biol. Chem. 2001, 276, 40190–40201. [Google Scholar] [CrossRef]
- Lee, Y.; Nishizawa, T.; Yamashita, K.; Ishitani, R.; Nureki, O. Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter. Nat. Commun. 2015, 6, 6112. [Google Scholar] [CrossRef]
- Liu, B.; Du, H.; Rutkowski, R.; Gartner, A. Europe PMC Funders Group LAAT-1 is the Lysosomal Lysine / Arginine Transporter that Maintains Amino Acid Homeostasis. Science 2013, 337, 351–354. [Google Scholar] [CrossRef]
- Welton, R.M.; Hoffman, C.S. Glucose monitoring in fission yeast via the gpa2 Gα, the git5 Gβ, and the git3 putative glucose receptor. Genetics 2000, 156, 513–521. [Google Scholar] [CrossRef]
- Johnston, C.A.; Siderovski, D.P. Receptor-mediated activation of heterotrimeric G-proteins: Current structural insights. Mol. Pharmacol. 2007, 72, 219–230. [Google Scholar] [CrossRef]
- Moussatche, P.; Lyons, T.J. Non-genomic progesterone signalling and its non-canonical receptor. Biochem. Soc. Trans. 2012, 40, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Hinterdobler, W.; Beier, S.; Monroy, A.A.; Berger, H.; Dattenböck, C.; Schmoll, M. The G-protein coupled receptor GPR8 regulates secondary metabolism in Trichoderma reesei. Front. Bioeng. Biotechnol. 2020, 8, 558996. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.; Brunk, M.; Avalos, J.; Terpitz, U. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Sci. Rep. 2015, 5, 7798. [Google Scholar] [CrossRef]
- DeZwaan, T.M.; Carroll, A.M.; Valent, B.; Sweigard, J.A. Magnaporthe grisea Pth11p Is a Novel Plasma Membrane Protein That Mediates Appressorium Differentiation in Response to Inductive Substrate Cues. Plant Cell 1999, 11, 2013–2030. [Google Scholar] [CrossRef] [PubMed]
- Muthumeenakshi, S.; Sreenivasaprasad, S.; Rogers, C.W.; Challen, M.P.; Whipps, J.M. Analysis of cDNA transcripts from Coniothyrium minitans reveals a diverse array of genes involved in key processes during sclerotial mycoparasitism. Fungal Genet. Biol. 2007, 44, 1262–1284. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Tan, Y.H.; Ramanujam, R.; Naqvi, N.I. Structure–function analyses of the Pth11 receptor reveal an important role for CFEM motif and redox regulation in rice blast. New Phytol. 2017, 214, 330–342. [Google Scholar] [CrossRef]
- Martín, J.F.; van den Berg, M.A.; Ver Loren van Themaat, E.; Liras, P. Sensing and transduction of nutritional and chemical signals in filamentous fungi: Impact on cell development and secondary metabolites biosynthesis. Biotechnol. Adv. 2019, 37, 107392. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, I.E.; Pacentine, I.V.; Lim, A.; Guerrero, N.; Krystofova, S.; Li, L.; Michkov, A.V.; Servin, J.A.; Ahrendt, S.R.; Carrillo, A.J.; et al. Global analysis of predicted G Protein−Coupled receptor genes in the filamentous fungus Neurospora crassa. G3 2015, 5, 2729–2743. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, H.; Wei, G.; Wang, G.; Li, F.; Wang, S. In vivo gene expression profiling of the entomopathogenic fungus Beauveria bassiana elucidates its infection stratagems in Anopheles mosquito. Sci. China Life Sci. 2017, 60, 839–851. [Google Scholar] [CrossRef]
- Choi, H.Y.; Saha, S.K.; Kim, K.; Kim, S.; Yang, G.M.; Kim, B.; Kim, J.H.; Cho, S.G. G protein-coupled receptors in stem cell maintenance and somatic reprogramming to pluripotent or cancer stem cells. BMB Rep. 2015, 48, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Martínez-Espinoza, A.D. Las Vías de Transducción de Señales en la Patogénesis y la Morfogénesis de Hongos: Los Casos de Ustilago maydis y Magnaporthe grisea. Rev. Mex. Fitopatol. 2000, 18, 55–60. [Google Scholar]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuo, C.Y.; Tay, R.J.; Lin, H.C.; Juan, S.C.; Vidal-Diez de Ulzurrun, G.; Chang, Y.C.; Hoki, J.; Schroeder, F.C.; Hsueh, Y.P. The nematode-trapping fungus Arthrobotrys oligospora detects prey pheromones via G protein-coupled receptors. Nat. Microbiol. 2024, 9, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Orejas, M.; Espeso, E.A.; Tilburn, J.; Sarkar, S.; Arst, H.N.; Peñalva, M.A. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 1995, 9, 1622–1632. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Arst, H.N. Regulation of Gene Expression by Ambient pH in Filamentous Fungi and Yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 426–446. [Google Scholar] [CrossRef]
- Su, S.S.; Mitchell, A.P. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics 1993, 133, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Montelongo, J.A.; Ruiz-Herrera, J. Identification of a novel member of the pH responsive pathway Pal/Rim in Ustilago maydis. J. Basic Microbiol. 2019, 59, 14–23. [Google Scholar] [CrossRef]
- Cruz-Magalhães, V.; Nieto-Jacobo, M.F.; Rostás, M.; Echaide-Aquino, J.F.; Esquivel-Naranjo, E.U.; Stewart, A.; Loguercio, L.L.; Mendoza-Mendoza, A. Histidine kinase two-component response regulators Ssk1, Skn7, and Rim15 differentially control growth, developmental and volatile organic compounds emissions as stress responses in Trichoderma atroviride. Curr. Res. Microb. Sci. 2022, 3, 100139. [Google Scholar] [CrossRef]
- Cervantes-Montelongo, J.A. Estudio del mecanismo de transmisión de la señal de pH en Ustilago maydis. Ph.D. Dissertation, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Irapuato, Mexico, 2017. [Google Scholar]
- Cervantes-Chávez, J.A.; Ortiz-Castellanos, L.; Tejeda-Sartorius, M.; Gold, S.; Ruiz-Herrera, J. Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet. Biol. 2010, 47, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Min, B.; Jang, Y.; Kim, J.; Lipzen, A.; Sharma, A.; Andreopoulos, B.; Johnson, J.; Riley, R.; Spatafora, J.W.; et al. Comprehensive genomic and transcriptomic analysis of polycyclic aromatic hydrocarbon degradation by a mycoremediation fungus Dentipellis sp. KUC8613. Appl. Microbiol. Biotechnol. 2019, 103, 8145–8155. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Liu, Z.; Yu, Y.; Wu, Y.; Jin, Y.; Yang, M.; Ma, L. Codon-Optimized Rhodotorula glutinis PAL Expressed in Escherichia coli With Enhanced Activities. Front. Bioeng. Biotechnol. 2020, 8, 610506. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-J.; Hou, J.; Zhang, H.; Lei, J.-H.; Lin, H.-Y.; Ding, J.-L.; Feng, M.; Ying, S. Systematic contributions of CFEM domain-containing proteins to iron acquisition are essential for interspecies interaction of the filamentous pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2022, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Yu, S.-F.; Wang, C.-L.; Wang, L. CAMP signalling pathway in biocontrol fungi. Curr. Issues Mol. Biol. 2022, 44, 2622–2634. [Google Scholar] [CrossRef] [PubMed]
- Krol, E.; Yau, H.C.L.; Lechner, M.; Schäper, S.; Bange, G.; Vollmer, W.; Becker, A. Tol-Pal system and Rgs proteins interact to promote unipolar growth and cell division in Sinorhizobium meliloti. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Sieber, B.; Coronas-Serna, J.M.; Martin, S.G. A focus on yeast mating: From pheromone signaling to cell-cell fusion. Semin. Cell Dev. Biol. 2022, 133, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Abah, F.; Kuang, Y.; Biregeya, J.; Abubakar, Y.S.; Ye, Z.; Wang, Z. Mitogen-activated protein kinases SvPmk1 and SvMps1 are critical for abiotic stress resistance, development, and pathogenesis of Sclerotiophoma versabilis. J. Fungi 2023, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wen, D.; Yang, Y.; Qiu, X.; Xiong, D.; Tian, C. Comparative transcriptomic analysis of MAPK-mediated regulation of pathogenicity, stress responses and development in Cytospora chrysosperma. Phytopathology 2023, 113, 239–251. [Google Scholar] [CrossRef]
- Lv, B.; Fan, L.; Li, S.; Sun, M. Screening and characterisation of proteins interacting with the mitogen-activated protein kinase Crmapk in the fungus Clonostachys chloroleuca. Sci. Rep. 2022, 12, 9997. [Google Scholar] [CrossRef]
- Fernandes, T.R.; Mariscal, M.; Serrano, A.; Segorbe, D.; Fernández-Acero, T.; Martín, H.; Turrà, D.; Di Pietro, A. Cytosolic pH controls fungal MAPK signaling and pathogenicity. mBio 2023, 14, e0028523. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Y.; Wang, Y.; Li, C.; Bian, Z.; Zhang, X.; Liu, H.; Xu, J.R.; Jiang, C. Deletion of all three MAP kinase genes results in severe defects in stress responses and pathogenesis in Fusarium graminearum. Stress Biol. 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.R.; Mariscal, M.; Serrano, A.; Segorbe, D.; Fernández-Acero, T.; Martín, H.; Turrà, D.; Di Pietro, A. Cytosolic pH controls fungal MAPK signaling and pathogenicity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Wang, Z.; Liao, L.; Zheng, Y.; Liu, Y. Mechanism of HOG-MAPK pathway in regulating mycotoxins formation under environmental stresses. Sheng Wu Gong Cheng Xue Bao 2022, 38, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Schalamun, M.; Beier, S.; Hinterdobler, W.; Wanko, N.; Schinnerl, J.; Brecker, L.; Engl, D.E.; Schmoll, M. MAP-kinases regulate secondary metabolism, sexual development, and light-dependent cellulase regulation in Trichoderma reesei. Sci. Rep. 2023, 13, 1912. [Google Scholar] [CrossRef] [PubMed]
- Manfiolli, A.O.; Siqueira, F.S.; dos Reis, T.F.; Van Dijck, P.; Schrevens, S.; Hoefgen, S.; Föge, M.; Straßburger, M.; de Assis, L.J.; Heinekamp, T.; et al. Mitogen-activated protein kinase cross-talk interaction modulates the production of melanins in aspergillus fumigatus. mBio 2019, 10, e00215-19. [Google Scholar] [CrossRef]
- González-Rubio, G.; Fernández-Acero, T.; Martín, H.; Molina, M. Mitogen-activated protein kinase phosphatases (MKPs) in fungal signaling: Conservation, function and regulation. Int. J. Mol. Sci. 2019, 20, 1709. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Xu, J.R. Assays for MAP kinase activation in Magnaporthe oryzae and other plant pathogenic fungi. Methods Mol. Biol. 2018, 1848, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Frawley, D.; Karahoda, B.; Sarikaya Bayram, Ö.; Bayram, Ö. The HamE scaffold positively regulates MpkB phosphorylation to promote development and secondary metabolism in Aspergillus nidulans. Sci. Rep. 2018, 8, 16588. [Google Scholar] [CrossRef]
- Carreras-Villaseñor, N.; Sánchez-Arreguín, J.A.; Herrera-Estrella, A.H. Trichoderma: Sensing the environment for survival and dispersal. Microbiology 2012, 158, 3–16. [Google Scholar] [CrossRef]
- Omann, M.R.; Lehner, S.; Escobar Rodríguez, C.; Brunner, K.; Zeilinger, S. The seven-transmembrane receptor Gpr1 governs processes relevant for the antagonistic interaction of Trichoderma atroviride with its host. Microbiology 2012, 158, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Latha, J.; Hadar, R.; Horwitz, B.A. Role of two G-protein alpha subunits, TgaA and TgaB, in the antagonism of plant pathogens by Trichoderma virens. Appl. Environ. Microbiol. 2004, 70, 542–549. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Kale, S.P. cAMP signalling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology 2007, 153, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Qu, W.; Liao, J.B.; Chen, L.; Cao, Y.J.; Li, H.L. Jiangtangjing ameliorates type 2 diabetes through effects on the gut microbiota and cAMP/PKA pathway. Tradit. Med. Res. 2022, 7, 7. [Google Scholar] [CrossRef]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Sakamoto, K.; Abe, K.; Gomi, K. Signaling pathways for stress responses and adaptation in Aspergillus species: Stress biology in the post-genomic era. Biosci. Biotechnol. Biochem. 2016, 80, 1667–1680. [Google Scholar] [CrossRef]
- El-Defrawy, M.M.H.; Hesham, A.E. G-protein-coupled receptors in fungi. In Fungal Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 37–126. [Google Scholar]
- Schmoll, M.; Hinterdobler, W. Tools for adapting to a complex habitat: G-protein coupled receptors in Trichoderma. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–97. [Google Scholar]
- Nikolaou, E.; Agrafioti, I.; Stumpf, M.; Quinn, J.; Stansfield, I.; Brown, A.J. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol. Biol. 2009, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Vande Zande, P.; Zhou, X.; Selmecki, A. The dynamic fungal genome: Polyploidy, aneuploidy, and copy number variation in response to stress. Annu. Rev. Microbiol. 2023, 77, 341–361. [Google Scholar] [CrossRef]
- Poltronieri, P.; Reca, I.B.; De Domenico, S.; Santino, A. Plant-microbe interactions in developing environmental stress resistance in plants. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Springer: Singapore, 2020; pp. 583–602. [Google Scholar]
- Xiao, W.; Zhang, J.; Huang, J.; Xin, C.; Li, M.J.; Song, Z. Response and regulatory mechanisms of heat resistance in pathogenic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 5415–5431. [Google Scholar] [CrossRef]
- Heaney, H.; Laing, J.; Paterson, L.; Walker, A.W.; Gow, N.A.R.; Johnson, E.M.; MacCallum, D.M.; Brown, A.J.P. The environmental stress sensitivities of pathogenic Candida species including Candida auris and implications for their spread in the hospital setting. Med. Mycol. 2020, 58, 744–755. [Google Scholar] [CrossRef]
- Yi, W.; Ziyu, Z.; Feng-Lan, L.; Zhang, S.-H. Molecular basis of stress-tolerant genes in extreme microorganisms. In Beneficial Microorganisms in Agriculture; Springer: Singapore, 2022; pp. 293–306. [Google Scholar]
- Shuryak, I.; Tkavc, R.; Matrosova, V.Y.; Volpe, R.P.; Grichenko, O.; Klimenkova, P.; Conze, I.H.; Balygina, I.A.; Gaidamakova, E.K.; Daly, M.J. Chronic gamma radiation resistance in fungi correlates with resistance to chromium and elevated temperatures but not with resistance to acute irradiation. Sci. Rep. 2019, 9, 11361. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.S.; McDonald, B.A.; Palma-Guerrero, J. A transcription factor and a phosphatase regulate temperature-dependent morphogenesis in a fungal plant pathogen. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kubová, Z.; Pagáč, T.; Víglaš, J.; Olejníková, P. Detoxification and adaptation mechanisms of Trichoderma atroviride to antifungal agents. Acta Chim. Slovaca 2022, 15, 85–96. [Google Scholar] [CrossRef]
- Bodnár, V.; Király, A.; Orosz, E.; Miskei, M.; Emri, T.; Karányi, Z.; Leiter, É.; de Vries, R.P.; Pócsi, I. Species-specific effects of the introduction of Aspergillus nidulans gfdB in osmophilic aspergilli. Appl. Microbiol. Biotechnol. 2023, 107, 2423–2436. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Zhu, G.; Yao, R.; Chen, X.; Liu, L. Cg Hog1-mediated Cg Rds2 phosphorylation alters glycerophospholipid composition to coordinate osmotic stress in Candida glabrata. Appl. Environ. Microbiol. 2019, 85, e02822-18. [Google Scholar] [CrossRef]
- Saldaña, C.; Villava, C.; Ramírez-Villarreal, J.; Morales-Tlalpan, V.; Campos-Guillen, J.; Chávez-Servín, J.; García-Gasca, T. Rapid and reversible cell volume changes in response to osmotic stress in yeast. Braz. J. Microbiol. 2021, 52, 895–903. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, X.; Li, M.; Si, H.; Zhou, Q.; Liu, X.; Fan, Y.; Zhang, X.; Han, J.; Gu, S.; et al. Effect of Osmotic Stress on the Growth, Development, and Pathogenicity of Setosphaeria turcica. Front. Microbiol. 2021, 12, 706349. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.H.; Feng, M.G.; Keyhani, N.O. A carbon responsive G-protein coupled receptor modulates broad developmental and genetic networks in the entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2013, 15, 2902–2921. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Crosstalk between the er Stress Pathway and Osmotic Stress in S. cerevisiae. Ph.D. Dissertation, Durham University, Durham, UK, 2011. Available online: https://etheses.dur.ac.uk/3435/ (accessed on 5 February 2024).
- Parmar, J.H.; Bhartiya, S.; Venkatesh, K.V. A model-based study delineating the roles of the two signaling branches of Saccharomyces cerevisiae Sho1 and Sln1 during adaptation to osmotic stress. Phys. Biol. 2009, 6, 036019. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, T.; Zhuo, J.; Zhan, C.; Zhang, F.; Linhardt, R.J.; Bai, Z.; Yang, Y. MAPK/HOG signaling pathway induced stress-responsive damage repair is a mechanism for Pichia pastoris to survive from hyperosmotic stress. J. Chem. Technol. Biotechnol. 2021, 96, 412–422. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Weng, Q.; Lin, S.; Wang, H.; Li, L.; Fuchs, B.B.; Tan, X.; Mylonakis, E. SPT20 regulates the Hog1-MAPK pathway and is involved in candida albicans response to hyperosmotic stress. Front. Microbiol. 2020, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Plemenitaš, A. Sensing and responding to hypersaline conditions and the HOG signal transduction pathway in fungi isolated from hypersaline environments: Hortaea werneckii and Wallemia ichthyophaga. Preprints 2021. [Google Scholar] [CrossRef] [PubMed]
- Yaakoub, H.; Mina, S.; Marot, A.; Papon, N.; Calenda, A.; Bouchara, J.-P. A stress hub in Scedosporium apiospermum: The High Osmolarity Glycerol (HOG) pathway. Kinases Phosphatases 2022, 1, 4–13. [Google Scholar] [CrossRef]
- Sánchez, N.S.; Calahorra, M.; González, J.; Defosse, T.; Papon, N.; Peña, A.; Coria, R. Contribution of the mitogen-activated protein kinase Hog1 to the halotolerance of the marine yeast Debaryomyces hansenii. Curr. Genet. 2020, 66, 1135–1153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, L.; Zhong, S.; Wei, X.; Zhao, Q.; Pan, Q.; Kang, Z.; Liu, J. A Cu-only superoxide dismutase from stripe rust fungi functions as a virulence factor deployed for counter defense against host-derived oxidative stress. Environ. Microbiol. 2020, 22, 5309–5326. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; McDonald, B.A.; Palma-Guerrero, J. Genetic architecture of oxidative stress tolerance in the fungal wheat pathogen Zymoseptoria tritici. bioRxiv 2020. [Google Scholar] [CrossRef]
- Breitenbach, M.; Weber, M.; Rinnerthaler, M.; Karl, T.; Breitenbach-Koller, L. Oxidative stress in fungi: Its function in signal transduction, interaction with plant hosts and lignocellulose degradation. Biomolecules 2015, 5, 318–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Li, Y.; Bi, Y.; Mao, R.; Yang, Y.; Jiang, Q.; Prusky, D. Cellular responses required for oxidative stress tolerance of the necrotrophic fungus Alternaria alternata, causal agent of pear black spot. Microorganisms 2022, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated alleviation of drought stress via non-enzymatic antioxidants: A meta-analysis. Plants 2022, 11, 2448. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Shi, H.; Ballauff, J.; Schnitzler, J.P.; Polle, A. Reactive oxygen species (ROS) in mycorrhizal fungi and symbiotic interactions with plants. In Oxidative Stress Response in Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–275. [Google Scholar]
- Herrero de Dios, C.; Roman, E.; Alonso Monge, R.; Pla, J. The role of MAPK signal transduction pathways in the response to oxidative stress in the fungal pathogen candida albicans: Implications in virulence. Curr. Protein Pept. Sci. 2010, 11, 693–703. [Google Scholar] [CrossRef]
- González, J.; Castillo, R.; García-Campos, M.A.; Noriega-Samaniego, D.; Escobar-Sánchez, V.; Romero-Aguilar, L.; Alba-Lois, L.; Segal-Kischinevzky, C. Tolerance to Oxidative Stress in Budding Yeast by Heterologous Expression of Catalases A and T from Debaryomyces hansenii. Curr. Microbiol. 2020, 77, 4000–4015. [Google Scholar] [CrossRef]
- Briones-Martin-Del-Campo, M.; Orta-Zavalza, E.; Juarez-Cepeda, J.; Gutierrez-Escobedo, G.; Cañas-Villamar, I.; Castaño, I.; De Las Peñas, A. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Rev. Iberoam. Micol. 2014, 31, 67–71. [Google Scholar] [CrossRef]
- Wesener, F.; Rillig, M.C.; Tietjen, B. Heat stress can change the competitive outcome between fungi: Insights from a modelling approach. Oikos 2023, 2023, e09377. [Google Scholar] [CrossRef]
- Veri, A.O.; Robbins, N.; Cowen, L.E. Regulation of the heat shock transcription factor Hsf1 in fungi: Implications for temperature-dependent virulence traits. FEMS Yeast Res. 2018, 18, foy041. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.J.; Cao, P.F.; Chen, T.X.; Zhang, G.; Shi, L.; Jiang, A.L.; Zhao, M.W. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef]
- Fabri, J.H.T.M.; Rocha, M.C.; Fernandes, C.M.; Persinoti, G.F.; Ries, L.N.A.; Cunha, A.F.; da Goldman, G.H.; Del Poeta, M.; Malavazi, I. The heat shock transcription factor HsfA is essential for thermotolerance and regulates cell wall integrity in Aspergillus fumigatus. Front. Microbiol. 2021, 12, 656548. [Google Scholar] [CrossRef]
- Dev, R. Exploring small heat shock proteins (sHSPs) for targeting drug resistance in candida albicans and other pathogenic fungi. J. Pure Appl. Microbiol. 2021, 15, 20–28. [Google Scholar] [CrossRef]
- Fabri, J.H.T.M.; Rocha, M.C.; Fernandes, C.M.; Campanella, J.E.M.; Cunha, A.F.; da Del Poeta, M.; Malavazi, I. The heat shock transcription factor HsfA plays a role in membrane lipids biosynthesis, connecting thermotolerance and unsaturated fatty acid metabolism in Aspergillus fumigatus. Microbiol. Spectr. 2023, 11, e0162723. [Google Scholar] [CrossRef]
- McNamara-Bordewick, N.K.; McKinstry, M.; Snow, J.W. Robust transcriptional response to heat shock impacting diverse cellular processes despite lack of heat shock factor in Microsporidia. mSphere 2019, 4, e00219-19. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, P.; Chen, Y.; Lu, Y.; Wang, C. MrSkn7 controls sporulation, cell wall integrity, autolysis, and virulence in Metarhizium robertsii. Eukaryot. Cell 2015, 14, 396–405. [Google Scholar] [CrossRef]
- Hussain, M.; Hamid, M.; Wang, N.; Bin, L.; Xiang, M.; Liu, X. The transcription factor SKN7 regulates conidiation, thermotolerance, apoptotic-like cell death, and parasitism in the nematode endoparasitic fungus Hirsutella minnesotensis. Sci. Rep. 2016, 6, 30047. [Google Scholar] [CrossRef]
- Zhou, G.; Ying, S.H.; Hu, Y.; Fang, X.; Feng, M.G.; Wang, J. Roles of three HSF domain-containing proteins in mediating heat-shock protein genes and sustaining asexual cycle, stress tolerance, and virulence in Beauveria bassiana. Front. Microbiol. 2018, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, S.; Bertolini, M.C. Functional diversity in the pH signaling pathway: An overview of the pathway regulation in Neurospora crassa. Curr. Genet. 2018, 64, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, L.; Wang, S.; Wang, Y.; Zhu, Y.; Jiang, J.; Li, R. Acidic/alkaline stress mediates responses to azole drugs and oxidative stress in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e0199921. [Google Scholar] [CrossRef] [PubMed]
- Picazo, I.; Etxebeste, O.; Requena, E.; Garzia, A.; Espeso, E.A. Defining the transcriptional responses of Aspergillus nidulans to cation/alkaline pH stress and the role of the transcription factor SltA. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, G.; Ding, Q.; Zhou, P.; Liu, L.; Chen, X. Cg Cmk1 activates Cg Rds2 to resist low-pH stress in candida glabrata. Appl. Environ. Microbiol. 2020, 86, e00302-20. [Google Scholar] [CrossRef]
- Csillag, K.; Emri, T.; Rangel, D.E.N.; Pócsi, I. pH-dependent effect of Congo Red on the growth of Aspergillus nidulans and Aspergillus niger. Fungal Biol. 2023, 127, 1180–1186. [Google Scholar] [CrossRef]
- Jin, Y.; Qin, S.; Gao, H.; Zhu, G.; Wang, W.; Zhu, W.; Wang, Y. An anti-HBV anthraquinone from aciduric fungus Penicillium sp. OUCMDZ-4736 under low pH stress. Extremophiles 2017, 22, 39–45. [Google Scholar] [CrossRef]
- Brown, H.E.; Pianalto, K.M.; Fernandes, C.M.; Mueller, K.D.; Del Poeta, M.; Alspaugh, J.A. Internalization of the host alkaline pH signal in a fungal pathogen. bioRxiv 2020. [Google Scholar] [CrossRef]
- Buensanteai, N.; Prathuangwong, S. The gene expression of the Tvmfs transporter and defense enzyme activities from Trichoderma harzianum response to pH stress. Afr. J. Microbiol. Res. 2012, 6, 944–952. [Google Scholar] [CrossRef]
- Pianalto, K.M.; Ost, K.S.; Brown, H.E.; Alspaugh, J.A. Characterization of additional components of the environmental pH-sensing complex in the pathogenic fungus Cryptococcus neoformans. J. Biol. Chem. 2018, 293, 9995–10008. [Google Scholar] [CrossRef] [PubMed]
- Höft, M.A.; Duvenage, L.; Hoving, J.C. Key thermally dimorphic fungal pathogens: Shaping host immunity. Open Biol. 2022, 12, 210219. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants and insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, R.; Wan, Z.; Liu, W.; Wang, X.; Qiao, J.; Wang, D.; Bulmer, G.; Calderone, R. The effects of temperature, pH, and salinity on the growth and dimorphism of Penicillium marneffei. Med. Mycol. 2007, 45, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Karuppayil, S.M.; Szaniszlo, P.J. Calcium regulates in vitro dimorphism in chromoblastomycotic fungi: Calcium reguliert in vitro den Dimorphismus von Chromoblastomykose-Erregern. Mycoses 1993, 36, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.F.; McEwen, J.G.; Clay, O.K.; Cuomo, C.A. Genome analysis reveals evolutionary mechanisms of adaptation in systemic dimorphic fungi. medRxiv 2017. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.A.; Baeza, L.C.; Almeida-Paes, R.; Bailão, A.M.; Borges, C.L.; Guimarães, A.J.; Soares, C.M.A.; Zancopé-Oliveira, R.M. Comparative proteomic analysis of Histoplasma capsulatum yeast and mycelium reveals differential metabolic shifts and cell wall remodeling processes in the different morphotypes. Front. Microbiol. 2021, 12, 640931. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.V.; de Barros, Y.N.; Segura, W.D.; Chaves, A.F.A.; Jannuzzi, G.P.; Ferreira, K.S.; Xander, P.; Batista, W.L. The role of dimorphism regulating histidine kinase (Drk1) in the pathogenic fungus Paracoccidioides brasiliensis cell wall. J. Fungi 2021, 7, 1014. [Google Scholar] [CrossRef]
- Farh, M.E.; Abdellaoui, N.; Seo, J.A. pH changes have a profound effect on gene expression, hydrolytic enzyme production, and dimorphism in Saccharomycopsis fibuligera. Front. Microbiol. 2021, 12, 672661. [Google Scholar] [CrossRef]
- Wang, B.; Han, Z.; Gong, D.; Xu, X.; Li, Y.; Sionov, E.; Prusky, D.; Bi, Y.; Zong, Y. The pH signalling transcription factor PacC modulate growth, development, stress response, and pathogenicity of Trichothecium roseum. Environ. Microbiol. 2022, 24, 1608–1621. [Google Scholar] [CrossRef]
- Gazengel, K.; Lebreton, L.; Lapalu, N.; Amselem, J.; Guillerm-Erckelboudt, A.Y.; Tagu, D.; Daval, S. pH effect on strain-specific transcriptomes of the take-all fungus. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.P.C.; Brancini, G.T.P.; de Castro, P.A.; Valero, C.; Ferreira Filho, J.A.; Silva, L.P.; Rocha, M.C.; Malavazi, I.; Pontes, J.G.D.M.; Fill, T.; et al. Aspergillus fumigatus G-protein coupled receptors GprM and GprJ are important for the regulation of the cell wall integrity pathway, secondary metabolite production, and virulence. mBio 2020, 11, e02458-20. [Google Scholar] [CrossRef] [PubMed]
- Garud, A.; Carrillo, A.J.; Collier, L.A.; Ghosh, A.; Kim, J.D.; Lopez-Lopez, B.; Ouyang, S.; Borkovich, K.A. Genetic relationships between the RACK1 homolog cpc-2 and heterotrimeric G protein subunit genes in Neurospora crassa. PLoS ONE 2019, 14, e0223334. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Sridhar, P.S.; Blackman, C.; Foote, S.J.; Allingham, J.S.; Subramaniam, R.; Loewen, M.C. Fusarium graminearum Ste3 G-protein coupled receptor: A mediator of hyphal chemotropism and pathogenesis. mSphere 2022, 7, e0045622. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Shang, Y.; Tang, G.; Wang, C. Identification of a key G-protein coupled receptor in mediating appressorium formation and fungal virulence against insects. Sci. China Life Sci. 2021, 64, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Dilks, T.; Halsey, K.; De Vos, R.P.; Hammond-Kosack, K.E.; Brown, N.A. Non-canonical fungal G-protein coupled receptors promote Fusarium head blight on wheat. PLoS Pathog. 2019, 15, e1007666. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, Z.; Jiang, C.; Xu, J.-R. Regulation of biotic interactions and responses to abiotic stresses by MAP kinase pathways in plant pathogenic fungi. Stress Biol. 2021, 1, 5. [Google Scholar] [CrossRef]
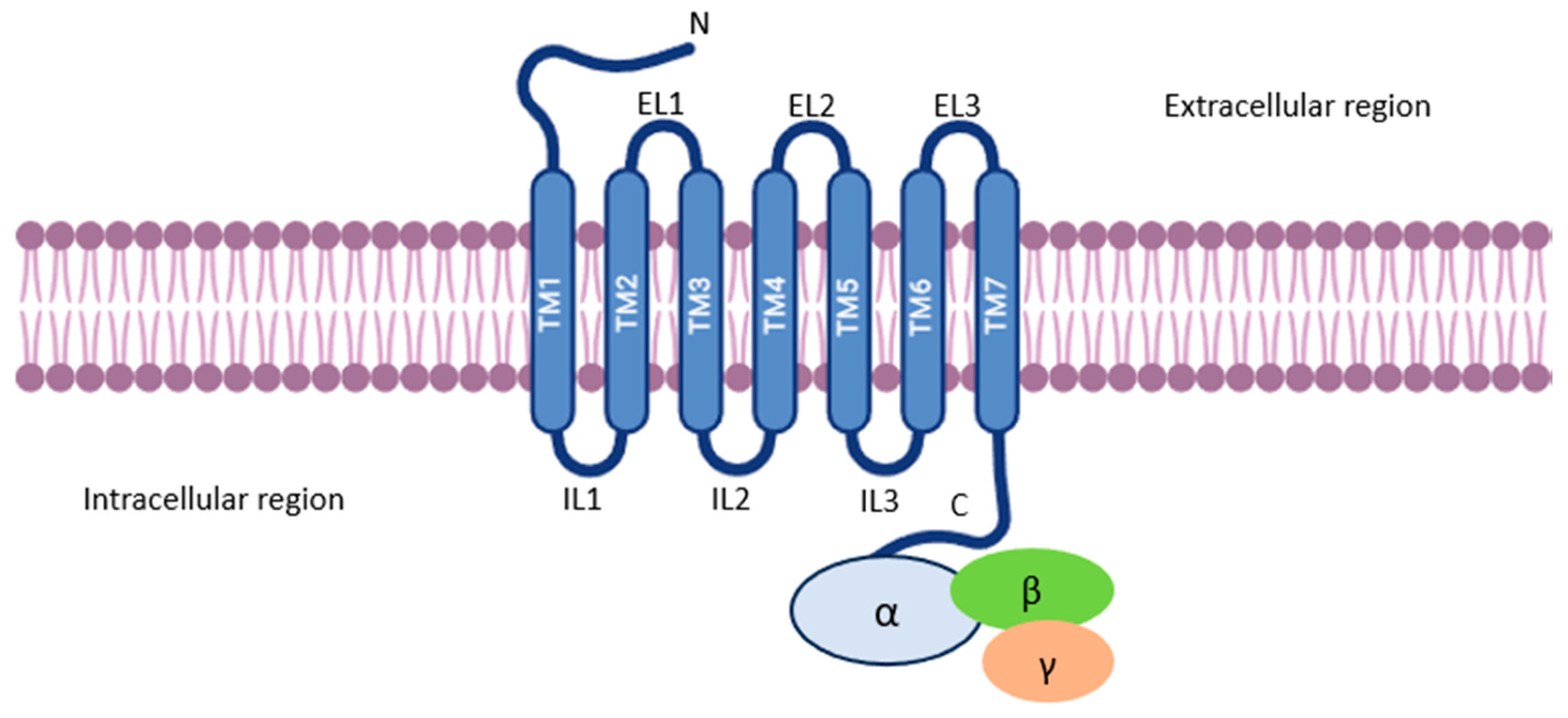

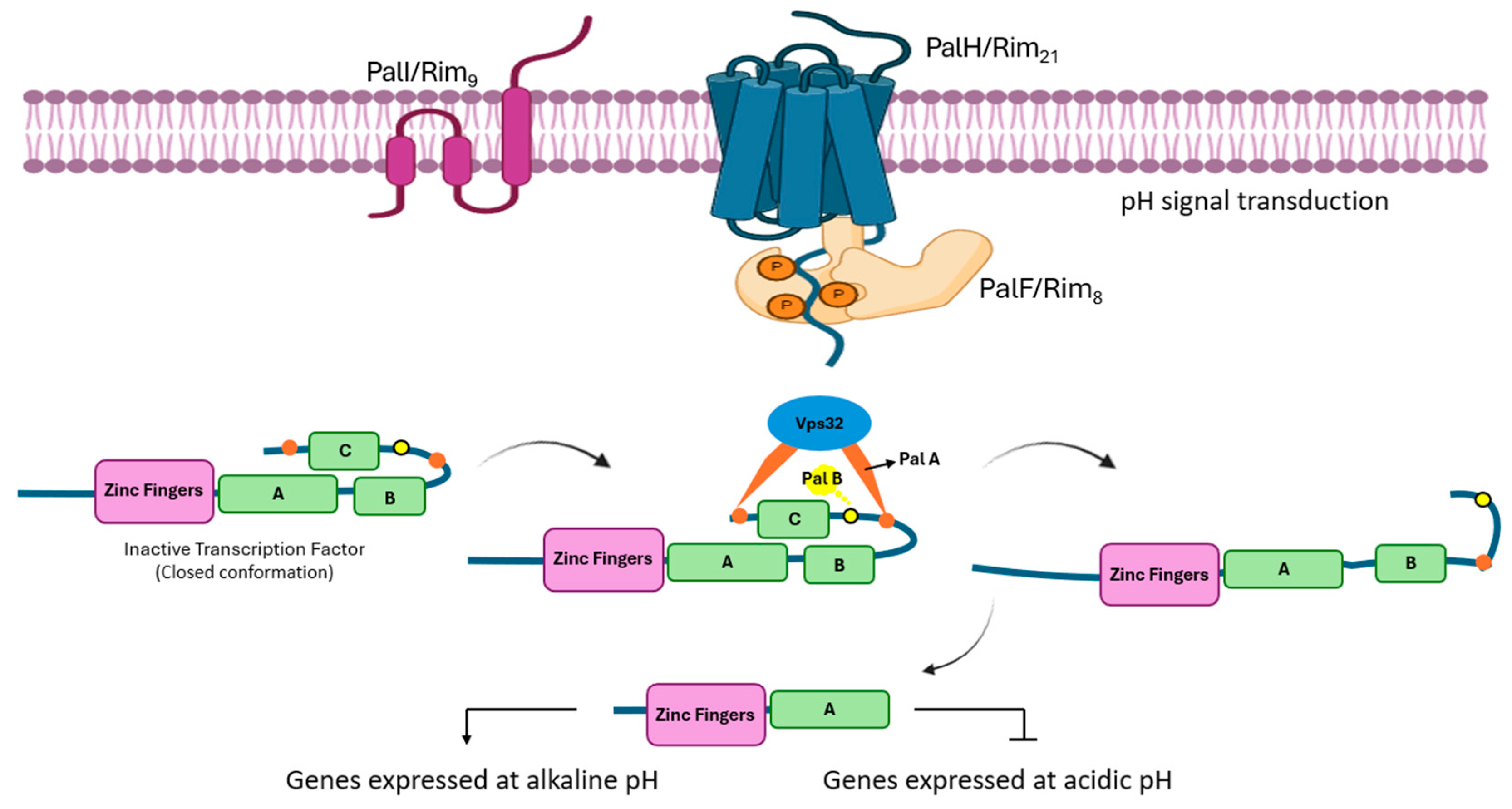
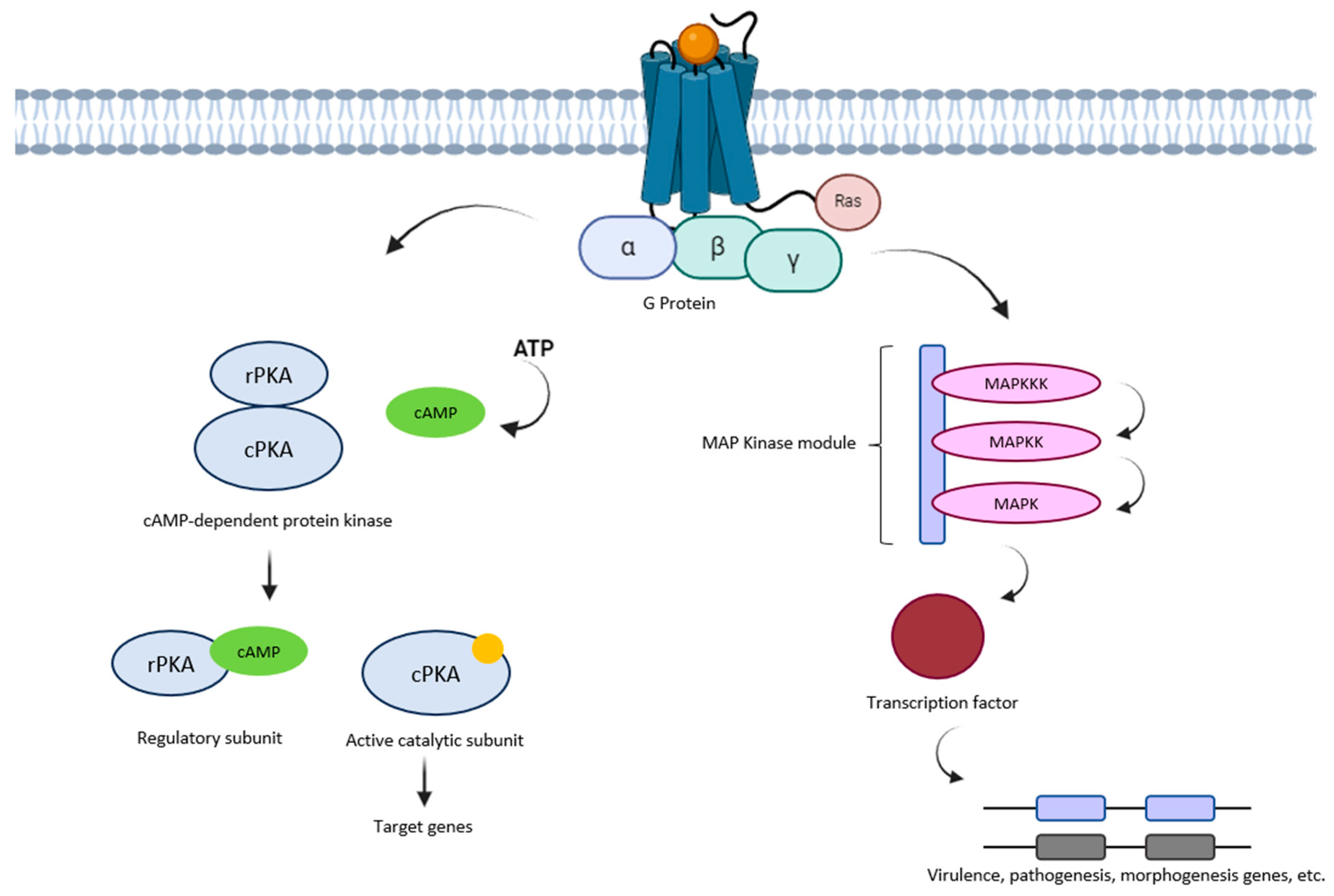
| Class | Description | Characteristics | Examples | Ref. |
|---|---|---|---|---|
| I and II | Pheromone Receptors | -Similar to Ste2 and Ste3 receptors, sensing α-factor and a-factor, respectively. -Expressed on a-cells and α-cells. | GprA, GprB (Aspergillus fumigatus) | [36,37] |
| III | Carbon Receptors | Involves the third intracellular loop and cytoplasmic tail in G-protein binding and receptor desensitization. | GprC, GprD (A. fumigatus) | [36,38] |
| IV | Putative Nitrogen Receptors | -Characterized by a PQ loop containing two repeats spanning two TM helices. -Possibly involved in transporting cysteine and cationic amino acids. | GprF. GprG, GprJ (A. fumigatus) | [39,40,41] |
| V | Putative Carbon and Amino Acid Receptors (cAMP receptor-like) | A unique organization with a Git3-carbon-sensing domain from S. pombe, CrlA-cAMP receptor domain from Dictyostelium discoideum, and extended cytoplasmic tail. | GprV, Gpr H (Aspergillus nidulans) | [12,13,18,42] |
| VI | RGS-Domain-Containing Receptors | Contain an intracellular RGS domain. | [43] | |
| VII | Orthologues of MG00532 with Weak Similarity to Rat Growth-Hormone-Releasing Factor | Show limited similarity to the rat growth hormone-releasing hormone receptor. | GprM (Aspergillus nidulans), Gpr8 (Trichoderma reesei) | [18,32,44,45] |
| VIII | Mammalian Progesterone Receptor-Like Receptors | Resemble mPRs that activate inhibitory G-proteins, suggesting their role as GPCRs | MoRgs7 (Magnaphorthe oryzae) | [32,44] |
| IX | Microbial Opsin Receptors | Functionally conserved, with FfCarO opsin receptor as a green-light-driven proton pump influencing hyphal development. | NopA (Aspergillus fumigatus) | [37,46] |
| X | Family Pth11 Receptors | The CFEM domain of the M. oryzae class X Pth11 receptor is critical for hydrophobic sensing | BBA_00828, BBA_05185 (B. bassiana), CmEST-463 (C. minitans) | [47,48,49,50] |
| Class | Description | Characteristics | Examples | Ref. |
|---|---|---|---|---|
| VI | GrpK-like/RGS Domain Receptors | Contain RGS domains, suggesting potential regulatory functions. | GprK (Aspergillus fumigatus) | [37] |
| VII | Rat Growth Hormone-Releasing Factor Receptor-Like Receptors | Exhibit similarities to rat growth hormone-releasing factor receptors. | GprM (Aspergillus fumigatus) | [37] |
| VIII | mPR-like/PAQR Receptors | Share characteristics with mammalian progesterone receptors. | GprO, GprP (Aspergillus fumigatus) | [37] |
| X | Lung 7TM Superfamily Receptors | Represent receptors similar to the Lung 7TM Superfamily. | [37] | |
| XI | GPCR89/ABA GPCR Receptors | Belong to the GPCR89/ABA GPCR class. Could be involved in signaling related to abscisic acid, a plant hormone. | Gpr-12 (N. crassa) | [52] |
| XII | Family C-like Receptors | Similar to receptors found in Family C, which include metabotropic glutamate receptors. | Gpr-13 (N. crassa) | [52] |
| XIII | DUF300 Superfamily/PsGPR11 Receptors | Associated with the DUF300 Superfamily and PsGPR11. | Gpr-14 (N. crassa) | [52] |
| XIV | Pth11-Like Receptors | Related to the Pth11 family of receptors, involved in signaling and pathogenicity in plants. | Gpr-15, Gpr-23, Gpr-29 (N. crassa) | [52] |
| Organism | MAPKs | Main Functions | Ref. |
|---|---|---|---|
| Aspergillus fumigatus | MpkA, MpkC, SakA, MpkB | Mpka: Regulates cell wall integrity. MpkC and SakA: Response to various types of stress (osmotic, oxidative, adaptation to high temperatures), cell wall damage, and virulence. MpkB: function yet uncharacterized. | [80] |
| Cytospora chrysosperma | CcPmk1, CcHog1, CcSlt2 | Core regulators of fungal pathogenicity, development, and stress responses; required for responses to hyperosmotic pressure, cell wall inhibition agents, and oxidative stress; CcPmk1 and CcSlt2 essential for hyphal growth and fungal pathogenicity. | [17] |
| Saccharomyces cerevisiae | MAPKs: Fus3; DUSPs: Sdp1, Msg5 | Crucial for modulating MAPK signaling flow; affects cell physiology and pathogenicity in various fungi. | [74] |
| Candida albicans | FUS3 (homolog) | Loss of mating efficiency and decreased biofilm formation. | [80] |
| Cryptococcus neoformans | |||
| Aspergillus flavus | Fus3 | Regulates aflatoxin biosynthesis by modulating substrate levels; demonstrates the role of MAPKs in secondary metabolism. | [74] |
| Aspergillus nidulans | Mpk8 | Inhibition of sexual reproduction and impact on the production of secondary metabolites such as sterigmatocystin, terrequinone A, and penicillin. | [80] |
| Fusarium oxysporum | Fmk1 | Utilized in response to host alkalinization, highlighting the role of MAPKs in fungal pathogenicity through environmental sensing. | [81] |
| Clonostachys chloroleuca | Crmapk | Significant for mycoparasitism and biocontrol activities; investigated through yeast two-hybrid screening to identify interacting proteins. | [82] |
| Aspergillus nidulans | Pheromone module | Regulates development and secondary metabolism; HamE proposed as a scaffold protein for this pathway | [83] |
| Magnaporthe oryzae | Pmk1, Mps1 | Essential for appressorium formation, penetration, and invasive growth; critical for fungal infection processes | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Martínez, D.; Tristán-Flores, F.E.; Cervantes-Montelongo, J.A.; Silva-Martínez, G.A. Fungal Stress Responses and the Importance of GPCRs. J. Fungi 2025, 11, 213. https://doi.org/10.3390/jof11030213
Lara-Martínez D, Tristán-Flores FE, Cervantes-Montelongo JA, Silva-Martínez GA. Fungal Stress Responses and the Importance of GPCRs. Journal of Fungi. 2025; 11(3):213. https://doi.org/10.3390/jof11030213
Chicago/Turabian StyleLara-Martínez, Daniela, Fabiola Estefania Tristán-Flores, Juan Antonio Cervantes-Montelongo, and Guillermo Antonio Silva-Martínez. 2025. "Fungal Stress Responses and the Importance of GPCRs" Journal of Fungi 11, no. 3: 213. https://doi.org/10.3390/jof11030213
APA StyleLara-Martínez, D., Tristán-Flores, F. E., Cervantes-Montelongo, J. A., & Silva-Martínez, G. A. (2025). Fungal Stress Responses and the Importance of GPCRs. Journal of Fungi, 11(3), 213. https://doi.org/10.3390/jof11030213







