Analysis of Whole-Genome for Alternaria Species Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Genome Sequences for the Target Species
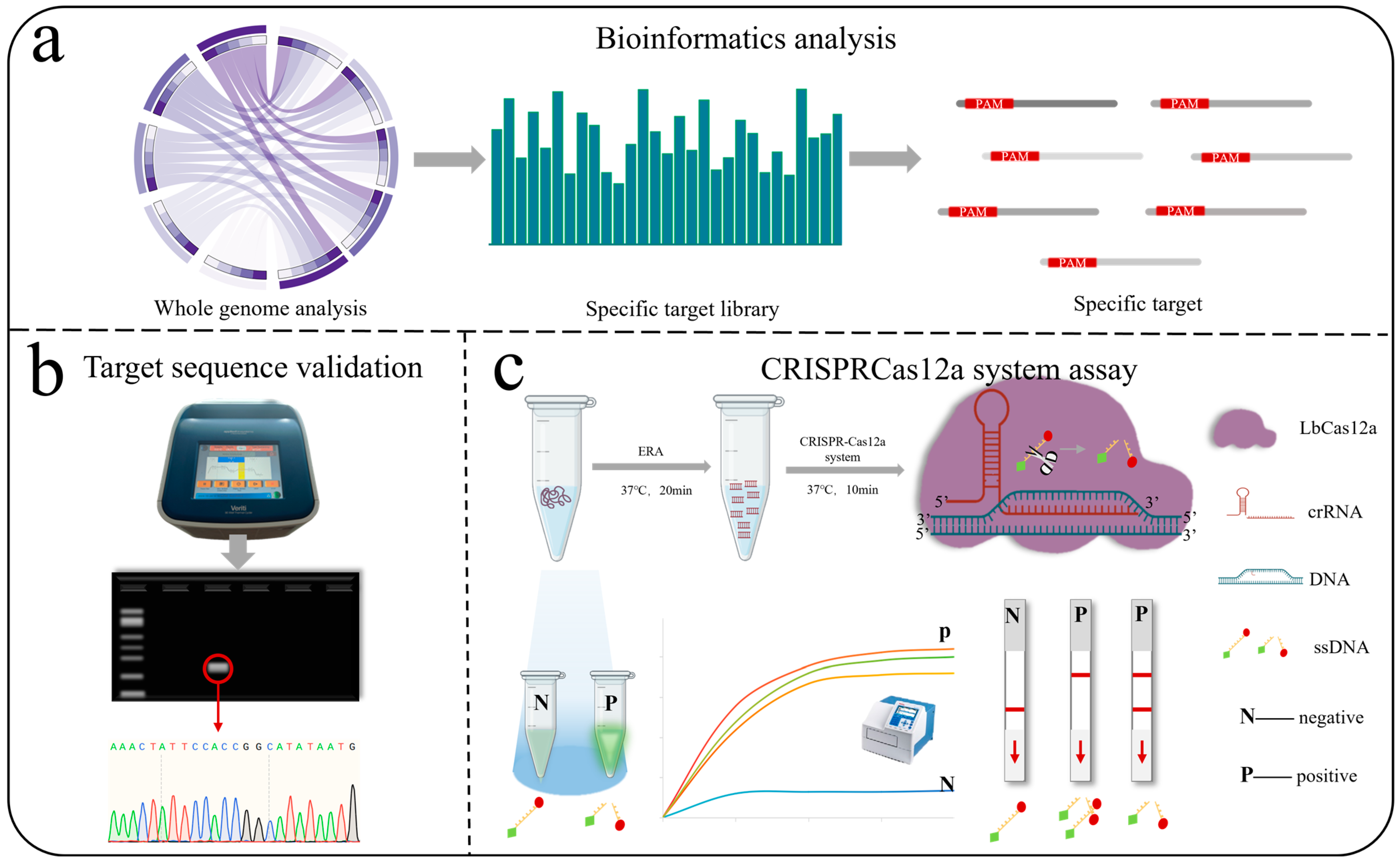
2.2. Bioinformatics Screening of Specific Target Sequences
2.2.1. Construction of a Species-Specific Target Library for Alternaria
2.2.2. Selection of Reference Targets for Each Alternaria Species
2.3. Cultivation and DNA Extraction of Strains
2.4. Detection of Specific Targets Using Sanger Sequencing
2.5. Rapid Detection of Specific Targets Using CRISPR-Cas12a
2.6. Statistical Analyses
3. Results
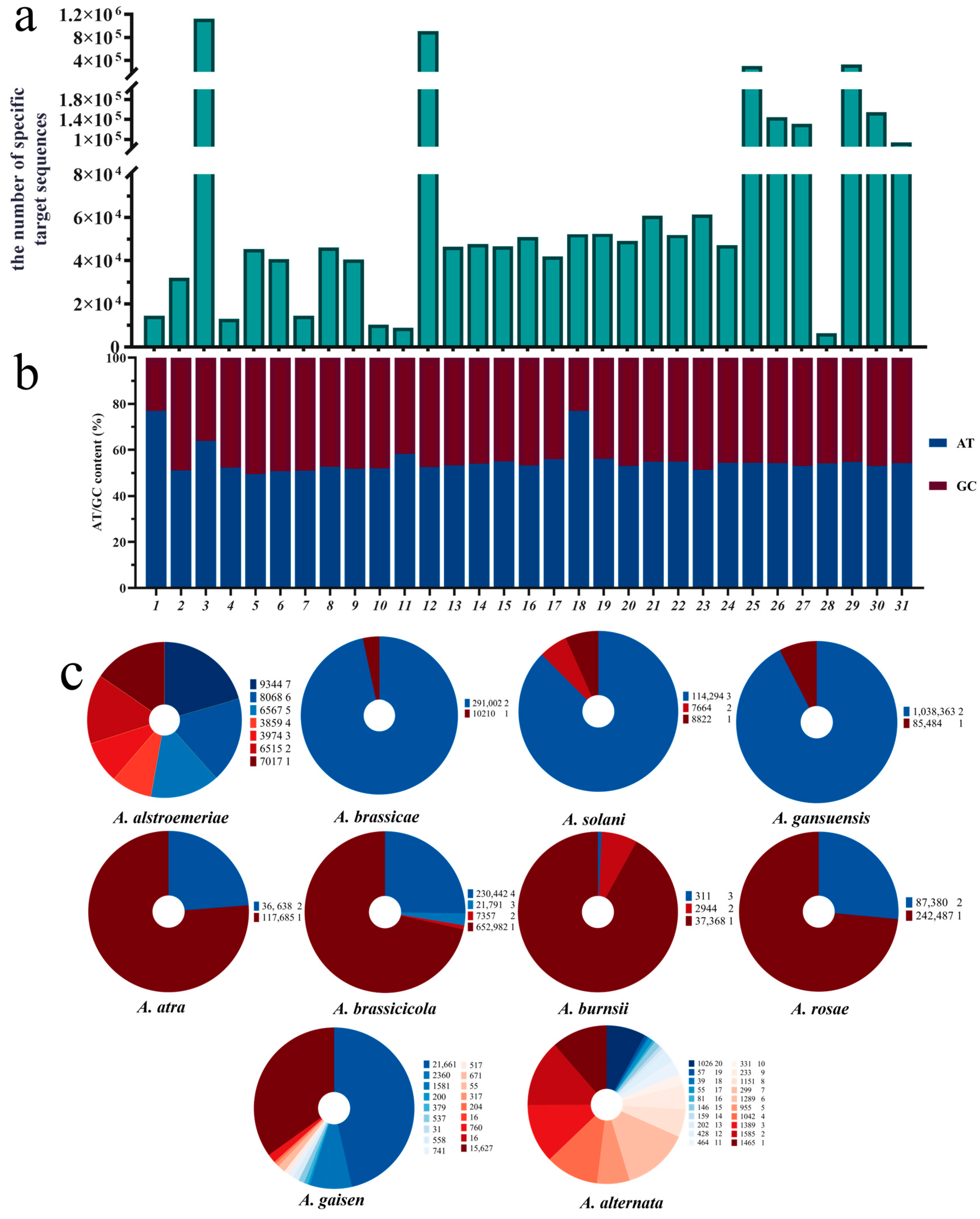
3.1. Construction of a Species-Specific Target Library for Alternaria Species
3.1.1. Main Factors Affecting the Number of Specific Target Sequences
3.1.2. GC Content Distribution in the Species-Specific Target Library
3.1.3. The Distribution Characteristics of Specific-Target Sequences Across the Whole Genome
3.2. Detection of Target Species Using Sanger Sequencing
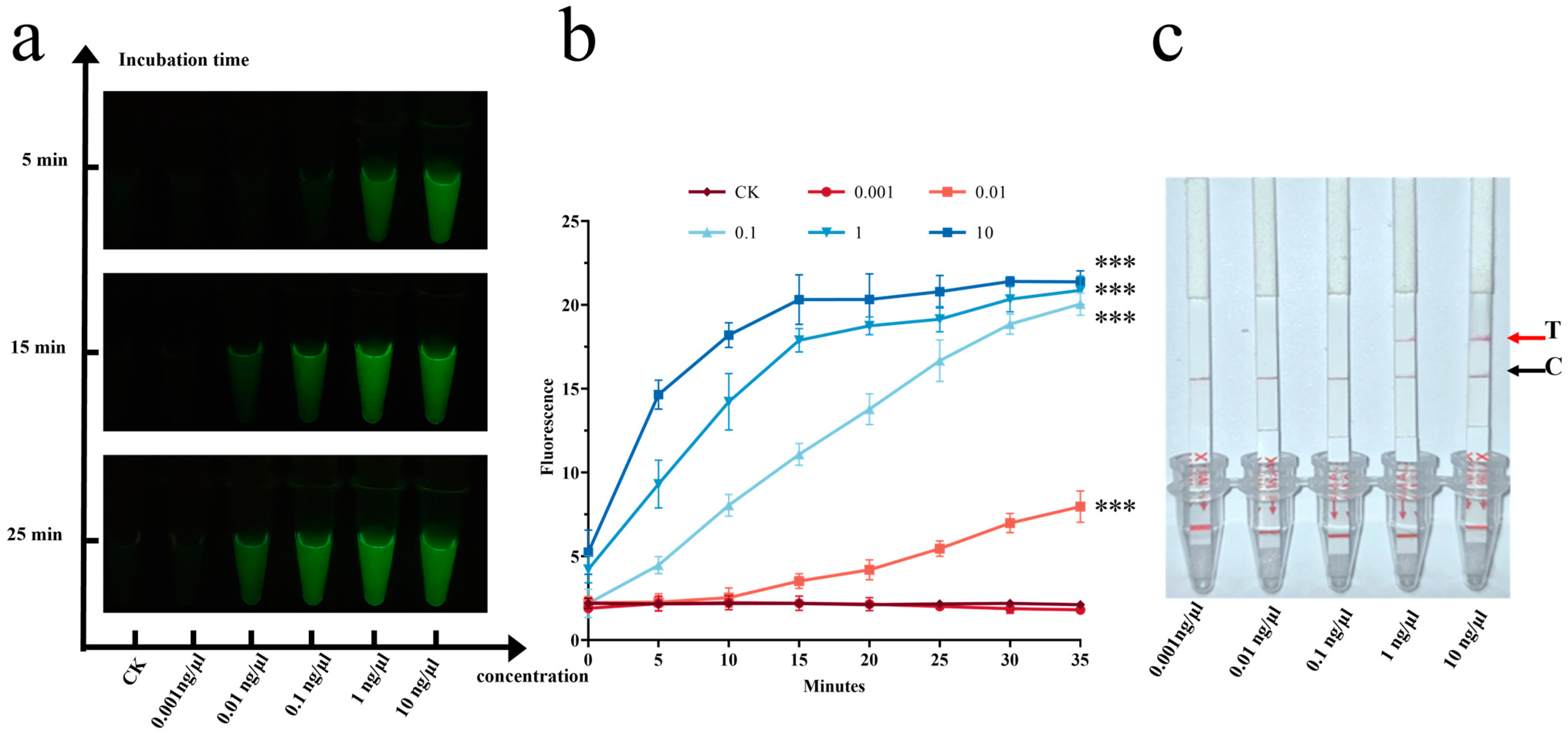
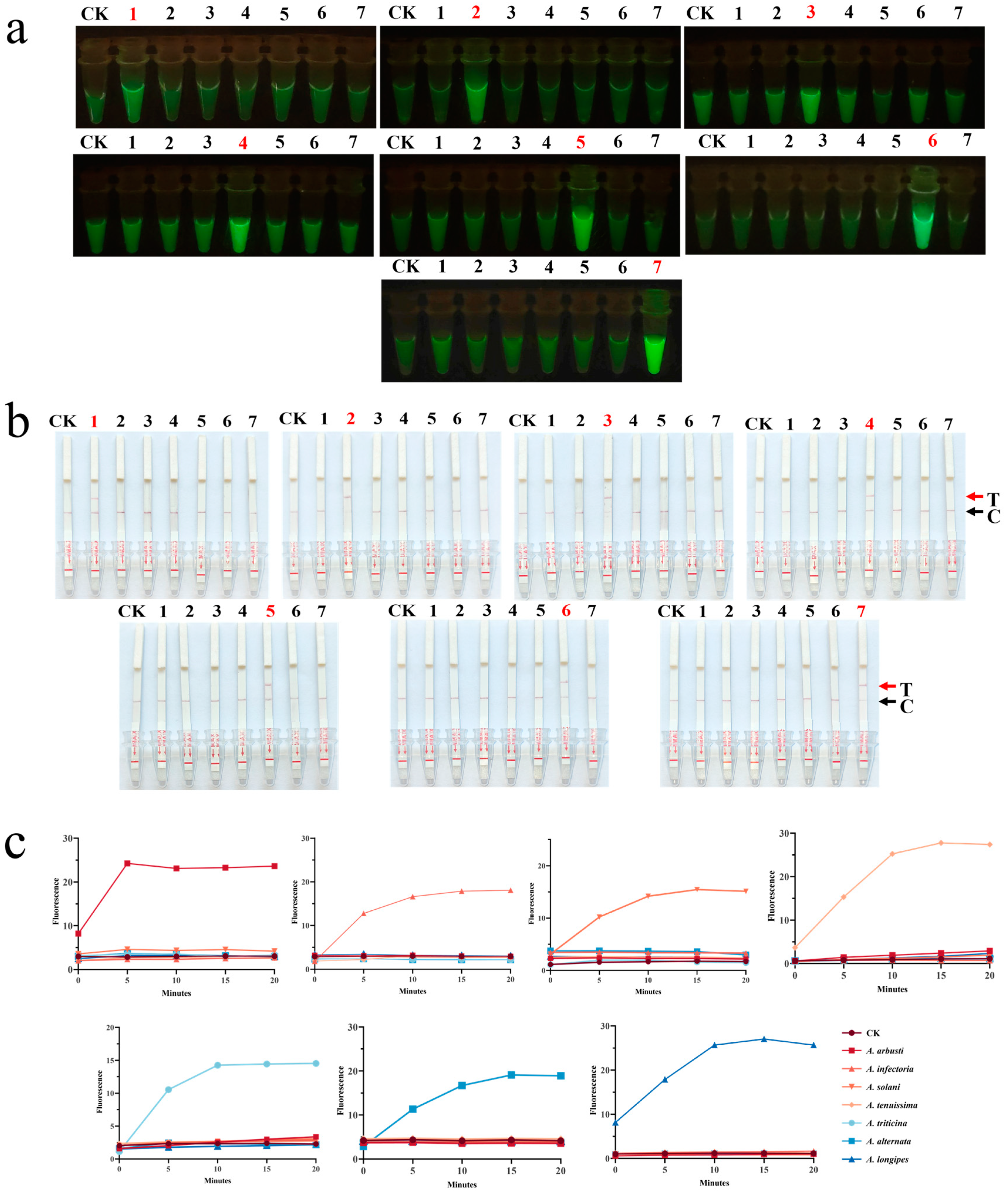
3.3. Detection of Target Species Using the CRISPR-Cas12a Dual System
3.3.1. Sensitivity Experiment of the CRISPR-Cas12a Dual System
3.3.2. Specificity Experiment of the CRISPR-Cas12a Dual System
4. Discussion
4.1. AGE Enables the Construction of a Species-Specific Target Library for Alternaria Genus Identification
4.2. AGE Demonstrates Accurate Species Identification Through Multiple Experimental Methods
4.3. AGE Enables Identification of Key Alternaria Species
4.4. AGE: Challenges and Developments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeMers, M. Alternaria alternata as endophyte and pathogen. Microbiology 2022, 168, 001153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Jiang, H.B.; Jeewon, R.; Hongsanan, S.; Bhat, D.J.; Tang, S.M.; Lumyong, S.; Mortimer, P.E.; Xu, J.C.; Camporesi, E. Alternaria: Update on species limits, evolution, multi-locus phylogeny, and classification. Stud. Fungi 2023, 8, 1–61. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.; Aptroot, A.; Leontyev, D.; Saxena, R. Outline of Fungi and fungus-like taxa. Fungal Biol. 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Stengel, A.; Stanke, K.M.; Quattrone, A.C.; Herr, J.R. Improving taxonomic delimitation of fungal species in the age of genomics and phenomics. Front. Microbiol. 2022, 13, 847067. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, W.; Gao, H.; Han, R.; Qi, F. Serious damage to crop production caused by Alternaria diseases and the safety of agricultural products. Plant Prot. 2017, 43, 9–15. [Google Scholar]
- Kang, Z.; Jiang, L.; Luo, Y.; Liu, C.; Li, X. The research advances of mechanism of pathogenicity of Alternaria phytopathogenic fungi. Chin. Bull. Life Sci. 2013, 9, 908–914. [Google Scholar]
- Feng, Z.; Li, Y.; Ma, X.; Duan, Y.; Zhang, R.; Hsiang, T.; Niu, Y.; Sun, G. Draft Genome Sequence of Alternaria longipes Causing Tobacco Brown Spot. Plant Dis. 2022, 106, 734–736. [Google Scholar] [CrossRef]
- Chen, Y.-h.; Wu, Y.Z.; Liu, Q.; Xia, Z.; Wang, J.; Luo, X.X. Streptomyces tamarix sp. nov.: Antagonism against Alternaria gaisen producing streptochlorin, isolated from Tamarix root soil. Front. Microbiol. 2023, 14, 1273842. [Google Scholar] [CrossRef]
- Al Lami, H.F.; You, M.P.; Barbetti, M.J. Temperature Drives Contrasting Alternaria Leaf Spot Epidemic Development in Canola and Mustard Rape from Alternaria japonica and A. brassicae. Plant Dis. 2020, 104, 1668–1674. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.L.; Wang, C.; Luo, M.; Hai Bo, M.A.; Zhang, Y. Detection of Alternaria triticina Prasada & Prabhu using PCR techniques. Biosafety 2015, 24, 72–77. [Google Scholar]
- Tozlu, E.; Tekiner, N.; Kotan, R.; Örtücü, S. Investigation on the biological control of Alternaria alternata. Indian J. Agric. Sci. 2018, 88, 1241–1247. [Google Scholar] [CrossRef]
- Fan, Q.; Zhou, Q.; Zhang, S.; Li, Y.; Li, J.; Chen, X.; Sun, L. First Report of Leaf Spot Disease Caused by Alternaria alternata on Polygonatum cyrtonema in Hunan Province of China. Plant Dis. 2024, 108, 530. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.Q.; Chen, Y.; Zhang, S.J.; Zhu, C.C. First Report of Leaf Spot on Chaste Tree Caused by Alternaria alternata in China. Plant Dis. 2024, 108, 520. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, Z.; Xu, T.; Lu, G.; Wang, X.; Ma, M.; Ni, R.; Shen, B.; Chang, J. First Report of Leaf Spot on Taraxacum mongolicum Caused by Alternaria solani in China. Plant Dis. 2024, 108, 796. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food: Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-host interactions: New insights on its pathogenesis, defense mechanisms and control strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Dalinova, A.; Salimova, D.; Berestetskiy, A. Fungi of the genera Alternaria as producers of biological active compounds and mycoherbicides. Appl. Biochem. Microbiol. 2020, 56, 256–272. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Liu, J.; Xiao, S.; Yang, S.; Mei, J.; Ren, M.; Wu, S.; Zhang, H.; Yang, X. Secondary metabolites of Alternaria: A comprehensive review of chemical diversity and pharmacological properties. Front. Microbiol. 2023, 13, 1085666. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, H.; Cheng, H.; Gou, Y.N.; Yu, Z.H.; Deng, J.X. New species of large-Spored Alternaria in section Porri associated with Compositae plants in China. Fungi 2022, 8, 607. [Google Scholar] [CrossRef]
- Iturrieta-González, I.; Gené, J. Alternaria muriformis sp. nov., a New Species in Section Chalastospora Isolated from Herbivore Dung in Spain. Diversity 2023, 15, 606. [Google Scholar] [CrossRef]
- Oviedo, M.S.; Sturm, M.E.; Reynoso, M.M.; Chulze, S.N.; Ramirez, M.L. Toxigenic profile and AFLP variability of Alternaria alternata and Alternaria infectoria occurring on wheat. Braz. J. Microbiol. 2013, 44, 447–455. [Google Scholar] [CrossRef]
- Elfar, K.; Bustamante, M.I.; Arreguin, M.; Nouri, M.T.; Eskalen, A. Identification and pathogenicity of Alternaria species causing leaf blotch and fruit spot of apple in California. Phytopathol. Mediterr. 2023, 62, 467–479. [Google Scholar] [CrossRef]
- Meyer, W.; Irinyi, L.; Hoang, M.T.V.; Robert, V.; Garcia-Hermoso, D.; Desnos-Ollivier, M.; Yurayart, C.; Tsang, C.-C.; Lee, C.-Y.; Woo, P.C. Database establishment for the secondary fungal DNA barcode translational elongation factor 1α (TEF1α). Genome 2019, 62, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Aylagas, E.; Borja, Á.; Irigoien, X.; Rodríguez-Ezpeleta, N. Benchmarking DNA metabarcoding for biodiversity-based monitoring and assessment. Front. Mar. Sci. 2016, 3, 96. [Google Scholar] [CrossRef]
- Laich, F.S.; Alcoba Flórez, J.; Pérez Roth, E.; Bahaya, Y.; Luis Delgado, J.; Méndez Álvarez, S. Molecular characterization of Alternaria alternata causing ocular infection: Detection of IGS-RFLP intraspecific polymorphism. Sabouraudia 2008, 46, 615–619. [Google Scholar] [CrossRef][Green Version]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef]
- Roberts, R.; Reymond, S.; Andersen, B. RAPD fragment pattern analysis and morphological segregation of small-spored Alternaria species and species groups. Mycol. Res. 2000, 104, 151–160. [Google Scholar] [CrossRef]
- Woudenberg, J.; Seidl, M.; Groenewald, J.; De Vries, M.; Stielow, J.; Thomma, B.; Crous, P. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Andersen, B.; Krøger, E.; Roberts, R.G. Chemical and morphological segregation of Alternaria arborescens, A. infectoria and A. tenuissima species-groups. Mycol. Res. 2002, 106, 170–182. [Google Scholar] [CrossRef]
- Zwickel, T.; Kahl, S.M.; Rychlik, M.; Müller, M.E. Chemotaxonomy of mycotoxigenic small-spored Alternaria fungi–do multitoxin mixtures act as an indicator for species differentiation? Front. Microbiol. 2018, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Peever, T.; Pryor, B. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia 2009, 101, 95–109. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.; Horré, R. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 2002, 45, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.J.; Cendoya, E.; Romero, C.J.; Humaran, J.F.; Zachetti, V.G.; Palacios, S.A.; Ramirez, M.L. Phylogenetic analysis and toxigenic profile of Alternaria species isolated from chickpeas (Cicer arietinum) in Argentina. Diversity 2022, 14, 924. [Google Scholar] [CrossRef]
- Ramires, F.A.; Masiello, M.; Somma, S.; Villani, A.; Susca, A.; Logrieco, A.F.; Luz, C.; Meca, G.; Moretti, A. Phylogeny and mycotoxin characterization of Alternaria species isolated from wheat grown in Tuscany, Italy. Toxins 2018, 10, 472. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, X.; Yang, X.; Xu, X.; Lin, J.; Bing, H.; Wang, X.; Zhao, J.; Xiang, W. Identification and pathogenicity of fungi associated with leaf spot of muskmelon in eastern Shandong Province, China. Plant Dis. 2022, 106, 872–890. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.; Cao, H.; Li, J.; Cao, P.; Guo, L.; Wang, X.; Zhao, J.; Xiang, W. Alternaria spp. associated with leaf blight of maize in Heilongjiang Province, China. Plant Dis. 2022, 106, 572–584. [Google Scholar] [CrossRef]
- Falcon, R.M.G.; Sumabat Dacones, L.G. Phylogeography of Fungicide Resistance of Alternaria alternata and Alternaria tenuissima to Demethylation Inhibitor Fungicides. Sci. Diliman 2022, 34, 5–27. [Google Scholar]
- He, L.; Cheng, H.; Zhao, L.; Htun, A.A.; Yu, Z.H.; Deng, J.X.; Li, Q.L. Morphological and molecular identification of two new Alternaria species (Ascomycota, Pleosporaceae) in section Radicina from China. MycoKeys 2021, 78, 187. [Google Scholar] [CrossRef]
- Gao, L.; Xu, W.; Xin, T.; Song, J. Application of third-generation sequencing to herbal genomics. Front. Plant Sci. 2023, 14, 1124536. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, Y.; Pu, X.; Gao, R.; Xu, Z.; Song, J. Trends in herbgenomics. Sci. China Life Sci. 2019, 62, 288–308. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Xin, T.; Xu, W.; Hao, L.; Qi, G.; Lou, Q.; Song, J. Principles and Strategies for Species Identification Based on Analysis of whole-Genome. Acta Pharm. Sin. 2023, 58, 2364–2374. [Google Scholar]
- Gan, Y.; Qi, G.; Hao, L.; Xin, T.; Lou, Q.; Xu, W.; Song, J. Analysis of Whole-Genome as a Novel Strategy for Animal Species Identification. Int. J. Mol. Sci. 2024, 25, 2955. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Xu, W.; Qi, G.; Xin, T.; Xu, Z.; Lei, H.; Song, J. GAGE is a method for identification of plant species based on whole genome analysis and genome editing. Commun. Biol. 2022, 5, 947. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Hao, L.; Gan, Y.; Xin, T.; Lou, Q.; Xu, W.; Song, J. Identification of closely related species in Aspergillus through Analysis of Whole-Genome. Front. Microbiol. 2024, 15, 1323572. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, L.; Gan, Y.; Qi, G.; Hao, L.; Xin, T.; Xu, W.; Song, J. Analysis of Whole-Genome for Identification of Seven Penicillium Species with Significant Economic Value. Int. J. Mol. Sci. 2024, 25, 8172. [Google Scholar] [CrossRef]
- Qi, G.; Hao, L.; Xin, T.; Gan, Y.; Lou, Q.; Xu, W.; Song, J. Analysis of whole-Genome facilitates rapid and precise identification of fungal species. Front. Microbiol. 2024, 15, 1336143. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, H.; Tang, D.; Liang, J.; Su, X.; Jiang, T.; Hu, X.; Ying, W.; Zhen, D.; Xiao, X. Ultrasensitive, Specific, and Rapid Detection of Mycoplasma pneumoniae Using the ERA/CRISPR–Cas12a Dual System. Front. Microbiol. 2022, 13, 811768. [Google Scholar] [CrossRef]
- Zi Ming, C.; Yi Feng, C. Genetic relationships of the specialized schizothoracine fishes inferred from random amplified polymorphic dna analysis. Zool. Res. 2000, 21, 262–268. [Google Scholar]
- He, X.-L.; Li, Q.; Peng, W.-H.; Zhou, J.; Cao, X.-L.; Wang, D.; Huang, Z.-Q.; Tan, W.; Li, Y.; Gan, B.-C. Intra-and inter-isolate variation of ribosomal and protein-coding genes in Pleurotus: Implications for molecular identification and phylogeny on fungal groups. BMC Microbiol. 2017, 17, 139. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Biochemical mutagens affect the preservation of fungi and biodiversity estimations. Appl. Microbiol. Biotechnol. 2013, 97, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; García, D.; Gené, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R. Genera of phytopathogenic fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–124. [Google Scholar] [CrossRef] [PubMed]
- Nwe, Z.M.; Htut, K.N.; Aung, S.L.L.; Gou, Y.-N.; Huang, C.-X.; Deng, J.-X. Two novel species and a new host record of Alternaria (Pleosporales, Pleosporaceae) from sunflower (Compositae) in Myanmar. MycoKeys 2024, 105, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Rybnicky, G.A.; Dixon, R.A.; Kuhn, R.M.; Karim, A.S.; Jewett, M.C. Development of a freeze-dried CRISPR-Cas12 sensor for detecting wolbachia in the secondary science classroom. ACS Synth. Biol. 2022, 11, 835–842. [Google Scholar] [CrossRef]
- Nie, Z.; Lü, P.; Zhang, R.; Tu, Y.; Liu, Z.; Li, Y.; Tang, C.; Li, X.; Zhao, K.; Zhou, Q. A simple and rapid method for fish sex identification based on recombinase-aided amplification and its use in Cynoglossus semilaevis. Sci. Rep. 2021, 11, 10429. [Google Scholar] [CrossRef]
- Cheng, C.; Fei, Z.; Xiao, P. Methods to improve the accuracy of next-generation sequencing. Front. Bioeng. Biotechnol. 2023, 11, 982111. [Google Scholar] [CrossRef]
- Swarts, D.C. Making the cut (s): How Cas12a cleaves target and non-target DNA. Biochem. Soc. Trans. 2019, 47, 1499–1510. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, Y.; Liu, D.; Wang, Y.; Sheng, Y.; Zhang, H. Leaf Spot caused by Alternaria tenuissima on Rhamnella franguloides in China. Plant Dis. 2024, 108, 1106. [Google Scholar] [CrossRef]
- Woudenberg, J.; Groenewald, J.; Binder, M.; Crous, P. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Tan, G.; Yang, Z.; Yuan, Z.; Zhang, S. Morphological, molecular and pathogenic characterization of Alternaria longipes, the fungal pathogen causing leaf spot on Atractylodes macrocephala. Microbiol. Res. 2013, 7, 2589–2595. [Google Scholar] [CrossRef]
- Masiello, M.; El Ghorayeb, R.; Somma, S.; Carine, S.; Giuseppe, M.; Logrieco, A.F.; Habib, W.; Moretti, A. Alternaria species and related mycotoxin detection in Lebanese durum wheat grain. Phytopathol. Mediterr. 2022, 61, 383–393. [Google Scholar] [CrossRef]
- Sánchez, P.; Vélez-del-Burgo, A.; Suñén, E.; Martínez, J.; Postigo, I. Fungal allergen and mold allergy diagnosis: Role and relevance of Alternaria alternata Alt a 1 protein family. J. Fungi 2022, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cao, W.; Xing, L.; Qin, G.; Wu, J.; Nagle, M.F.; Xiong, Q.; Chen, J.; Yang, L.; Bajaj, P. A novel high-accuracy genome assembly method utilizing a high-throughput workflow. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ciufo, S.; Kannan, S.; Sharma, S.; Badretdin, A.; Clark, K.; Turner, S.; Brover, S.; Schoch, C.L.; Kimchi, A.; DiCuccio, M. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int. J. Syst. Evol. Microbiol. 2018, 68, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]

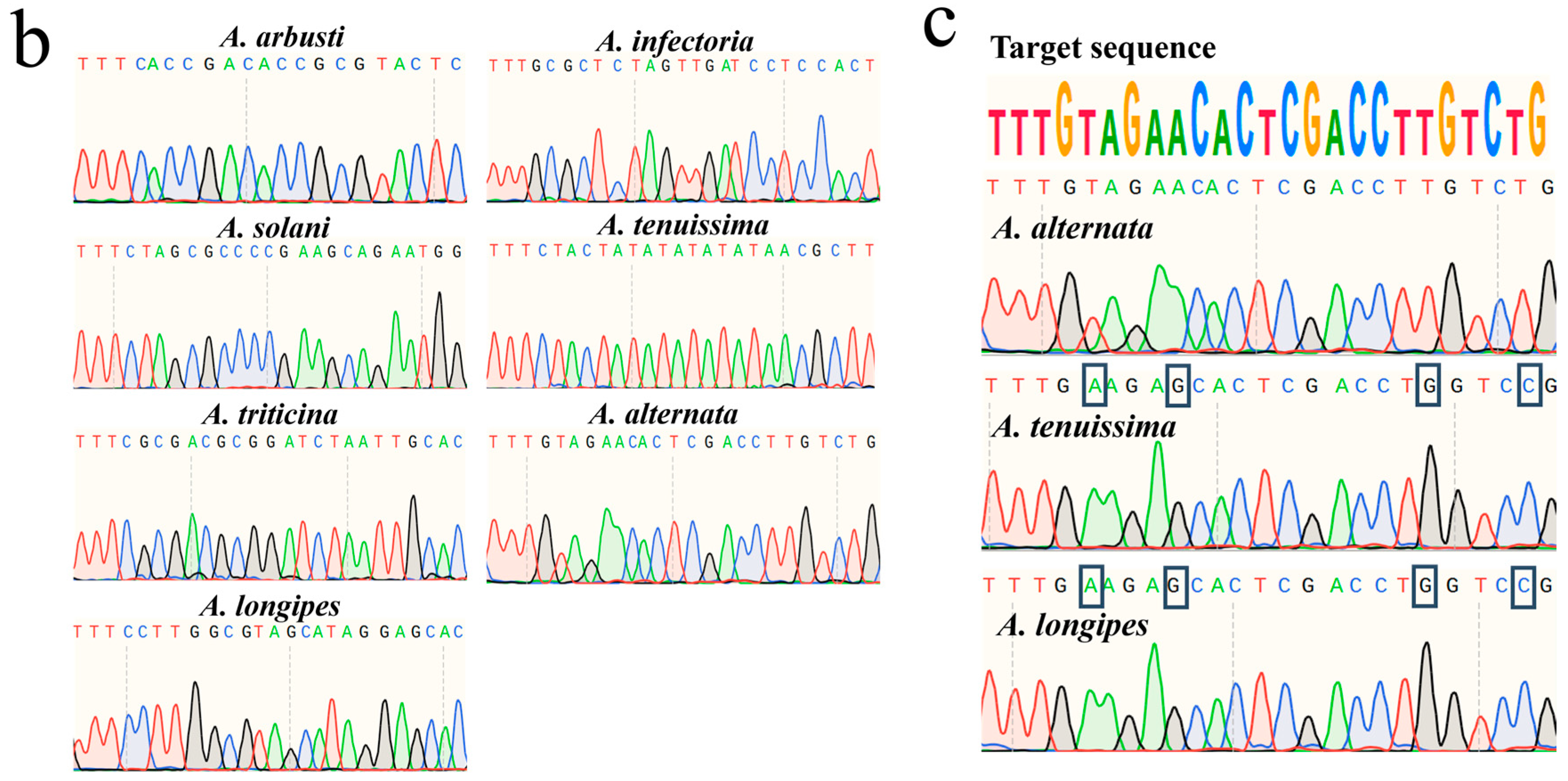
| Species | Reference Target Sequence | Chromosomal Location | Annotation | Sequence Range |
|---|---|---|---|---|
| A. arbusti | TTTCACCGACACCGCGTACTCTGTC | no assembled | / | 1,506,110–1,506,134 |
| A. infectoria | TTTGCGCTCTAGTTGATCCTCCACT | no assembled | / | 897,690–897,714 |
| A. solani | TTTCTAGCGCCCCGAAGCAGAATGG | chromosome 2 | / | 632,284–632,308 |
| A. tenuissima | TTTCTACTATATATATATAACGCTT | no assembled | O-methyltransferase | 825–849 |
| A. triticina | TTTCGCGACGCGGATCTAATTGCAC | no assembled | / | 62,527–62,551 |
| A. alternata | TTTGTAGAACACTCGACCTTGTCTG | no assembled | Nnf1-domain-containing protein | 370,978–371,002 |
| A. longipes | TTTCCTTGGCGTAGCATAGGAGCAC | chromosome 3 | / | 554,291–554,315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Gan, Y.; Xu, W.; Huang, Y.; Xin, T.; Tan, R.; Song, J. Analysis of Whole-Genome for Alternaria Species Identification. J. Fungi 2025, 11, 185. https://doi.org/10.3390/jof11030185
Yang Y, Gan Y, Xu W, Huang Y, Xin T, Tan R, Song J. Analysis of Whole-Genome for Alternaria Species Identification. Journal of Fungi. 2025; 11(3):185. https://doi.org/10.3390/jof11030185
Chicago/Turabian StyleYang, Ying, Yutong Gan, Wenjie Xu, Yuanhao Huang, Tianyi Xin, Rui Tan, and Jingyuan Song. 2025. "Analysis of Whole-Genome for Alternaria Species Identification" Journal of Fungi 11, no. 3: 185. https://doi.org/10.3390/jof11030185
APA StyleYang, Y., Gan, Y., Xu, W., Huang, Y., Xin, T., Tan, R., & Song, J. (2025). Analysis of Whole-Genome for Alternaria Species Identification. Journal of Fungi, 11(3), 185. https://doi.org/10.3390/jof11030185







