Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Design
2.2. Physical and Chemical Analytical Procedures
2.3. DNA Extraction, Gene Amplification, and Sequencing
2.4. Bioinformatics and FUNGuild
2.5. Statistical Analyses
3. Results
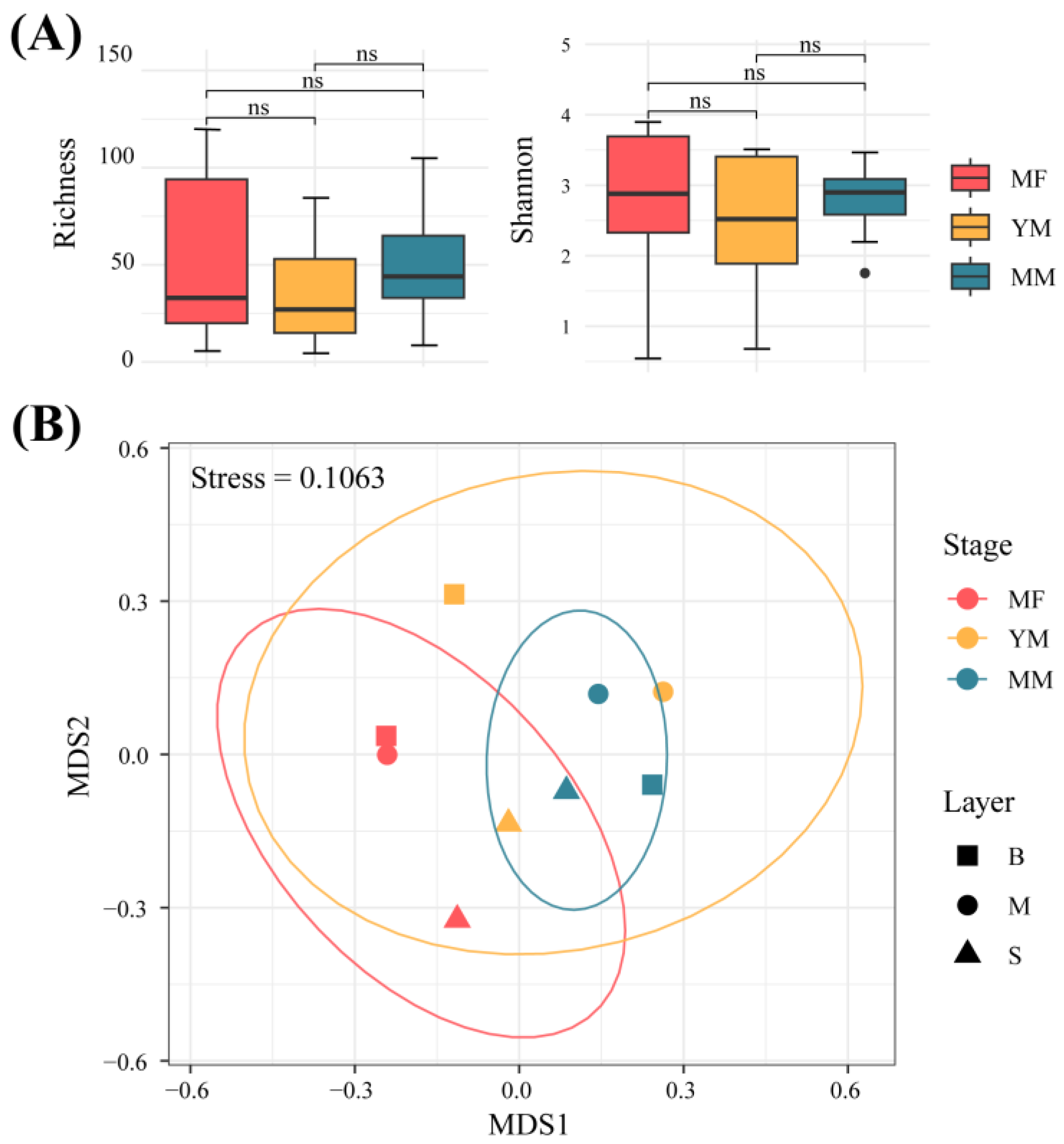
3.1. Soil Fungal Diversity and Community Composition
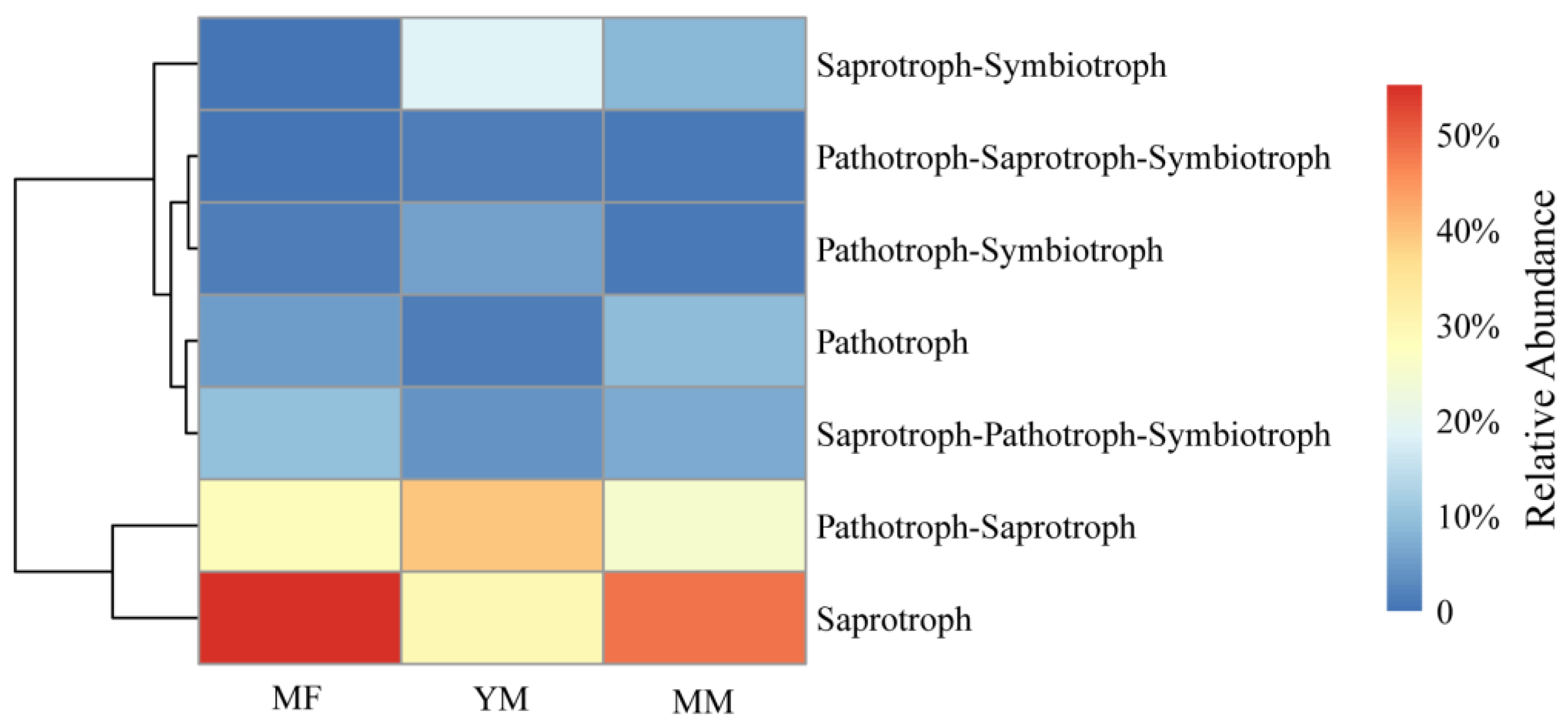
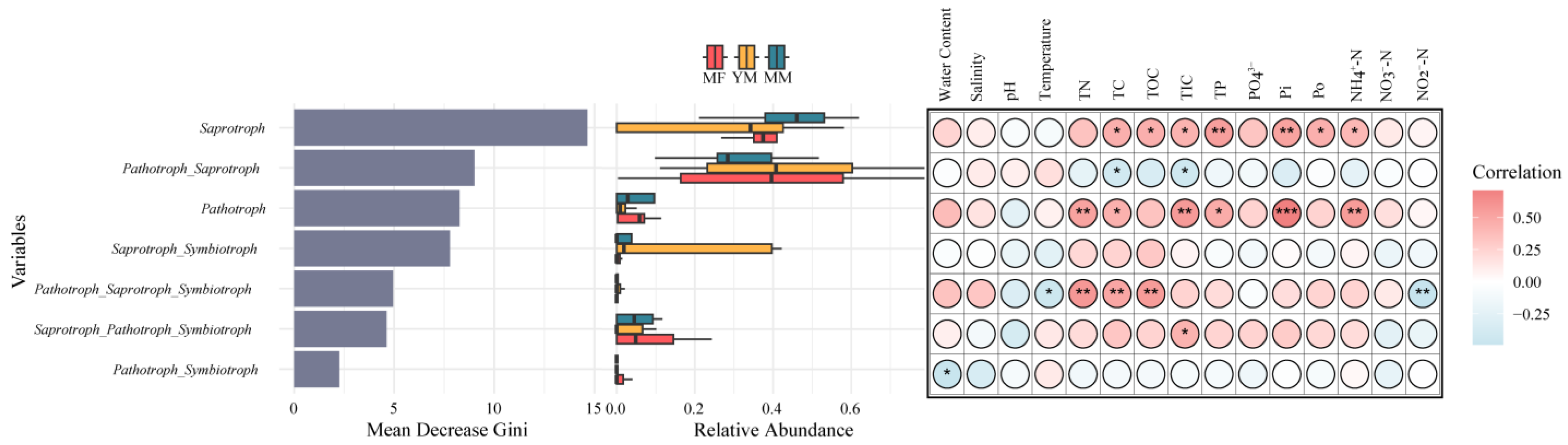
3.2. Trophic Mode Features of the Soil Fungal Community
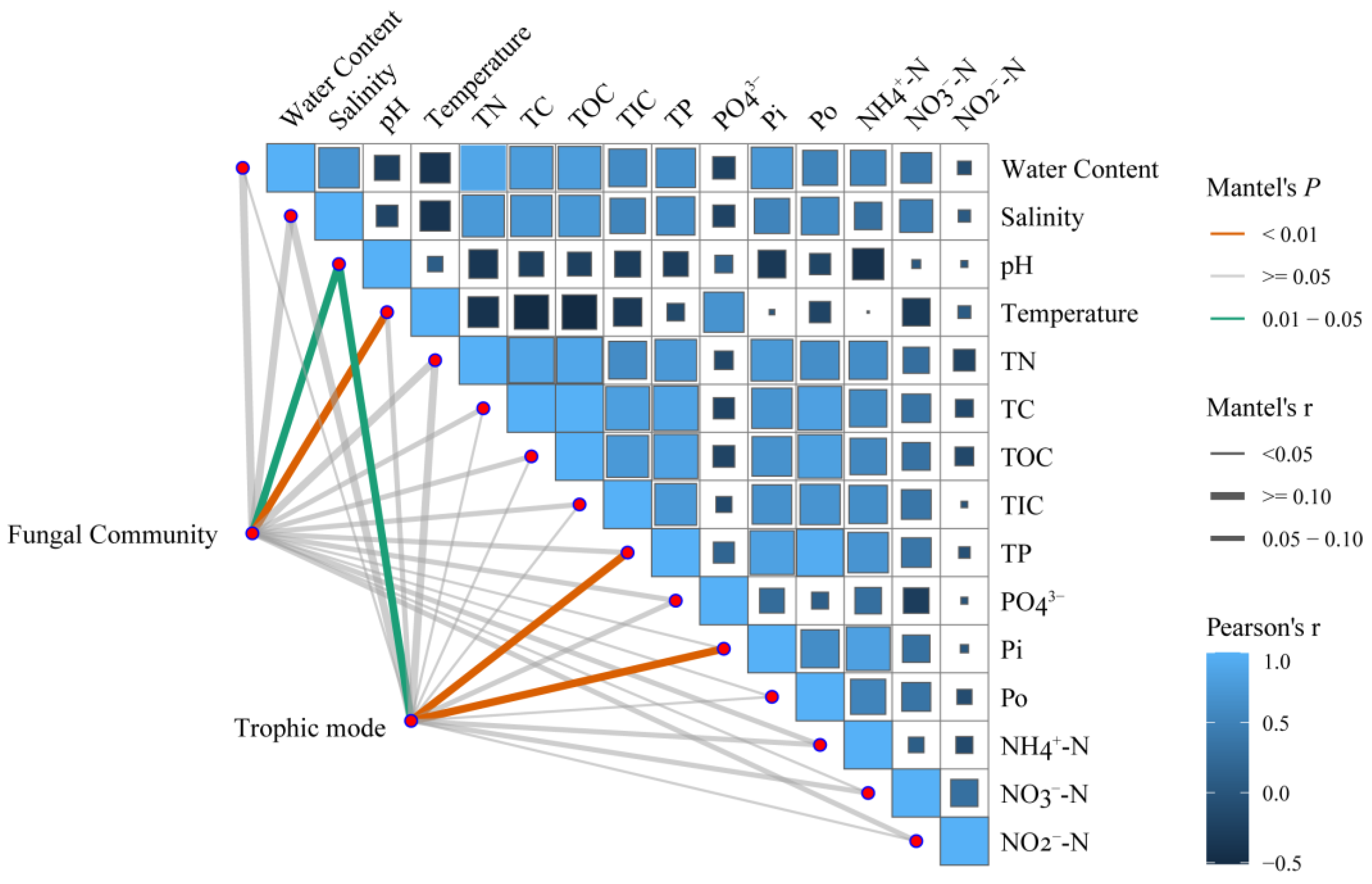
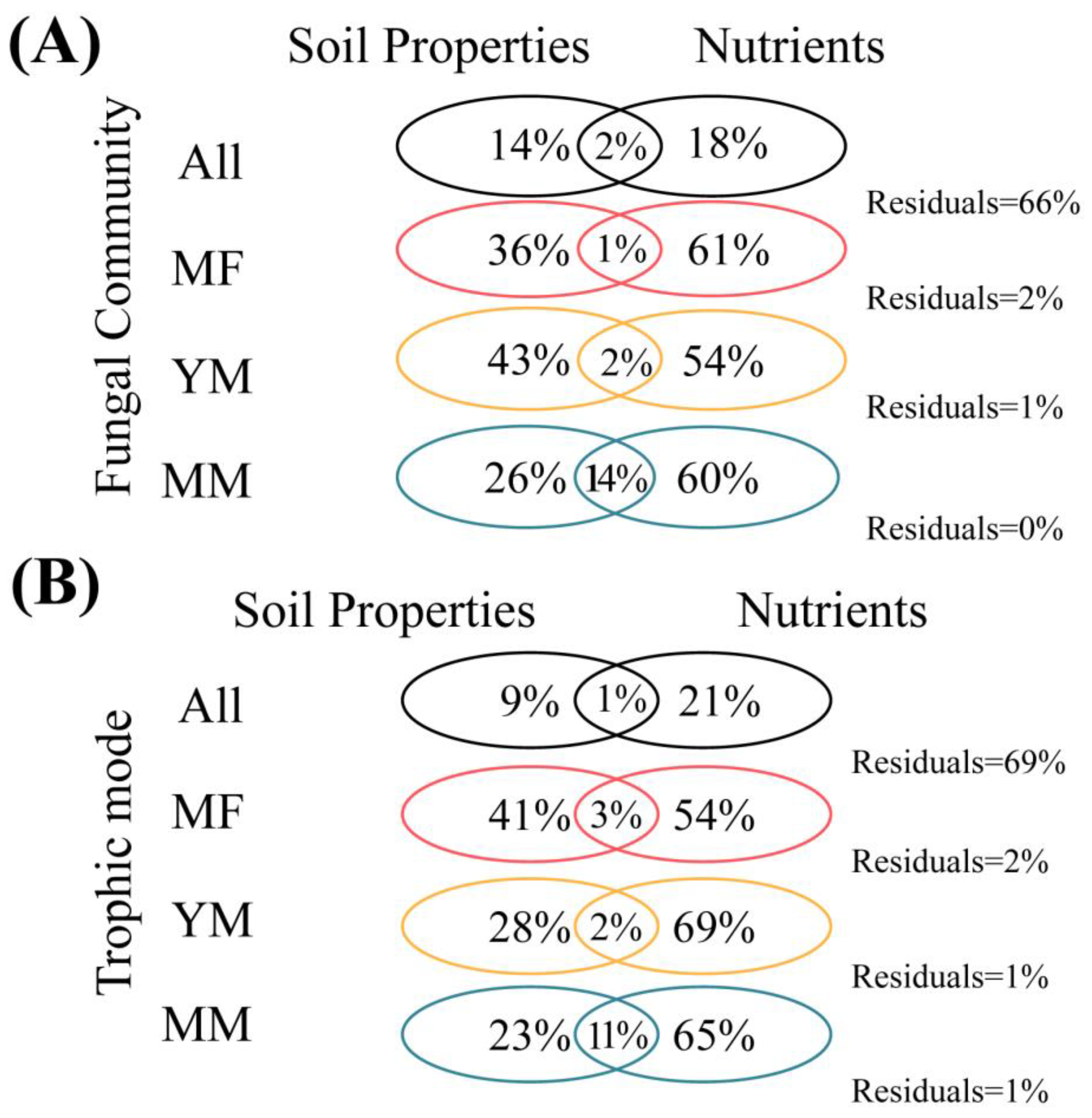
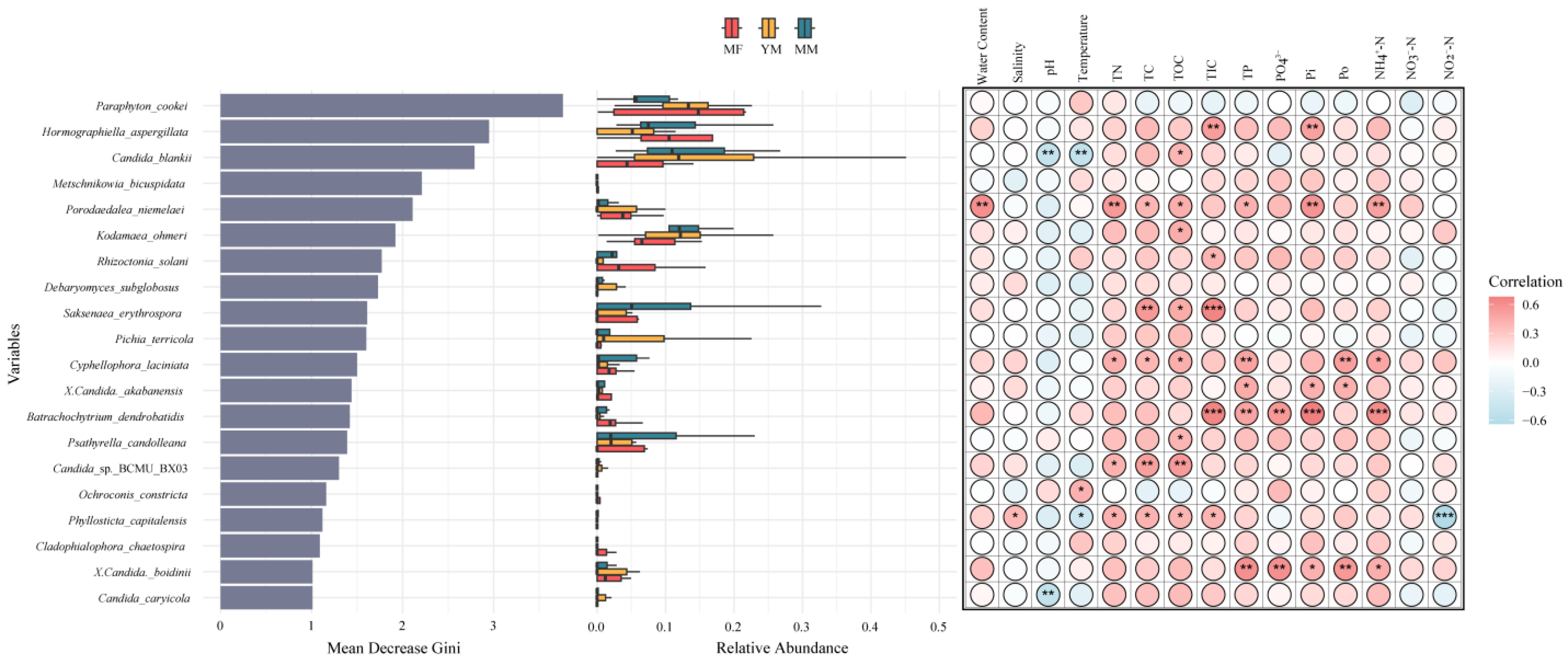
3.3. Environmental Effects on the Fungal Community and Trophic Modes
3.4. Bioindicators in Different Successional Environments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and Death in the Soil Microbiome: How Ecological Processes Influence Biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, F. Biodiversity and Ecosystem Functioning in Naturally Assembled Communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, D.; Wu, J.; Zhang, X.; Zhuang, X.; Kong, W. The Interaction of Climate, Plant, and Soil Factors Drives Putative Soil Fungal Pathogen Diversity and Community Structure in Dry Grasslands. Environ. Microbiol. Rep. 2024, 16, e13223. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bing, H.; Fang, L.; Wu, Y.; Yu, J.; Shen, G.; Jiang, M.; Wang, X.; Zhang, X. Diversity Patterns of the Rhizosphere and Bulk Soil Microbial Communities along an Altitudinal Gradient in an Alpine Ecosystem of the Eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil PH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Nugent, A.; Allison, S.D. A Framework for Soil Microbial Ecology in Urban Ecosystems. Ecosphere 2022, 13, e3968. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Yin, R.; Xing, S.; Fu, W.; Wu, H.; Hao, Z.; Ma, Y.; Zhang, X. Long-Term Nickel Contamination Increased Soil Fungal Diversity and Altered Fungal Community Structure and Co-Occurrence Patterns in Agricultural Soils. J. Hazard. Mater. 2022, 436, 129113. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Huang, M.; Zhou, S. Plant Diversity Promotes Soil Fungal Pathogen Richness under Fertilization in an Alpine Meadow. J. Plant Ecol. 2021, 14, 323–336. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiao, F.; Huang, Y.; Li, N.; Wang, B.; Gao, H.; An, S. Response of Soil Fungal Community Composition and Functions on the Alteration of Precipitation in the Grassland of Loess Plateau. Sci. Total Environ. 2021, 751, 142273. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Lei, X.; Zhao, L.; Brookes, P.C.; Wang, F.; Chen, C.; Yang, W.; Xing, S. Fungal Communities and Functions Response to Long-Term Fertilization in Paddy Soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Likulunga, L.E.; Rivera Pérez, C.A.; Schneider, D.; Daniel, R.; Polle, A. Tree Species Composition and Soil Properties in Pure and Mixed Beech-Conifer Stands Drive Soil Fungal Communities. For. Ecol. Manag. 2021, 502, 119709. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in Soil Bacterial and Fungal Community Composition and Functional Groups during the Succession of Boreal Forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients Addition Decreases Soil Fungal Diversity and Alters Fungal Guilds and Co-Occurrence Networks in a Semi-Arid Grassland in Northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and Distribution of Mangrove Forests of the World Using Earth Observation Satellite Data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Allard, S.M.; Costa, M.T.; Bulseco, A.N.; Helfer, V.; Wilkins, L.G.E.; Hassenrück, C.; Zengler, K.; Zimmer, M.; Erazo, N.; Mazza Rodrigues, J.L.; et al. Introducing the Mangrove Microbiome Initiative: Identifying Microbial Research Priorities and Approaches To Better Understand, Protect, and Rehabilitate Mangrove Ecosystems. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-X.; Jiang, Z.-Y.; Wu, P.; Wang, Y.-F.; Cheng, H.; Wang, Y.-S.; Gu, J.-D. Effect of Mangrove Restoration on Sediment Properties and Bacterial Community. Ecotoxicology 2021, 30, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Huang, Y.; Wang, Z.; Tang, X.; Ye, W.; Cao, H.; Shen, H. Soil Microbial Community Structure, Function and Network along a Mangrove Forest Restoration Chronosequence. Sci. Total Environ. 2024, 913, 169704. [Google Scholar] [CrossRef]
- Bai, S.; Li, J.; He, Z.; Van Nostrand, J.D.; Tian, Y.; Lin, G.; Zhou, J.; Zheng, T. GeoChip-Based Analysis of the Functional Gene Diversity and Metabolic Potential of Soil Microbial Communities of Mangroves. Appl. Microbiol. Biotechnol. 2013, 97, 7035–7048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-F.; Pan, Y.-P.; Liu, Y.; Li, M. High-Level Diversity of Basal Fungal Lineages and the Control of Fungal Community Assembly by Stochastic Processes in Mangrove Sediments. Appl. Environ. Microbiol. 2021, 87, e00928-21. [Google Scholar] [CrossRef]
- Arfi, Y.; Buée, M.; Marchand, C.; Levasseur, A.; Record, E. Multiple Markers Pyrosequencing Reveals Highly Diverse and Host-Specific Fungal Communities on the Mangrove Trees Avicennia Marina and Rhizophora Stylosa. FEMS Microbiol. Ecol. 2012, 79, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zeng, R.; Tian, C.; Wang, J.; Qu, W. The Importance of Conditionally Rare Taxa for the Assembly and Interaction of Fungal Communities in Mangrove Sediments. Appl. Microbiol. Biotechnol. 2022, 106, 3787–3798. [Google Scholar] [CrossRef]
- Hou, N.; Yang, X.; Wang, W.; Sardans, J.; Yin, X.; Jiang, F.; Song, Z.; Li, Z.; Tian, J.; Ding, X.; et al. Mangrove Wetland Recovery Enhances Soil Carbon Sequestration Capacity of Soil Aggregates and Microbial Network Stability in Southeastern China. Sci. Total Environ. 2024, 951, 175586. [Google Scholar] [CrossRef]
- Devadatha, B.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Hyde, K.D.; Sakayaroj, J.; Bahkali, A.H.; Calabon, M.S.; Sarma, V.V.; Sutreong, S.; et al. Occurrence and Geographical Distribution of Mangrove Fungi. Fungal Divers. 2021, 106, 137–227. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A Few Ascomycota Taxa Dominate Soil Fungal Communities Worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Cunliffe, M. Multi-Year Assessment of Coastal Planktonic Fungi Reveals Environmental Drivers of Diversity and Abundance. ISME J. 2016, 10, 2118–2128. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root Exudates Regulate Soil Fungal Community Composition and Diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Zhao, H.; Brearley, F.Q.; Huang, L.; Tang, J.; Xu, Q.; Li, X.; Huang, Y.; Zou, S.; Chen, X.; Hou, W.; et al. Abundant and Rare Taxa of Planktonic Fungal Community Exhibit Distinct Assembly Patterns Along Coastal Eutrophication Gradient. Microb. Ecol. 2023, 85, 495–507. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of Mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Wu, R.; Gu, J.-D. Impact of Nitrogen Pollution/Deposition on Extracellular Enzyme Activity, Microbial Abundance and Carbon Storage in Coastal Mangrove Sediment. Chemosphere 2017, 177, 275–283. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Kathiresan, K.; MubarakAli, D.; Kayalvizhi, K.; Rajendran, N.; Hemalatha, S.; Chen, J. Soil-Microbial Communities Indexing from Mangroves Rhizosphere and Barren Sandy Habitats. Physiol. Mol. Plant Pathol. 2018, 104, 58–68. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature Mediates Continental-Scale Diversity of Microbes in Forest Soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhai, Y.; Jia, J.; Zhao, Q.; Wang, W.; Hu, X. Microbial Diversity and Functions in Saline Soils: A Review from a Biogeochemical Perspective. J. Adv. Res. 2024, 59, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.E.P.; van Noordwijk, C.G.E.; Hildrew, A.G. Delivering on a Promise: Integrating Species Traits to Transform Descriptive Community Ecology into a Predictive Science. Freshw. Sci. 2013, 32, 531–547. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, H.; Yang, S.; Qin, X.; Liao, N.; Li, X.; Wei, Q.; Li, W.; Jiang, G.; Li, N.; et al. Mycoplanktonic Community Structure and Their Roles in Monitoring Environmental Changes in a Subtropical Estuary in the Beibu Gulf. J. Mar. Sci. Eng. 2022, 10, 1940. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Li, N.; Zhao, H.; Pu, Y.; Huang, J.; Yang, S.; Qin, X.; Dong, K.; Li, M.; et al. Effects of Environmental Factors on Mycoplankton Diversity and Trophic Modes in Coastal Surface Water. Ecol. Indic. 2023, 146, 109778. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, F.; Wang, Y.; Wang, J.; Li, J.; Zhang, Z. Variation and Drivers of Soil Fungal and Functional Groups among Different Forest Types in Warm Temperate Secondary Forests. Glob. Ecol. Conserv. 2023, 45, e02523. [Google Scholar] [CrossRef]
- Lacerda, A.L.d.F.; Proietti, M.C.; Secchi, E.R.; Taylor, J.D. Diverse Groups of Fungi Are Associated with Plastics in the Surface Waters of the Western South Atlantic and the Antarctic Peninsula. Mol. Ecol. 2020, 29, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gong, L.; Pang, K.-L.; Luo, Z.-H. Fungal Diversity in Deep-Sea Sediments of a Hydrothermal Vent System in the Southwest Indian Ridge. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 16–26. [Google Scholar] [CrossRef]
- Xie, C.; Ma, X.; Zhao, Y.; Dai, T.; Song, W.; Qi, Q.; Feng, J.; Cui, X.; Zhou, J.; Huang, X.; et al. Nitrogen Addition and Warming Rapidly Alter Microbial Community Compositions in the Mangrove Sediment. Sci. Total Environ. 2022, 850, 157992. [Google Scholar] [CrossRef] [PubMed]
- Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Solazzo, E.; Monforti-Ferrario, F.; Olivier, J.G.J.; Vignati, E. Fossil CO2 Emissions of All World Countries—2020 Report, EUR 30358 EN; JRC121460; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-21515-8. [Google Scholar] [CrossRef]
- Purahong, W.; Sadubsarn, D.; Tanunchai, B.; Wahdan, S.F.M.; Sansupa, C.; Noll, M.; Wu, Y.-T.; Buscot, F. First Insights into the Microbiome of a Mangrove Tree Reveal Significant Differences in Taxonomic and Functional Composition among Plant and Soil Compartments. Microorganisms 2019, 7, 585. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, J.; Hua, J.; Song, B.; Wang, T.; Xing, W.; Wang, G.; Mao, L.; Ruan, H. Differentiation of Fungal Trophic Guilds to Long-Term Nitrogen Addition in a Poplar Plantation. For. Ecol. Manag. 2024, 555, 121699. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Ran, Y.; Li, W.; Shao, M.; Zhang, Y.; Ji, Q.; Ding, X. Soil Saprophytic Fungi Could Be Used as an Important Ecological Indicator for Land Management in Desert Steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover Cropping and No-till Increase Diversity and Symbiotroph:Saprotroph Ratios of Soil Fungal Communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi Mediate Long Term Sequestration of Carbon and Nitrogen in Soil through Their Priming Effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
| 0.145 *** | MF | YM |
| MF | ||
| YM | 0.192 *** | |
| MM | 0.004 *** | 0.238 *** |
| 0.141 *** | MF | YM |
| MF | ||
| YM | 0.129 *** | |
| MM | 0.018 *** | 0.153 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhou, S.; Xu, L.; Nethmini, R.T.; Zhang, Y.; Huang, L.; Dong, K.; Zhao, H.; Pan, L. Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration. J. Fungi 2025, 11, 146. https://doi.org/10.3390/jof11020146
Shi X, Zhou S, Xu L, Nethmini RT, Zhang Y, Huang L, Dong K, Zhao H, Pan L. Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration. Journal of Fungi. 2025; 11(2):146. https://doi.org/10.3390/jof11020146
Chicago/Turabian StyleShi, Xiaofang, Shengyao Zhou, Lanzi Xu, Rajapakshalage Thashikala Nethmini, Yu Zhang, Liangliang Huang, Ke Dong, Huaxian Zhao, and Lianghao Pan. 2025. "Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration" Journal of Fungi 11, no. 2: 146. https://doi.org/10.3390/jof11020146
APA StyleShi, X., Zhou, S., Xu, L., Nethmini, R. T., Zhang, Y., Huang, L., Dong, K., Zhao, H., & Pan, L. (2025). Shifts in Soil Fungal Community and Trophic Modes During Mangrove Ecosystem Restoration. Journal of Fungi, 11(2), 146. https://doi.org/10.3390/jof11020146







