Fusarium Species Associated with Spikes and Grains of Cereal Crops in the Volga Region: Virulence and Toxin-Producing Potential
Abstract
1. Introduction
2. Materials and Methods
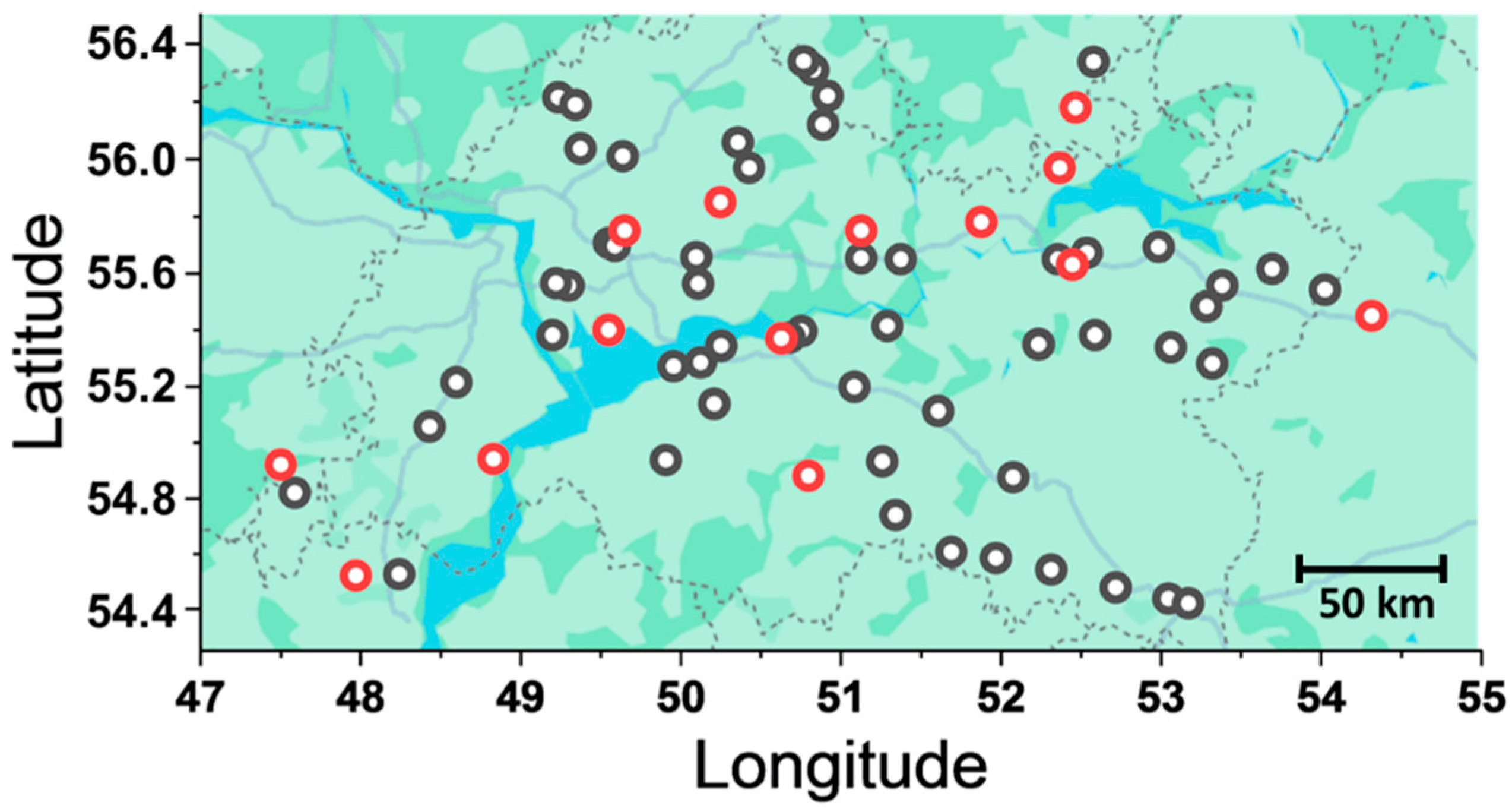
2.1. Plant Sample Collection
2.2. Isolation of Fusarium Fungi and Their Morphological Description
2.3. The Analysis of DNA Sequences
2.4. Virulence Assays
2.4.1. Laboratory Assay
2.4.2. Field Assay
2.5. Detection of Mycotoxin-Related DNA Loci
2.6. Determination of Mycotoxins
2.7. Statistics
3. Results
3.1. Isolation of Fusarium ssp. Strains Inhabiting the Volga Region
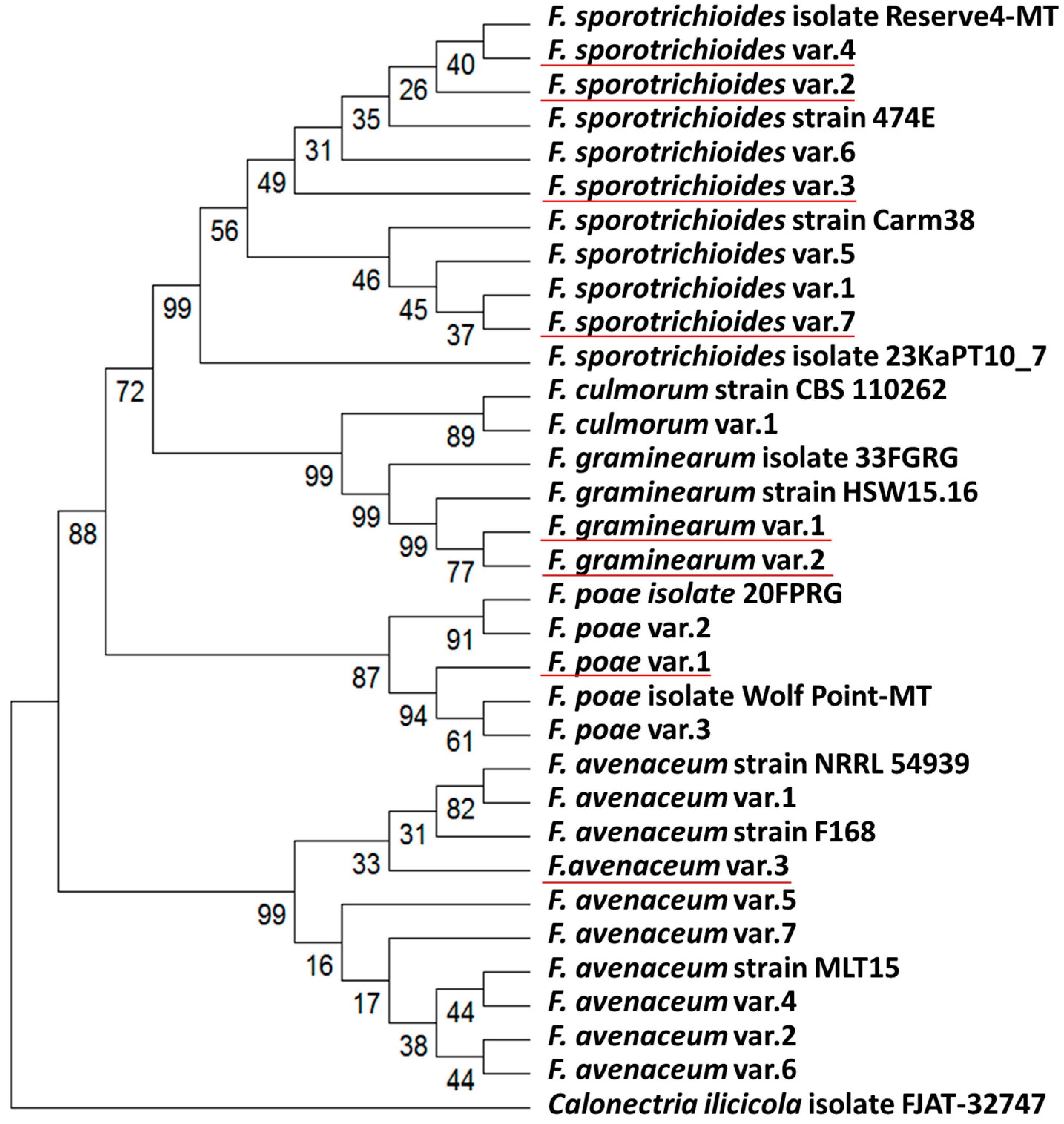
3.2. Genotyping and Phylogenetic Analysis of Fusarium ssp. Strains Inhabiting the Volga Region
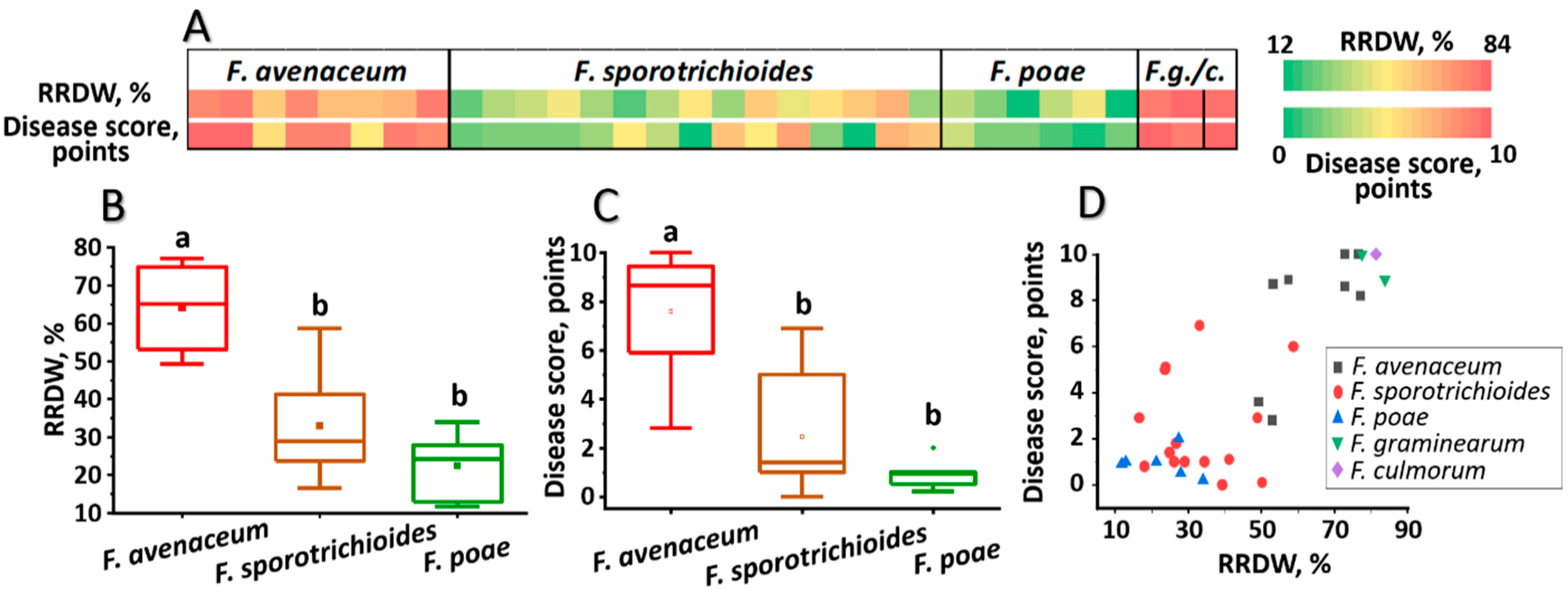
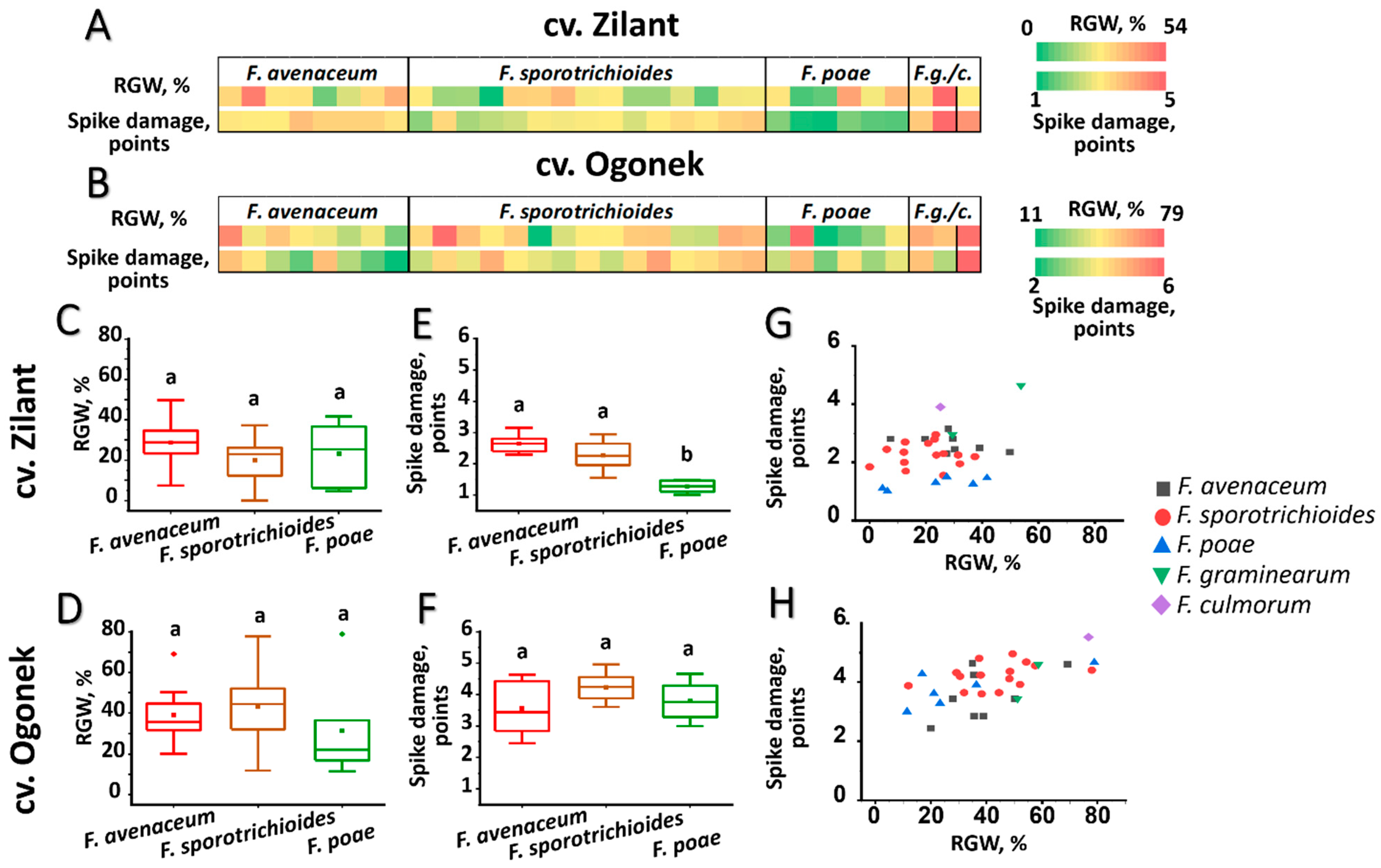
3.3. Virulence of Fusarium ssp. Strains Inhabiting the Volga Region
3.4. Mycotoxin-Producing Potential of Fusarium ssp. Strains Inhabiting the Volga Region
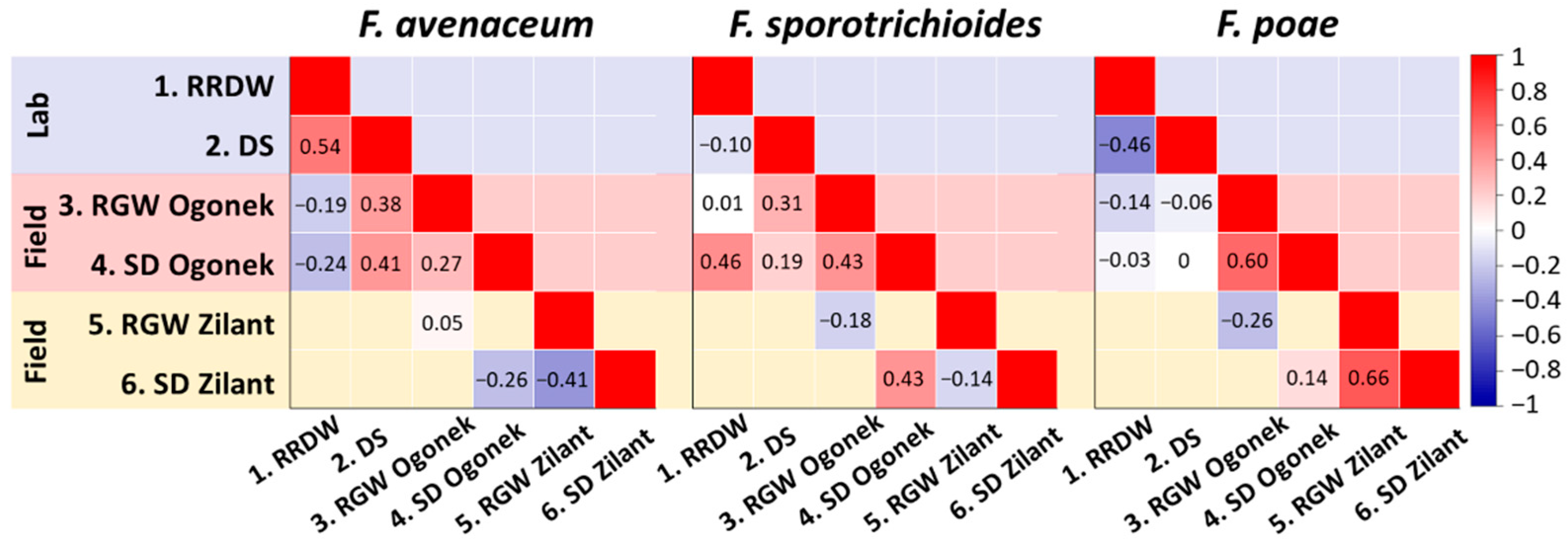
3.5. Analysis of Potential Relationships Between Various Virulence Parameters of Fusarium ssp. Strains Inhabiting the Volga Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a microbiome perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Alisaac, E.; Mahlein, A.K. Fusarium Head Blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Chen, Y.J.; Phukhamsakda, C.; Boekhout, T.; Groenewald, J.Z.; McKenzie, E.H.C.; Francisco, E.C.; Frisvad, J.C.; Groenewald, M.; Hurdeal, V.G.; et al. What are the 100 most cited fungal genera? Stud. Mycol. 2024, 108, 1–411. [Google Scholar] [CrossRef] [PubMed]
- Champeil, A.; Doré, T.; Fourbet, J.F. Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Dill-Macky, R. Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 184–210. [Google Scholar]
- Mesterhazy, A. What is Fusarium Head Blight (FHB) resistance and what are its food safety risks in wheat? Problems and solutions—A review. Toxins 2024, 16, 31. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Gaikpa, D.S.; Lieberherr, B.; Maurer, H.P.; Longin, C.F.H.; Miedaner, T. Comparison of rye, triticale, durum wheat and bread wheat genotypes for Fusarium head blight resistance and deoxynivalenol contamination. Plant Breed. 2020, 139, 251–262. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Wang, D.; Brûlé-Babel, A.; Larsen, J.; Henriquez, M.A. Comparative pathogenicity of four Fusarium species causing Fusarium head blight on rye. Can. J. Plant Pathol. 2025, 47, 393–408. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Xu, F.; Liu, W.; Song, Y.; Zhou, Y.; Xu, X.; Yang, G.; Wang, J.; Zhang, J.; Liu, L. The distribution of Fusarium graminearum and Fusarium asiaticum causing Fusarium Head Blight of wheat in relation to climate and cropping system. Plant Dis. 2021, 105, 2830–2835. [Google Scholar] [CrossRef]
- Molto, G.A.; Gonzalez, H.H.; Resnik, S.L.; Pereyra Gonzalez, A. Production of trichothecenes and zearalenone by isolates of Fusarium spp. from Argentinian maize. Food Addit. Contam. 1997, 14, 263–268. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Colleate, A.; Baxter, E.S. Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Eur. J. Plant Pathol. 2002, 108, 685–690. [Google Scholar] [CrossRef]
- Schollenberger, M.; Drochner, W.; Müller, H.M. Fusarium toxins of the scirpentriol subgroup: A review. Mycopathologia 2007, 164, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In vitro and in vivo mycotoxin production of Fusarium species isolated from Finnish grains. Arch. Phytopath. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Gagkaeva, T.Y.; Gavrilova, O.P.; Levitin, M.M.; Novozhilov, K.V. Fuzarioz zernovykh kulʹtur [The fusariosis of cereal crops]. Zashchita i karantin rasteniy [Plant Prot. Quar.] 2011, 5, 69–120. (In Russian) [Google Scholar]
- Miedaner, T.; Gwiazdowska, D.; Waśkiewicz, A. Editorial: Management of Fusarium species and their mycotoxins in cereal food and feed. Front. Microbiol. 2017, 8, 1543. [Google Scholar] [CrossRef]
- Miedaner, T.; Reinbrecht, C.; Schilling, A.G. Association among aggressiveness, fungal colonization, and mycotoxin production of 26 isolates of Fusarium graminearum in winter rye head blight / Beziehung zwischen aggressivität, myzelwachstum und mycotoxin produktion von 26 Fusarium-graminearum-isolaten bei der ährenfusariose des winterroggens. Zeitschrift Für Pflanzenkrankheiten Und Pflanzenschutz/J. Plant. Dis. Prot. 2000, 107, 124–134. [Google Scholar]
- Kolomiets, T.M.; Kiseleva, M.I.; Zhemchuzhina, N.S.; Pankratova, L.F.; Elizarova, S.A. A characteristic of the species composition of pathogenic fungi of the genus Fusarium in corn biocenoses of the Voronezh region. Vavilovskii Zhurnal Genet. Selektsii. 2022, 26, 583–592. [Google Scholar] [CrossRef]
- Alouane, T.; Rimbert, H.; Bormann, J.; González-Montiel, G.A.; Loesgen, S.; Schäfer, W.; Freitag, M.; Langin, T.; Bonhomme, L. Comparative genomics of eight Fusarium graminearum strains with contrasting aggressiveness reveals an expanded open pangenome and extended effector content signatures. Int. J. Mol. Sci. 2021, 22, 6257. [Google Scholar] [CrossRef] [PubMed]
- Zhemchuzhina, N.S.; Kiseleva, M.I.; Kolomiets, T.M.; Ablova, I.B.; Glinushkin, A.P.; Elizarova, S.A. Revealing the diversity of Fusarium micromycetes in agroecosystems of the North Caucasus plains for replenishing the State Collection of Phytopathogenic Microorganisms of the All-Russian Scientific Research Institute of a Phytopathology. Vavilovskii Zhurnal Genet. Selektsii. 2021, 25, 874–881. [Google Scholar] [CrossRef]
- Gang, G.; Miedaner, T.; Schuhmacher, U.; Schollenberger, M.; Geiger, H.H. Deoxynivalenol and nivalenol production by Fusarium culmorum isolates differing in aggressiveness toward winter rye. Phytopathology 1998, 88, 879–884. [Google Scholar] [CrossRef]
- Muthomi, J.W.; Schütze, A.; Dehne, H.-W.; Mutitu, E.W.; Oerke, E.-C. Characterization of Fusarium culmorum isolates by mycotoxin production and aggressiveness to winter wheat. J. Plant Dis. Prot. 2000, 107, 113–123. [Google Scholar]
- Miedaner, T.; Schilling, A.G.; Geiger, H.H. Molecular genetic diversity and variation for aggressiveness in populations of Fusarium graminearum and Fusarium culmorum sampled from wheat fields in different countries. J. Phytopathol. 2001, 149, 641–648. [Google Scholar] [CrossRef]
- Krnjaja, V.S.; Levic, J.; Stanković, S.; Živanov, S.T. Pathogenic and genetic characterisation of Fusarium sporotrichioides. Cereal Res. Comm. 2008, 36 (Suppl. B), 511–512. [Google Scholar]
- Vogelgsang, S.; Sulyok, M.; Bänziger, I.; Krska, R.; Schuhmacher, R.; Forrer, H.R. Effect of fungal strain and cereal substrate on in vitro mycotoxin production by Fusarium poae and Fusarium avenaceum. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 745–757. [Google Scholar] [CrossRef]
- Živanov, S.T.; Stanković, S.; Levic, J.; Krnjaja, V.S. Correlation of deoxynivalenol and zearalenone production by Fusarium species originating from wheat and maize grain. Pesticidi i Fitomedicina 2015, 30, 99–105. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.-R. Toxigenicity and pathogenicity of Fusarium poae and Fusarium avenaceum on wheat. Eur. J. Plant Pathol. 2008, 122, 265–276. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; de Rijk, T.C.; Booij, C.J.; Goedhart, P.W.; Boers, E.A.; Zhao, C.; Waalwijk, C.; Mol, H.G.; van der Lee, T.A. Occurrence of Fusarium Head Blight species and Fusarium mycotoxins in winter wheat in the Netherlands in 2009. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium Head Blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Homdork, S.; Fehrmann, H.; Beck, R. Effects of field application of tebuconazole on yield, yield components and the mycotoxin content of Fusarium-infected wheat grain. J. Phytopathol. 2000, 148, 1–6. [Google Scholar] [CrossRef]
- Hestbjerg, H.; Felding, G.; Elmholt, S. Fusarium culmorum infection of barley seedlings: Correlation between aggressiveness and deoxynivalenol content. J. Phytopathol. 2002, 150, 308–312. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Bockus, W.W.; Nopsa, J.H.; De Wolf, E.D.; Eskridge, K.M.; Peiris, K.H.S.; Dowell, F.E. Effects of integrating cultivar resistance and fungicide application on Fusarium Head Blight and deoxynivalenol in winter wheat. Plant Dis. 2011, 95, 554–560. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Lemańczyk, G.; Twarużek, M.; Błajet-Kosicka, A.; Kazek, M.; Grajewski, J. Fusarium head blight incidence and detection of Fusarium toxins in wheat in relation to agronomic factors. Eur. J. Plant Pathol. 2017, 149, 515–531. [Google Scholar] [CrossRef]
- Liu, W.; Langseth, W.; Skinnes, H.; Elen, O.N.; Sundheim, L. Comparison of visual head blight ratings, seed infection levels, and deoxynivalenol production for assessment of resistance in cereals inoculated with Fusarium culmorum. Eur. J. Plant Pathol. 1997, 103, 589–595. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.F.; Doré, T.; Rossignol, L. Influence of cropping system on Fusarium head blight and mycotoxin levels in winter wheat. Crop Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Christ, D.S.; Gödecke, R.; von Tiedemann, A.; Varrelmann, M. Pathogenicity, symptom development, and mycotoxin formation in wheat by Fusarium species frequently isolated from sugar beet. Phytopathology 2011, 101, 1338–1345. [Google Scholar] [CrossRef]
- Stenglein, S.A.; Dinolfo, M.I.; Barros, G.; Bongiorno, F.; Chulze, S.N.; Moreno, M.V. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant Dis. 2014, 98, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, M.; Blandino, M.; Bruni, R.; Capo, L.; Righetti, L.; Dall’Asta, C. Mycotoxin occurrence in kernels and straws of wheat, barley, and tritordeum. Mycotoxin Res. 2024, 40, 203–210. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.A.; Klink, H. Composition and predominance of Fusarium species causing Fusarium Head Blight in winter wheat grain depending on cultivar susceptibility and meteorological factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Kiecana, I.; Mielniczuk, E. Fusarium Head Blight of winter rye (Secale cereale L.). Acta Agrobot. 2010, 63, 129–135. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kiecana, I.; Cegiełko, M.; Mielniczuk, E.; Perkowski, J. The occurrence of Fusarium poae (Peck) Wollenw. on oat (Avena sativa L.) panicles and its harmfulness. Acta Agrobot. 2012, 65, 169–178. [Google Scholar] [CrossRef]
- Jedidi, I.; Soldevilla, C.; Lahouar, A.; Marín, P.; González-Jaén, M.T.; Said, S. Mycoflora isolation and molecular characterization of Aspergillus and Fusarium species in Tunisian cereals. Saudi J. Biol. Sci. 2018, 25, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Parikka, P.; Konstantinova, P.; Gagkaeva, T.Y. Molecular and morphological diversity of Fusarium species in Finland and northwestern Russia. Eur. J. Plant Pathol. 2004, 110, 573–585. [Google Scholar] [CrossRef]
- Kazartsev, I.A.; Gagkaeva, T.Y.; Gavrilova, O.P.; Gannibal, P.B. Fungal microbiome of barley grain revealed by NGS and mycological analysis. Foods Raw Mater. 2020, 8, 286–297. [Google Scholar] [CrossRef]
- Gagkaeva, T.; Orina, A.; Gavrilova, O. Fusarium head blight in the Russian Far East: 140 years after description of the ‘drunken bread’ problem. PeerJ 2021, 9, e12346. [Google Scholar] [CrossRef]
- Gavrilova, O.P.; Gagkaeva, T.Y.; Orina, A.S.; Gogina, N.N. Diversity of Fusarium species and their mycotoxins in cereal crops from the Asian territory of Russia. Dokl. Biol. Sci. 2023, 508, 9–19. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Gagkaeva, T.; Ward, T.J.; Aoki, T.; Kistler, H.C.; O’Donnell, K. A novel Asian clade within the Fusarium graminearum species complex includes a newly discovered cereal head blight pathogen from the Russian Far East. Mycologia 2009, 101, 841–852. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Ward, T.J.; O’Donnell, K.; Proctor, R.H.; Burkin, A.A.; Kononenko, G.P.; Gavrilova, O.P.; Aoki, T.; McCormick, S.P.; Gagkaeva, T.Y. Fusarium sibiricum sp. nov, a novel type A trichothecene-producing Fusarium from northern Asia closely related to F. sporotrichioides and F. langsethiae. Int. J. Food Microbiol. 2011, 147, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Stakheev, A.A.; Khairulina, D.R.; Zavriev, S.K. Four-locus phylogeny of Fusarium avenaceum and related species and their species-specific identification based on partial phosphate permease gene sequences. Int. J. Food Microbiol. 2016, 225, 27–37. [Google Scholar] [CrossRef]
- Gagkaeva, T.; Gavrilova, O.; Orina, A.; Lebedin, Y.; Shanin, I.; Petukhov, P.; Eremin, S. Analysis of toxigenic Fusarium species associated with wheat grain from three regions of Russia: Volga, Ural, and West Siberia. Toxins 2019, 11, 252. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell: Oxford, UK, 2006; pp. 1–388. [Google Scholar]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed]
- Cobo-D’ıaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. A novel metabarcoding approach to investigate Fusarium species composition in soil and plant samples. FEMS Microbiol. Ecol. 2019, 95, fiz084. [Google Scholar] [CrossRef] [PubMed]
- Wingett, S.W.; Andrews, S. FastQ screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 10, 11030–11035. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetics Analysis Version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Gorshkov, V.; Osipova, E.; Ponomareva, M.; Ponomarev, S.; Gogoleva, N.; Petrova, O.; Gogoleva, O.; Meshcherov, A.; Balkin, A.; Vetchinkina, E.; et al. Rye snow mold-associated Microdochium nivale strains inhabiting a common area: Variability in genetics, morphotype, extracellular enzymatic activities, and virulence. J. Fungi 2020, 6, 335. [Google Scholar] [CrossRef]
- Ponomareva, M.L.; Ponomarev, S.N.; Mannapova, G.S.; Gilmullina, L.F.; Ilalova, L.V.; Vafina, G.S. The new winter rye variety ‘Zilant’ with broad adaptability. Grain Econ. Russ. 2021, 1, 8–13. [Google Scholar] [CrossRef]
- Ponomareva, M.; Gorshkov, V.; Ponomarev, S.; Mannapova, G.; Askhadullin, D.; Askhadullin, D.; Gogoleva, O.; Meshcherov, A.; Korzun, V. Resistance to snow mold as a target trait for rye breeding. Plants 2022, 11, 2516. [Google Scholar] [CrossRef]
- Edwards, S.G.; Pirgozliev, S.R.; Hare, M.C.; Jenkinson, P. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl. Environ. Microbiol. 2001, 67, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.K.; Jensen, J.D.; Rodríguez, A.; Jørgensen, L.N.; Justesen, A.F. TRI12 based quantitative real-time PCR assays reveal the distribution of trichothecene genotypes of F. graminearum and F. culmorum isolates in Danish small grain cereals. Int. J. Food Microbiol. 2012, 157, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Dinolfo, M.I.; Barros, G.G.; Stenglein, S.A. Development of a PCR assay to detect the potential production of nivalenol in Fusarium poae. FEMS Microbiol. Lett. 2012, 332, 99–104. [Google Scholar] [CrossRef]
- Meng, K.; Wang, Y.; Yang, P.; Luo, H.; Bai, Y.; Shi, P.; Yuan, T.; Ma, R.; Yao, B. Rapid detection and quantification of zearalenone-producing Fusarium species by targeting the zearalenone synthase gene PKS4. Food Control 2010, 21, 207–211. [Google Scholar] [CrossRef]
- Kulik, T.; Pszczółkowska, A.; Fordoński, G.; Olszewski, J. PCR approach based on the esyn1 gene for the detection of potential enniatin-producing Fusarium species. Int. J. Food Microbiol. 2007, 116, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals: A review. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Stack, R.W.; Frohberg, R.C.; Casper, H. Reaction of spring wheats incorporating Sumai#3-derived resistance to inoculation with seven Fusarium species. Cereal Res. Comm. 1997, 25, 667–671. [Google Scholar] [CrossRef]
- Xue, A.G.; Ho, K.M.; Butler, G.; Vigier, B.J.; Babcock, C. Pathogenicity of Fusarium species causing head blight in barley. Phytoprotection 2006, 87, 55–61. [Google Scholar] [CrossRef]
- Zavtrikoviene, E.; Gorash, A.; Kadžienė, G.; Matelionienė, N.; Supronienė, S. Pathogenicity of asymptomatically residing Fusarium species in non-gramineous plants and weeds to spring wheat under greenhouse conditions. Pathogens 2022, 11, 1467. [Google Scholar] [CrossRef]
- Tóth, B.; Mesterházy, Á.; Nicholson, P.; Téren, J.; Varga, J. Mycotoxin production and molecular variability of European and American isolates of Fusarium culmorum. In Molecular Diversity and PCR-Detection of Toxigenic Fusarium Species and Ochratoxigenic Fungi, 1st ed.; Mulè, G., Bailey, J.A., Cooke, B.M., Logrieco, A., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 587–599. [Google Scholar] [CrossRef]
- Miedaner, T.; Cumagun, C.J.R.; Chakraborty, S. Population genetics of three important head blight pathogens Fusarium graminearum, F. pseudograminearum and F. culmorum. J. Phytopathol. 2008, 156, 129–139. [Google Scholar] [CrossRef]
- Inbaia, S.; Farooqi, A.; Ray, R.V. Aggressiveness and mycotoxin profile of Fusarium avenaceum isolates causing Fusarium seedling blight and Fusarium head blight in UK malting barley. Front. Plant Sci. 2023, 14, 1121553. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Pattori, E.; Somma, S.; Manstretta, V.; Waalwijk, C.; Moretti, A.; Meca, G.; Rossi, V. Infection incidence, kernel colonisation, and mycotoxin accumulation in durum wheat inoculated with Fusarium sporotrichioides, F. langsethiae or F. poae at different growth stages. Eur. J. Plant Pathol. 2019, 153, 715–729. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Gagkaeva, T. Molecular chemotyping of Fusarium graminearum, F. culmorum, and F. cerealis isolates from Finland and Russia. In Molecular Identification of Fungi, 1st ed.; Gherbawy, Y., Voigt, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 159–177. [Google Scholar]
- Shen, C.M.; Hu, Y.C.; Sun, H.Y.; Li, W.; Guo, J.H.; Chen, H.G. Geographic distribution of trichothecene chemotypes of the Fusarium graminearum species complex in major winter wheat production areas of China. Plant Dis. 2012, 96, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Muthomi, J.; Dehne, H.; Oerke, E.; Mutitu, E.; Hindorf, H. Characterisation of Fusarium graminearum and F. culmorum isolates by mycotoxin production and aggressiveness to wheat. Mycotoxin Res. 2000, 16 (Suppl. S1), 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Walker, S.L.; Leath, S.; Hagler, W.M., Jr.; Murphy, J.P. Variation among isolates of Fusarium graminearum associated with Fusarium head blight in North Carolina. Plant Dis. 2001, 85, 404–410. [Google Scholar] [CrossRef]
- Kachuei, R.; Rezaie, S.; Yadegari, M.H.; Safaie, N.; Allameh, A.; Aref-Poor, M.-A.; Fooladi, A.A.I.; Riazipour, M. Determination of T-2 mycotoxin in Fusarium strains by HPLC with fluorescence detector. J. Appl. Biotechnol. Rep. 2014, 1, 38–43. [Google Scholar]
- Kulik, T.; Jestoi, M. Quantification of Fusarium poae DNA and associated mycotoxins in asymptomatically contaminated wheat. Int. J. Food Microbiol. 2009, 130, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.A.; Von Scheidt, B.; Gacser, A.; Kassner, H.; Lieberei, R.; Schäfer, W.; Salomon, S. Enhanced mycotoxin production of a lipase-deficient Fusarium graminearum mutant correlates to toxin-related gene expression. Eur. J. Plant Pathol. 2007, 117, 1–12. [Google Scholar] [CrossRef]
- Viñas, M.; Karlovsky, P. A Comprehensive review of hypotheses about the biological function of zearalenone, and a new hypothesis for the function of resorcylic and dihydroxyphenylacetic macrolactones in fungi. Toxins 2025, 17, 226. [Google Scholar] [CrossRef] [PubMed]
- Moraes, W.B.; Laurence, V.; Madden, J.G.; Pierce, A.P. Environment, Grain Development, and Harvesting Strategy Effects on Zearalenone Contamination of Grain from Fusarium Head Blight-Affected Wheat Spikes. Phytopathology 2023, 113, 225–238. [Google Scholar] [CrossRef] [PubMed]

| Toxin Type | Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Tested Species | Reference |
|---|---|---|---|---|---|
| Trichotecene | tri5 | CAGATGGAGAACTGGATGGT | GCACAAGTGCCACGTGAC | F. graminearum, F. culmorum | [74] |
| 3ADON | tri12 | AACATGATCGGTGAGGTATCGA | CCATGGCGCTGGGAGTT | F. graminearum, F. culmorum | [75] |
| 15ADON | tri12 | GTTTCGATATTCATTGGAAAGCTAC | CAAATAAGTATCGTCTGAAATTGGAAA | ||
| NIV | tri12 | GCCCATATTCGCGACAATGT | GGCGAACTGATGAGTAACAAAACC | ||
| NIV | tri7 | TATCCTTGCATGGCAATGCC | AAATGGCGATACGAGTATTGA | F. poae | [76] |
| T-2 | tri16 | GGTGAGATTGCTTCGATGTG | CTCAAAGGGCGAATCAACTAC | F. sporotrichioides, F. poae | This study |
| ZEA | PKS4 | CGTCTTCGAGAAGATGACAT | TGTTCTGCAAGCACTCCGA | F. graminearum, F. culmorum F. sporotrichioides | [77] |
| ENN | esyn1 | GGTCTCGATCCATCCAAGTC | GTGAAGAAGGCAGGCTCAAC | F. avenaceum | [78] |
| GGCCTTGAGCCATCCAGATC | CTCGTTGGTAGCCTGCGATCG | F. poae, F. sporotrichioides |
| Strain | Mycotoxin | ||
|---|---|---|---|
| DON | T-2 | ZEA | |
| F. sporotrichioides 10001 | N/A | 23,799.8 | 135.6 |
| F. sporotrichioides 10005 | N/A | 36,627.7 | 151.9 |
| F. sporotrichioides 10011 | N/A | 25,988.7 | 303.9 |
| F. sporotrichioides 10012 | N/A | 32,668.9 | 165.8 |
| F. sporotrichioides 10031 | N/A | 33,542.7 | 0.0 |
| F. sporotrichioides 10034 | N/A | 32,668.9 | 196.6 |
| F. sporotrichioides 10035 | N/A | 27,883.9 | 0.0 |
| F. sporotrichioides 10039 | N/A | 27,397.5 | 0.0 |
| F. sporotrichioides 10046 | N/A | 17,646.3 | 146.9 |
| F. sporotrichioides 10051 | N/A | 22,378.2 | 236.7 |
| F. sporotrichioides 10053 | N/A | 28,882.7 | 264.4 |
| F. sporotrichioides 10055 | N/A | 28,130.3 | 0.0 |
| F. sporotrichioides 10056 | N/A | 33,839.1 | 0.0 |
| F. sporotrichioides 10057 | N/A | 27,397.5 | 0.0 |
| F. sporotrichioides 10058 | N/A | 32,668.9 | 0.0 |
| F. graminearum 10030 | N/A | N/A | 62.6 |
| F. graminearum 10048 | 260,811.9 | N/A | 84.3 |
| F. culmorum 10028 | 302,257.6 | N/A | 918.4 |
| Control mock-inoculated grain | 730.7 | 4394.5 | 119.4 |
| Detection limit | 100.0 | 24.0 | 100.0 |
| Maximum permissible concentrations | |||
| Russia | 700.0 | 100.0 | 1000.0 |
| EU | 1000.0 | 50.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chastukhina, I.B.; Ryazanov, E.A.; Ponomarev, S.N.; Ivanova, I.O.; Pavlova, S.Y.; Sakhabutdinov, I.T.; Osipova, E.V.; Ponomareva, M.L.; Gorshkov, V.Y. Fusarium Species Associated with Spikes and Grains of Cereal Crops in the Volga Region: Virulence and Toxin-Producing Potential. J. Fungi 2025, 11, 841. https://doi.org/10.3390/jof11120841
Chastukhina IB, Ryazanov EA, Ponomarev SN, Ivanova IO, Pavlova SY, Sakhabutdinov IT, Osipova EV, Ponomareva ML, Gorshkov VY. Fusarium Species Associated with Spikes and Grains of Cereal Crops in the Volga Region: Virulence and Toxin-Producing Potential. Journal of Fungi. 2025; 11(12):841. https://doi.org/10.3390/jof11120841
Chicago/Turabian StyleChastukhina, Inna B., Egor A. Ryazanov, Sergey N. Ponomarev, Irina O. Ivanova, Svetlana Y. Pavlova, Ildar T. Sakhabutdinov, Elena V. Osipova, Mira L. Ponomareva, and Vladimir Y. Gorshkov. 2025. "Fusarium Species Associated with Spikes and Grains of Cereal Crops in the Volga Region: Virulence and Toxin-Producing Potential" Journal of Fungi 11, no. 12: 841. https://doi.org/10.3390/jof11120841
APA StyleChastukhina, I. B., Ryazanov, E. A., Ponomarev, S. N., Ivanova, I. O., Pavlova, S. Y., Sakhabutdinov, I. T., Osipova, E. V., Ponomareva, M. L., & Gorshkov, V. Y. (2025). Fusarium Species Associated with Spikes and Grains of Cereal Crops in the Volga Region: Virulence and Toxin-Producing Potential. Journal of Fungi, 11(12), 841. https://doi.org/10.3390/jof11120841






