Aspergillus Endocarditis in Native Valves in Non-Traditional Hosts: A Systematic Review of a Case in a Patient with CREST Syndrome and Advanced Liver Cirrhosis
Abstract
1. Introduction
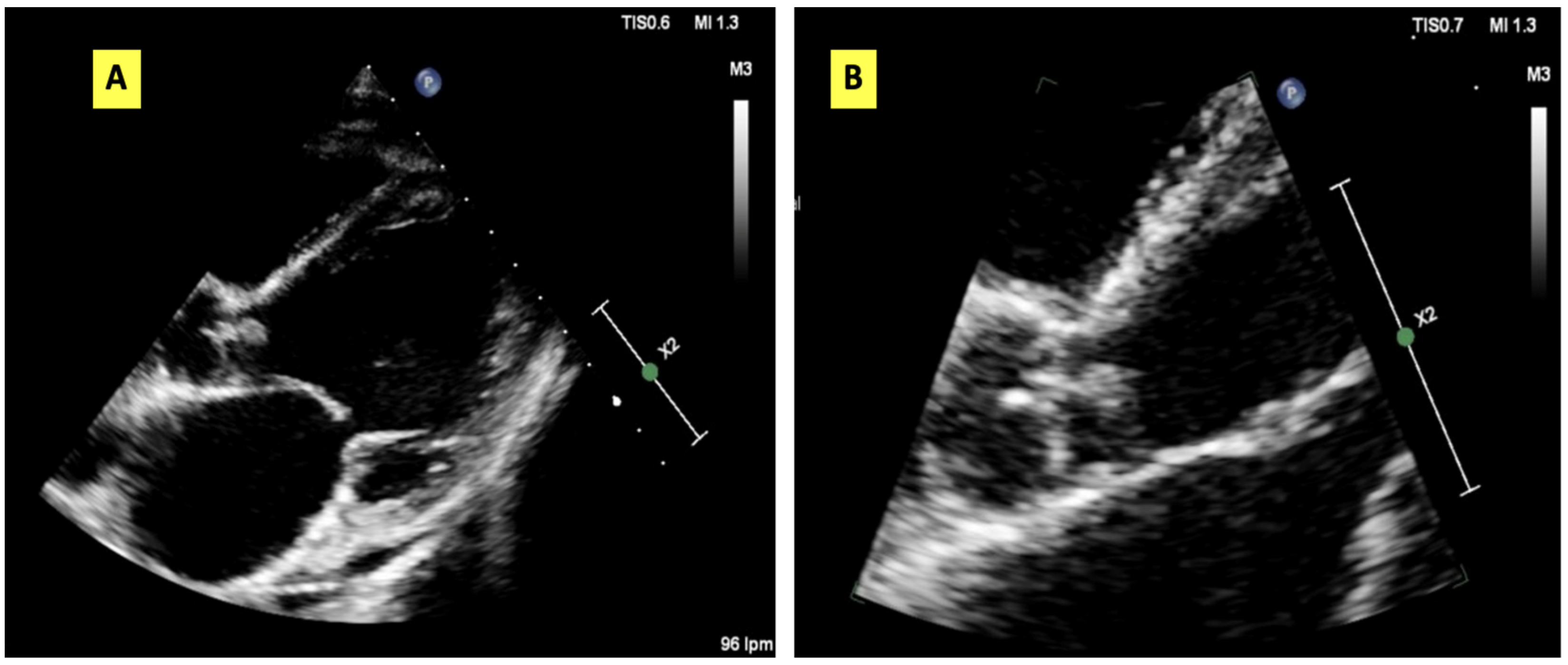
2. Case Presentation
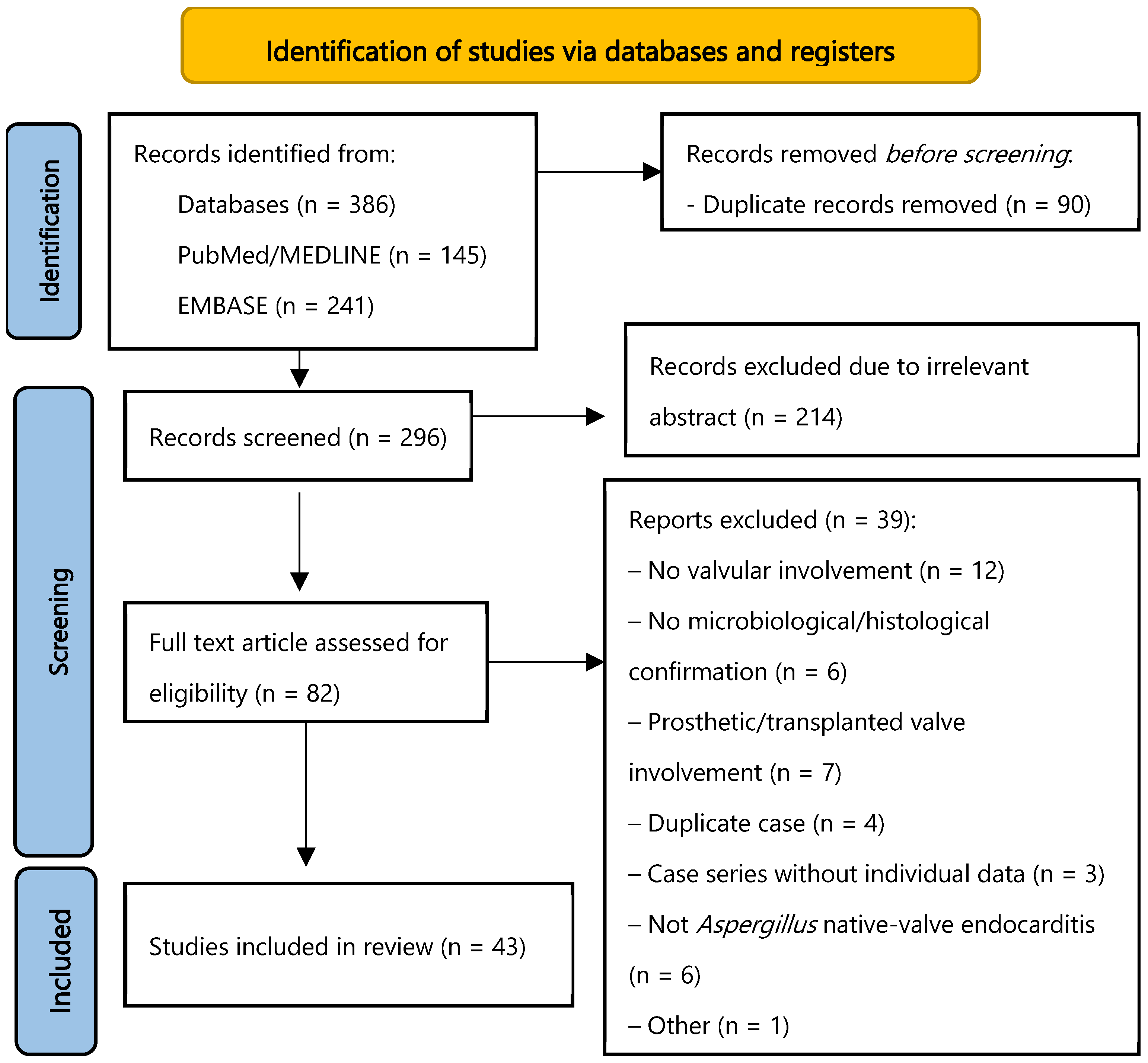
3. Material and Methods
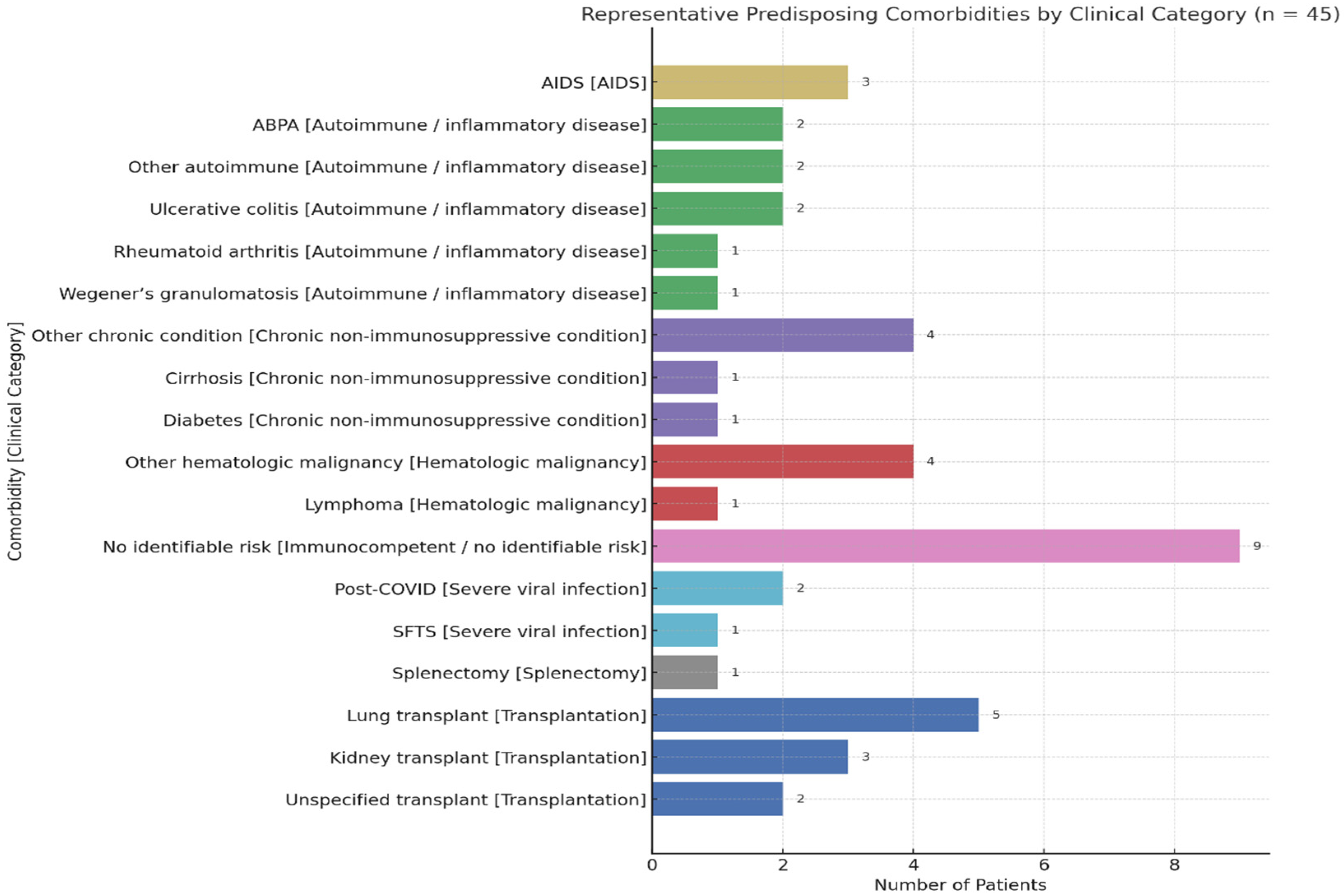
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Aspergillus endocarditis |
| BDG | 1,3-β-D-glucan |
| CAR-T | Chimeric antigen receptor T-cell therapy |
| CT | Computed tomography |
| ESBL | Extended-spectrum beta-lactamase |
| GM | Galactomannan |
| MRI | Magnetic resonance imaging |
| NVAE | Native-valve Aspergillus endocarditis |
| PCR | Polymerase chain reaction |
| TIPS | Transjugular intrahepatic portosystemic shunt |
| TTE | Transthoracic echocardiography |
Appendix A. Full Search Strategy
- -
- No date or language restrictions were applied.
- -
- References were imported into Rayyan, where duplicate records were automatically identified and removed.
- -
- Two reviewers independently screened titles and abstracts using Rayyan.
- -
- Full-text selection and reference management were performed using Zotero.
- -
- The search strategy was peer-reviewed and designed to ensure reproducibility and sensitivity in identifying relevant literature on Aspergillus native-valve endocarditis.
References
- Thompson, G.R., 3rd; Jenks, J.D.; Baddley, J.W.; Lewis, J.S., 2nd; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.; Pappas, P.G.; et al. Fungal endocarditis: Pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin. Microbiol. Rev. 2023, 36, e0001923. [Google Scholar] [CrossRef]
- Caroselli, C.; Suardi, L.R.; Besola, L.; Fiocco, A.; Colli, A.; Falcone, M. Native-valve Aspergillus endocarditis: Case report and literature review. Antibiotics 2023, 12, 1190. [Google Scholar] [CrossRef]
- Born, T.; Aruanno, M.; Kampouri, E.; Mombelli, M.; Monney, P.; Schrenzel, J.; Tozzi, P.; Lamoth, F. Aspergillus tubingensis endocarditis: A case report and review of the literature. Mycopathologia 2022, 187, 249–258. [Google Scholar] [CrossRef]
- Gioia, F.; Walti, L.N.; Orchanian-Cheff, A.; Husain, S. Risk factors for COVID-19-associated pulmonary aspergillosis: A systematic review and meta-analysis. Lancet Respir. Med. 2024, 12, 207–216. [Google Scholar] [CrossRef]
- Yassin, Z.; Hajsadeghi, S.; Shavazi, M.T.; Fattahi, M.; Ahmadzadeh, K.; Farid, A.; Karimi, Y.; Seirafianpour, F.; Babaheidarian, P.; Goodarzi, A. Endocarditis caused by Aspergillus fumigatus in a patient 9 months after COVID-19 infection recovery: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 519. [Google Scholar] [CrossRef]
- Evangelidis, P.; Tragiannidis, K.; Vyzantiadis, A.; Evangelidis, N.; Kalmoukos, P.; Vyzantiadis, T.A.; Tragiannidis, A.; Kourti, M.; Gavriilaki, E. Invasive fungal disease after chimeric antigen receptor-T immunotherapy in adult and pediatric patients. Pathogens 2025, 14, 170. [Google Scholar] [CrossRef]
- Marr, K.A.; Laverdière, M.; Gugel, A.; Leisenring, W.; Blanchard, T.; Bowden, R.A. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 2005, 40, 1762–1769. [Google Scholar] [CrossRef]
- Valerio, M.; Camici, M.; Machado, M.; Galar, A.; Olmedo, M.; Sousa, D.; Antorrena-Miranda, I.; Fariñas, M.C.; Hidalgo-Tenorio, C.; Montejo, M.; et al. Aspergillus endocarditis in the recent years, report of cases of a multicentric national cohort and literature review. Mycoses 2022, 65, 362–373. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef]
- Aggarwal, A.; Hogan, K.; Paez, A. Aspergillus fumigatus endocarditis in a splenectomized patient with no risk factors. IDCases 2020, 19, e00694. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Alghamdi, M.H.; Alhamdan, A.A.; Alamri, M.M.; Ahmed, A.M.; Aziz, M.S. Native valve fungal endocarditis caused by Aspergillus fumigatus: Management dilemma. Oxf. Med. Case Rep. 2020, 2020, omz147. [Google Scholar] [CrossRef]
- Attia, R.Q.; Nowell, J.L.; Roxburgh, J.C. Aspergillus endocarditis: A case of near complete left ventricular outflow obstruction. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Amirghofran, A.A.; Ghazi Nour, M.; Shafa, M.; Nemati, M.H. Incidence and outcome of documented fungal endocarditis. Int. Cardiovasc. Res. J. 2014, 8, 152–155. [Google Scholar] [PubMed]
- Caplan, H.I.; Frisch, E.; Houghton, J.D.; Climo, M.S.; Natsios, G.A. Aspergillus fumigatus endocarditis: Report of a case diagnosed during life. Ann. Intern. Med. 1968, 68, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, K.; Barde, F.; Benhamida, S.; Le Meur, M.; Thyrault, M.; Bentoumi, Y.; Lau, N.; Lebut, J. Invasive aspergillosis and endocarditis. Rev. Méd. Interne 2021, 42, 678–685. [Google Scholar] [CrossRef]
- Cox, J.N.; di Dió, F.; Pizzolato, G.P.; Lerch, R.; Pochon, N. Aspergillus endocarditis and myocarditis in a patient with the acquired immunodeficiency syndrome (AIDS): A review of the literature. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 417, 255–259. [Google Scholar] [CrossRef]
- Elzi, L.; Laifer, G.; Bremerich, J.; Vosbeck, J.; Mayr, M. Invasive apergillosis with myocardial involvement after kidney transplantation. Nephrol. Dial. Transplant. 2005, 20, 631–634. [Google Scholar] [CrossRef]
- Fayad, G.; Legout, L.; Colombie, V.; Modine, T.; Senneville, E.; Leroy, O. Aspergillus fumigatus native mitral valve endocarditis. J. Heart Valve Dis. 2009, 18, 472–473. [Google Scholar]
- Fitzpatrick, R.P.; Botros, M.B.; Dolan, B.; Aurigemma, G.P.; Bai, S.; Harrington, C.M. Mitral Valve Aspergilloma in an Immunocompromised Patient with Recurrent Cerebrovascular Accidents. CASE 2021, 5, 377–379. [Google Scholar] [CrossRef]
- Fullin, K.; Aiyer, A.N. Coronary Emboli Resulting in Nstemi in a Patient with Native Mitral Valve Aspergillus Endocarditis After Covid Infection. J. Am. Coll. Cardiol. 2022, 79. [Google Scholar] [CrossRef]
- García, C.G.; García-Fernández, M.A.; Cebada, F.S. Aspergillus endocarditis. Echocardiography 2005, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Gilbey, J.G.; Chalermskulrat, W.; Aris, R.M. Aspergillus endocarditis in a lung transplant recipient. A case report and review of the transplant literature. Ann. Transplant. 2000, 5, 48–53. [Google Scholar] [PubMed]
- Grossman, K.R.; Zacharioudakis, I.; Fleming, A.; Kupferman, T. Fungal endocarditis: Environmental risk factors and challenging diagnosis of a fatal disease. J. Gen. Intern. Med. 2020, 35 (Suppl. 1), S468. [Google Scholar]
- Gupta, K.; Das, A.; Joshi, K.; Nijhawan, R. Aspergillus endocarditis in a known case of allergic bronchopulmonary aspergillosis: An autopsy report. Cardiovasc. Pathol. 2010, 19, e137–e139. [Google Scholar] [CrossRef] [PubMed]
- Ikediobi, U.; Sutton, R.E. Aspergillus fumigatus native valve infective endocarditis in an otherwise healthy adult. JMM Case Rep. 2016, 3, e005018. [Google Scholar] [CrossRef]
- Jan, M.F.; Elias, H.; Mahboob, H. Footprints under the aorta. Chest 2011, 140, 95A. [Google Scholar] [CrossRef]
- Kanda, Y.; Akiyama, H.; Onozawa, Y.; Nannya, Y. Aspergillus endocarditis in a leukemia patient diagnosed by a PCR assay. Kansenshogaku Zasshi J. Jpn. Assoc. Infect. Dis. 1997, 71, 269–272. [Google Scholar] [CrossRef]
- Katsoulis, J.; Aggarwal, A.; Darling, A.H. Very rapid echocardiographic appearance of Aspergillus endocarditis. Aust. N. Z. J. Med. 1998, 28, 60–61. [Google Scholar] [CrossRef]
- Kuijer, P.M.; Kuijper, E.J.; van den Tweel, J.G.; van der Lelie, J. Aspergillus fumigatus, a rare cause of fatal coronary artery occlusion. Infection 1992, 20, 45–47. [Google Scholar] [CrossRef]
- Kuroki, K.; Murakami, T. Aspergillus endocarditis in a native valve without prior cardiac surgery. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Toby, M.M.; Martin, R.C.; Anne, V.H.; Banner, N.R. Native valve Aspergillus endocarditis complicating lung transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2008, 27, 910–913. [Google Scholar] [CrossRef]
- Kavcic, M.; Hojs, M.; Kuder, J.; Jereb, M. Native valve Aspergillus fumigatus endocarditis in a patient with autoimmune hepatitis on low dose systemic corticosteroids: A case report. IDCases 2023, 31, e01728. [Google Scholar] [CrossRef]
- Marín, P.; García-Martos, P.; García-Doncel, A.; Ruiz-Aragón, J. Endocarditis by Aspergillus fumigatus in a renal transplant. Mycopathologia 1999, 145, 127–129. [Google Scholar] [CrossRef]
- Minhas, H.S.; Jain, G.; Mangukia, C.; Sanghvi, S. Pulmonary endarterectomy for saddling pulmonary embolism by Aspergillus fungus in an immunocompetent patient. Indian Heart J. 2014, 66, 539–542. [Google Scholar] [CrossRef]
- Najafi, N.; Moslemi, A.; Ghafari, R.; Heydari, H. Post-COVID-19 fatal Aspergillus endocarditis: A case report. J. Clin. Lab. Anal. 2023, 37, e24816. [Google Scholar] [CrossRef] [PubMed]
- Ngampongpan, W.; Meemook, K.; Khajarern, S.; Romphothong, K. Acute Cardiovascular Care 2019. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 5–440. [Google Scholar] [CrossRef]
- Nusbaum, D.; Lu, L.; Chow, L. A rare cause of leg claudication: A fungal infection of the femoral artery. In Proceedings of the Society of General Internal Medicine 33rd Annual Meeting, Minneapolis, MN, USA, 28 April–1 May 2010; pp. S484–S485. [Google Scholar]
- Palomares, J.C.; Bernal, S.; Marín, M.; Martín-Rabadán, P.; Bouza, E. Molecular diagnosis of Aspergillus fumigatus endocarditis. Diagn. Microbiol. Infect. Dis. 2011, 70, 534–537. [Google Scholar] [CrossRef]
- Pemán, J.; Ortiz, R.; Osseyran, F.; Salavert, M.; Zaragoza, R.; Viudes, A.; Gobernado, M. Native valve Aspergillus fumigatus endocarditis with blood culture positive and negative for galactomannan antigen. Case report and literature review. Rev. Iberoam. Micol. 2007, 24, 157–160. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, M.; Kundi, A. Aspergillus fumigatus endocarditis. JPMA J. Pak. Med. Assoc. 1990, 40, 95–96. [Google Scholar]
- Regueiro, F.; Gutiérrez, F.; Mons, R.; Merino, E.; Ros, L.; Belda, J.; Martínez, E. Aspergillus endocarditis in lung transplant recipient: Successful surgical treatment. Ann. Thorac. Surg. 2013, 96, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Rofaiel, R.; Turkistani, Y.; McCarty, D.; Adalja, A.; Raza, S.; Cunningham, M.W. Fungal mobile mass on echocardiogram: Native mitral valve Aspergillus fumigatus endocarditis. BMJ Case Rep. 2016, 2016, bcr2016217281. [Google Scholar] [CrossRef]
- Saxena, P.; Clarke, B.; Dunning, J. Aspergillus endocarditis of the mitral valve in a lung-transplant patient. Tex. Heart Inst. J. 2007, 34, 95–97. [Google Scholar]
- Scherer, M.; Fieguth, H.-G.; Aybek, T.; Mohr, F.W.; Haverich, A. Disseminated Aspergillus fumigatus infection with consecutive mitral valve endocarditis in a lung transplant recipient. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2005, 24, 2297–2300. [Google Scholar] [CrossRef]
- Sloane, K.L.; Raymond, S.B.; Rabinov, J.D.; Kiser, T.H.; Mistry, E.A.; Ziai, W.C. Mechanical Thrombectomy in Stroke from Infective Endocarditis: Case Report and Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 104501. [Google Scholar] [CrossRef]
- Van Meensel, B.; Meersseman, W.; Bammens, B.; Peetermans, W.E.; Herregods, M.-C.; Herijgers, P.; Lagrou, K. Fatal right-sided endocarditis due to Aspergillus in a kidney transplant recipient. Med. Mycol. 2007, 45, 565–568. [Google Scholar] [CrossRef]
- Vassiloyanakopoulos, A.; Falagas, M.E.; Allamani, M.; Papageorgiou, T.; Alexandrou, P.; Gkegkes, I.; Zissimopoulos, A. Aspergillus fumigatus tricuspid native valve endocarditis in a non-intravenous drug user. J. Med. Microbiol. 2006, 55, 635–638. [Google Scholar] [CrossRef]
- Vohra, S.; Taylor, R.; Aronowitz, P. The tell-tale heart: Aspergillus fumigatus endocarditis in an immunocompetent patient. Hosp. Pract. 2013, 41, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Wu, J.-L.; Zeng, X.-D.; Liu, J.; Guo, H.-M.; Chen, J.-M. Native mitral valve fungal endocarditis caused by Aspergillus fumigatus: A case report. Int. J. Surg. Case Rep. 2024, 114, 109128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, X.; Wang, X.; Li, L. Severe fever with thrombocytopenia syndrome complicated with aspergillus endocarditis and multiple organ infarctions after glucocorticoid treatment in an immunocompetent man: A case report. BMC Infect. Dis. 2025, 25, 116. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, W.; Yeo, D.G.D.; Chua, W.C.; Tan, T.L.; Lim, S.Y.; Goh, B.H.; Ong, K.C.; Ng, C.C.; Lee, W.K.; et al. Mitral Valve Aspergillus Endocarditis with Aortal Embolization in an Immunocompetent Patient. JACC Case Rep. 2025, 30, 103325. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. COVID-19 Study Group. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses. 2021, 64, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Gaudin, M.; Melenotte, C.; Chasson, L.; Edouard, S.; Verdonk, C.; Prudent, E.; Amphoux, B.; Meresse, S.; Dorent, R.; et al. Metagenomic Analysis of Microdissected Valvular Tissue for Etiological Diagnosis of Blood Culture-Negative Endocarditis. Clin. Infect. Dis. 2020, 70, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Number of Patients | Predisposing Condition | Valve | Diagnosis | Antifungals | Outcome | Surgery |
|---|---|---|---|---|---|---|---|---|
| Aggarwal [11] | United States | 1 | Post-traumatic splenectomy | Mitral | Valvular histology + culture | Amphotericin B | Deceased | Yes |
| Aldosari [12] | Saudi Arabia | 1 | Wegener’s granulomatosis | Mitral | Valvular histology + culture | Azole + amphotericin B | Survivor | Yes |
| Attia [13] | United Kingdom | 1 | Allogeneic transplant + lymphoma | Aortic | Valvular histology + culture | Azole + amphotericin B | Deceased | Yes |
| Badiee [14] | Iran | 1 | None | Aortic | Culture + PCR on valvular tissue | Azole + amphotericin B | Deceased | Yes |
| Caplan [15] | United States | 1 | Liver cirrhosis | Aortic | Valvular histology + culture | Amphotericin B | Deceased | No |
| Chevalier [16] | France | 2 | Kidney transplant/IgA vasculitis | Aortic/Mitral | Valvular culture only | Not reported/Azole | Deceased | No |
| Cox [17] | France | 1 | Kidney transplant | Mitral | Valvular histology + culture | No treatment (postmortem) | Deceased | No |
| Elzi [18] | Switzerland | 1 | Kidney transplant | Aortic | Valvular histology + culture | Azole + amphotericin B | Deceased | No |
| Fayad [19] | France | 1 | None | Mitral | Valvular culture only | Azole + echinocandin | Survivor | Yes |
| Fitzpatrick [20] | United States | 1 | Ulcerative colitis | Mitral | Valvular histology + culture | Azole | Survivor | Yes |
| Fullin [21] | United States | 1 | Post-COVID | Mitral | Valvular culture only | Azole | Survivor | Yes |
| García [22] | Spain | 1 | HIV | Mitral | Valvular culture only | Not reported | Deceased | No |
| Gilbey [23] | United States | 1 | Lung transplant | Mitral | Valvular histology only | Sequential polyene → azole | Deceased | No |
| Grossman [24] | United States | 1 | None | Aortic | Valvular culture only | Azole + amphotericin B | Deceased | Yes |
| Gupta [25] | India | 1 | COPD | Tricuspid | Valvular histology only | Not reported | Deceased | No |
| Ikediobi [26] | United States | 1 | None | Mitral | Valvular histology + culture | Azole + amphotericin B | Deceased | Yes |
| Jan [27] | United States | 1 | Leukemia | Aortic | Valvular histology + culture | No treatment (postmortem) | Deceased | Yes |
| Kanda [28] | Japan | 1 | Chronic myeloid leukemia | Mitral | PCR on valvular tissue (no culture) | Amphotericin B | Deceased | No |
| Katsoulis [29] | Australia | 1 | None | Mitral | Valvular histology + culture | No treatment (postmortem) | Deceased | No |
| Kuijer [30] | Netherlands | 1 | Hairy cell leukemia | Aortic | Valvular culture only | Amphotericin B | Deceased | No |
| Kuroki [31] | Japan | 1 | Colon cancer | Mitral | Valvular histology + culture | Azole | Survivor | Yes |
| Maher [32] | United Kingdom | 1 | Lung transplant | Aortic/Mitral | Valvular culture only | Azole + amphotericin B | Deceased | Yes |
| Manja [33] | Slovenia | 1 | Autoimmune hepatitis | Mitral | Valvular histology + culture | Azole | Survivor | Yes |
| Marín [34] | Spain | 1 | Kidney transplant | Mitral | Valvular histology + culture | Not reported | Deceased | No |
| Minhas [35] | India | 1 | Congenital heart disease (restrictive VSD) | Tricuspid | Valvular histology + culture | Amphotericin B + echinocandin | Survivor | Yes |
| Najafi [36] | Iran | 1 | Post-COVID-19 | Mitral | Culture + PCR on valvular tissue | Azole + amphotericin B | Deceased | Yes |
| Ngampongpan [37] | United Kingdom | 1 | No clinical data available | Aortic | Valvular histology + culture | Amphotericin B | Deceased | Yes |
| Nusbaum [38] | United States | 1 | HIV | Mitral | Valvular culture only | Amphotericin B | Survivor | Yes |
| Palomares [39] | Spain | 1 | Chronic lymphocytic leukemia | Mitral | Histology + PCR on valvular tissue | Azole | Deceased | No |
| Pemán [40] | Spain | 1 | COPD | Mitral | Valvular histology + culture | Azole + echinocandin | Deceased | Yes |
| Rahman [41] | Pakistan | 1 | ABPA | Aortic | Valvular histology + culture | Amphotericin B | Survivor | Yes |
| Regueiro [42] | Spain | 1 | Lung transplant | Aortic | Valvular culture only | Amphotericin B + echinocandin | Deceased | Yes |
| Rofaiel [43] | United States | 1 | Acute promyelocytic leukemia | Mitral | Valvular histology + culture | Amphotericin B monotherapy | Deceased | Yes |
| Saxena [44] | Australia | 1 | None | Mitral | Valvular histology + culture | Amphotericin B + echinocandin | Survivor | Yes |
| Scherer [45] | Germany | 1 | Lung transplant | Mitral | Valvular histology + culture | Azole + amphotericin B | Deceased | Yes |
| Sloane [46] | United States | 1 | Ulcerative colitis | Mitral | Valvular histology + culture | Azole monotherapy | Survivor | Yes |
| Van Meensel [47] | Belgium | 1 | Kidney transplant | Tricuspid | Valvular histology + culture | Azole + amphotericin B | Deceased | Yes |
| Vassiloyanakopoulos [48] | Greece | 1 | Asthma | Tricuspid | Valvular histology + culture | Azole + amphotericin B | Survivor | Yes |
| Vohra [49] | United States | 1 | None | Tricuspid | Valvular histology + culture | Sequential azole → polyene | Deceased | No |
| Yassin [5] | Iran | 1 | Rheumatoid arthritis | Mitral | Culture + PCR on valvular/tissue | Azole monotherapy | Survivor | Yes |
| Zang [50] | China | 1 | None | Mitral | Combined histology, culture, and PCR on valvular tissue | Azole + echinocandin | Survivor | Yes |
| Zhao [51] | China | 1 | SFTS | Aortic | Valvular histology + culture | Azole monotherapy | Survivor | Yes |
| Zhu [52] | Singapore | 1 | Type 2 diabetes | Mitral | Valvular histology + culture | Azole + echinocandin | Deceased | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-del-Barrio, L.; Gómez G. de la Pedrosa, E.; Álvarez-Díaz, N.; Guzmán Martínez, J.; Corbacho Loarte, M.D.; Escudero Sánchez, R.; Martín-Dávila, P.; Fortún Abete, J.; Cobo Reinoso, J.; Pintado García, V.; et al. Aspergillus Endocarditis in Native Valves in Non-Traditional Hosts: A Systematic Review of a Case in a Patient with CREST Syndrome and Advanced Liver Cirrhosis. J. Fungi 2025, 11, 836. https://doi.org/10.3390/jof11120836
Espinosa-del-Barrio L, Gómez G. de la Pedrosa E, Álvarez-Díaz N, Guzmán Martínez J, Corbacho Loarte MD, Escudero Sánchez R, Martín-Dávila P, Fortún Abete J, Cobo Reinoso J, Pintado García V, et al. Aspergillus Endocarditis in Native Valves in Non-Traditional Hosts: A Systematic Review of a Case in a Patient with CREST Syndrome and Advanced Liver Cirrhosis. Journal of Fungi. 2025; 11(12):836. https://doi.org/10.3390/jof11120836
Chicago/Turabian StyleEspinosa-del-Barrio, Leticia, Elia Gómez G. de la Pedrosa, Noelia Álvarez-Díaz, Javier Guzmán Martínez, María Dolores Corbacho Loarte, Rosa Escudero Sánchez, Pilar Martín-Dávila, Jesús Fortún Abete, Javier Cobo Reinoso, Vicente Pintado García, and et al. 2025. "Aspergillus Endocarditis in Native Valves in Non-Traditional Hosts: A Systematic Review of a Case in a Patient with CREST Syndrome and Advanced Liver Cirrhosis" Journal of Fungi 11, no. 12: 836. https://doi.org/10.3390/jof11120836
APA StyleEspinosa-del-Barrio, L., Gómez G. de la Pedrosa, E., Álvarez-Díaz, N., Guzmán Martínez, J., Corbacho Loarte, M. D., Escudero Sánchez, R., Martín-Dávila, P., Fortún Abete, J., Cobo Reinoso, J., Pintado García, V., & Gioia, F. (2025). Aspergillus Endocarditis in Native Valves in Non-Traditional Hosts: A Systematic Review of a Case in a Patient with CREST Syndrome and Advanced Liver Cirrhosis. Journal of Fungi, 11(12), 836. https://doi.org/10.3390/jof11120836







