Improvement of Biocontrol Efficiency of Hanseniaspora thailandica Induced by Alginate Oligosaccharide Against Banana Anthracnose Caused by Colletotrichum musae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Fruits
2.2. Biocontrol Assay of AOS-Induced H. thailandica Lg 3
2.3. Determination of Polyphenol Oxidase (PPO), Peroxidase (POD), Chitinase (CHI) and Beta-1,3-Glucanase (GLU) Activity in Banana Fruits
2.4. Biofilm Formation of AOS-Induced H. thailandica Lg 3
2.5. Population Dynamics Analysis of AOS-Induced H. thailandica Lg 3 in Banana Wounds and NYDB Medium
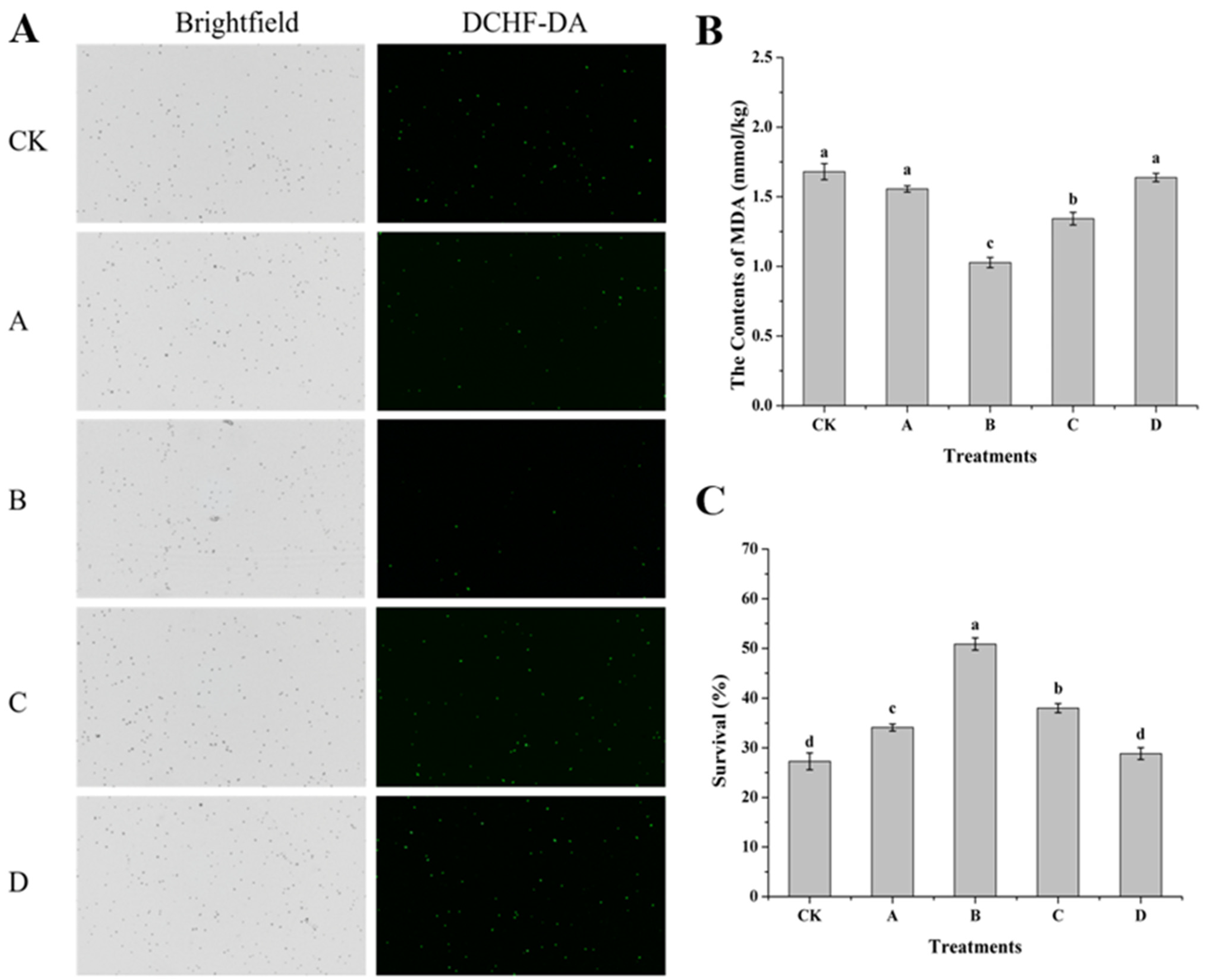
2.6. Determination of Intracellular Reactive Oxygen Species (ROS), MDA Content and Survival Rate of H. thailandica Lg 3 Under Oxidative Stress
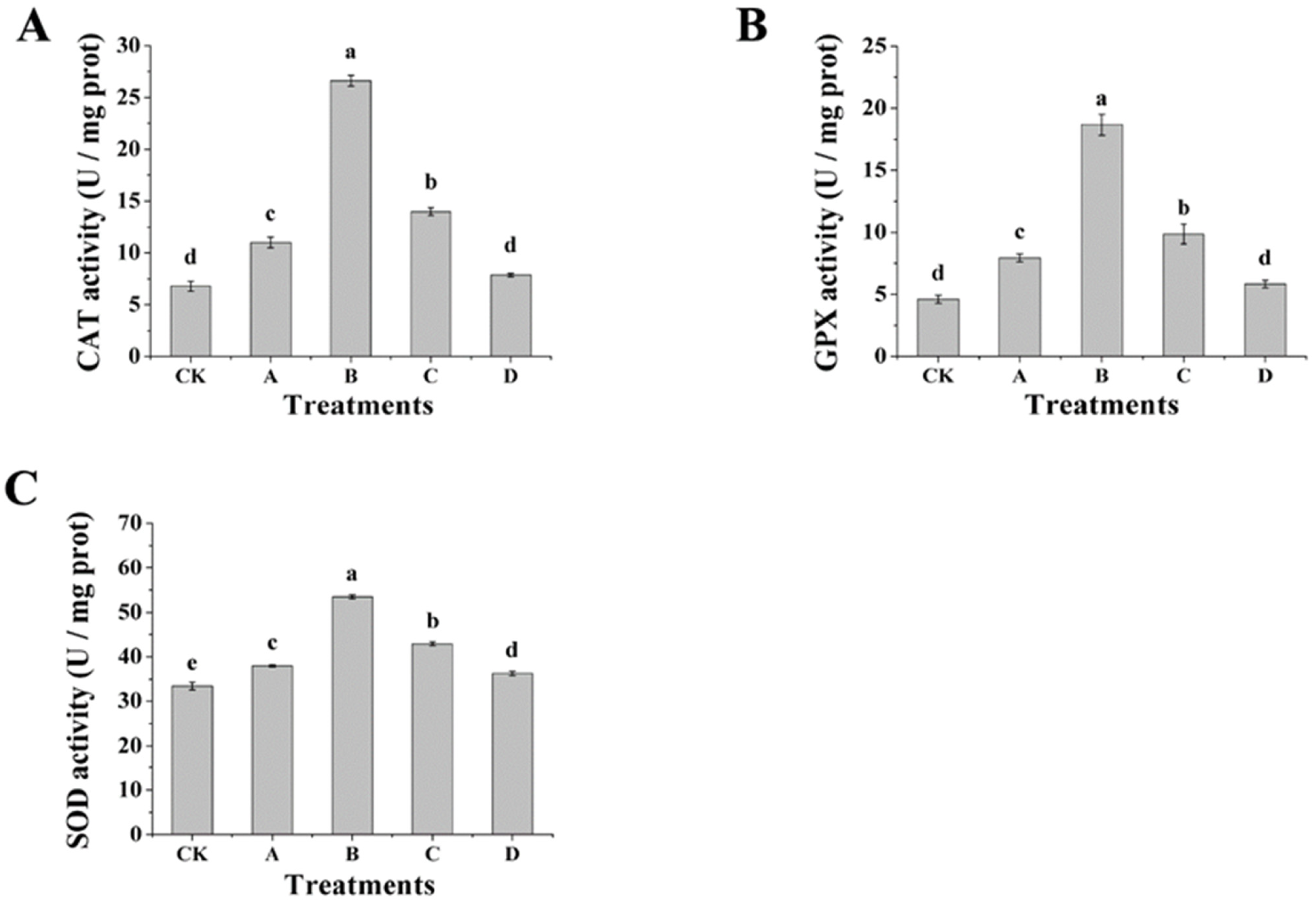
2.7. Determination of the Enzyme Activities of CAT, SOD and GPX
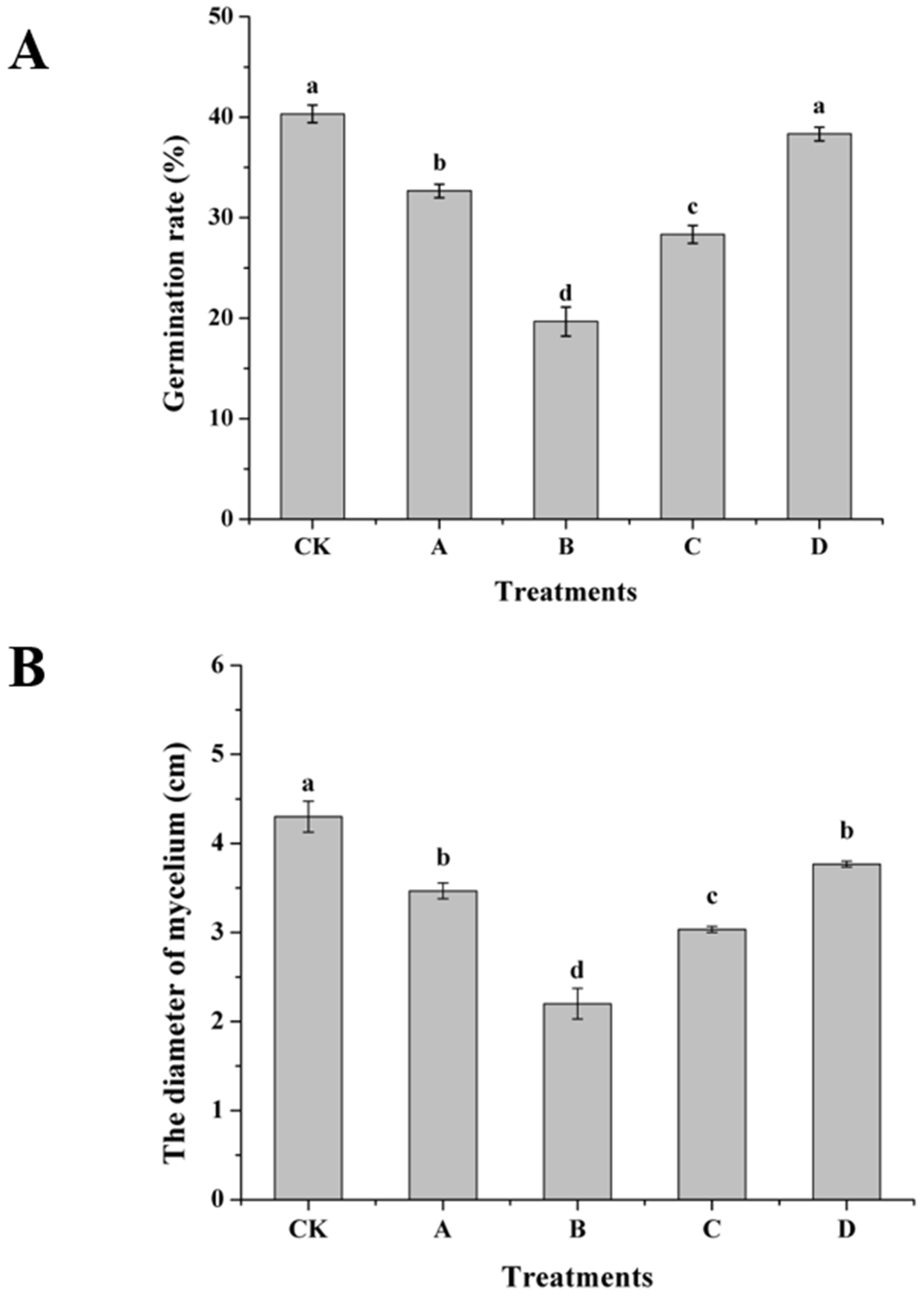
2.8. Inhibition of Spore Germination and Growth In Vitro by AOS-Induced H. thailandica Lg 3
2.9. Transcriptomics Analysis of AOS-Induced H. thailandica Lg 3
2.10. RT-qPCR Verification
2.11. Statistical Analysis
3. Results
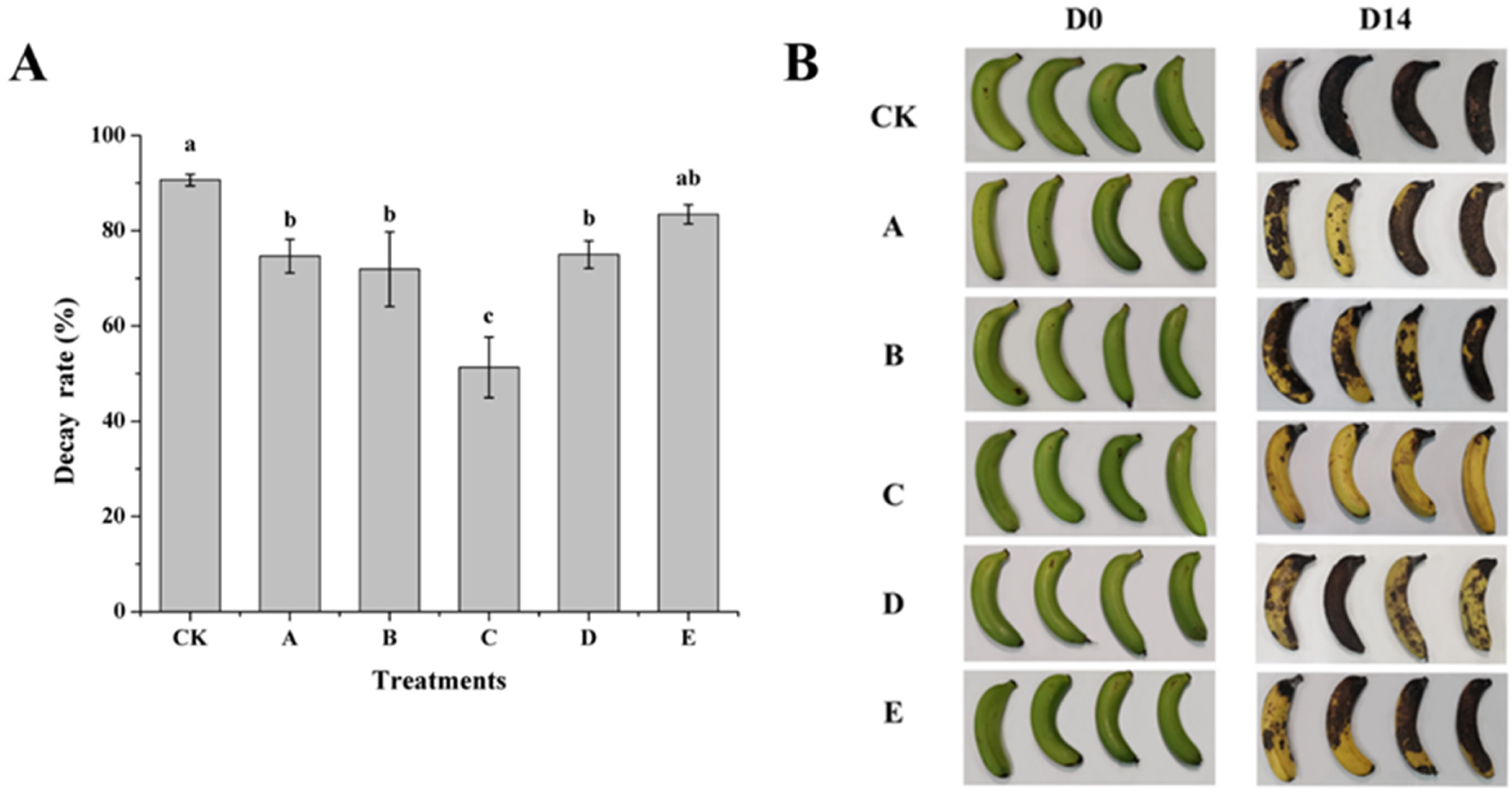
3.1. Effect of AOS on Biocontrol Efficiency of H. thailandica Lg 3 Against C. musae on Banana Fruits
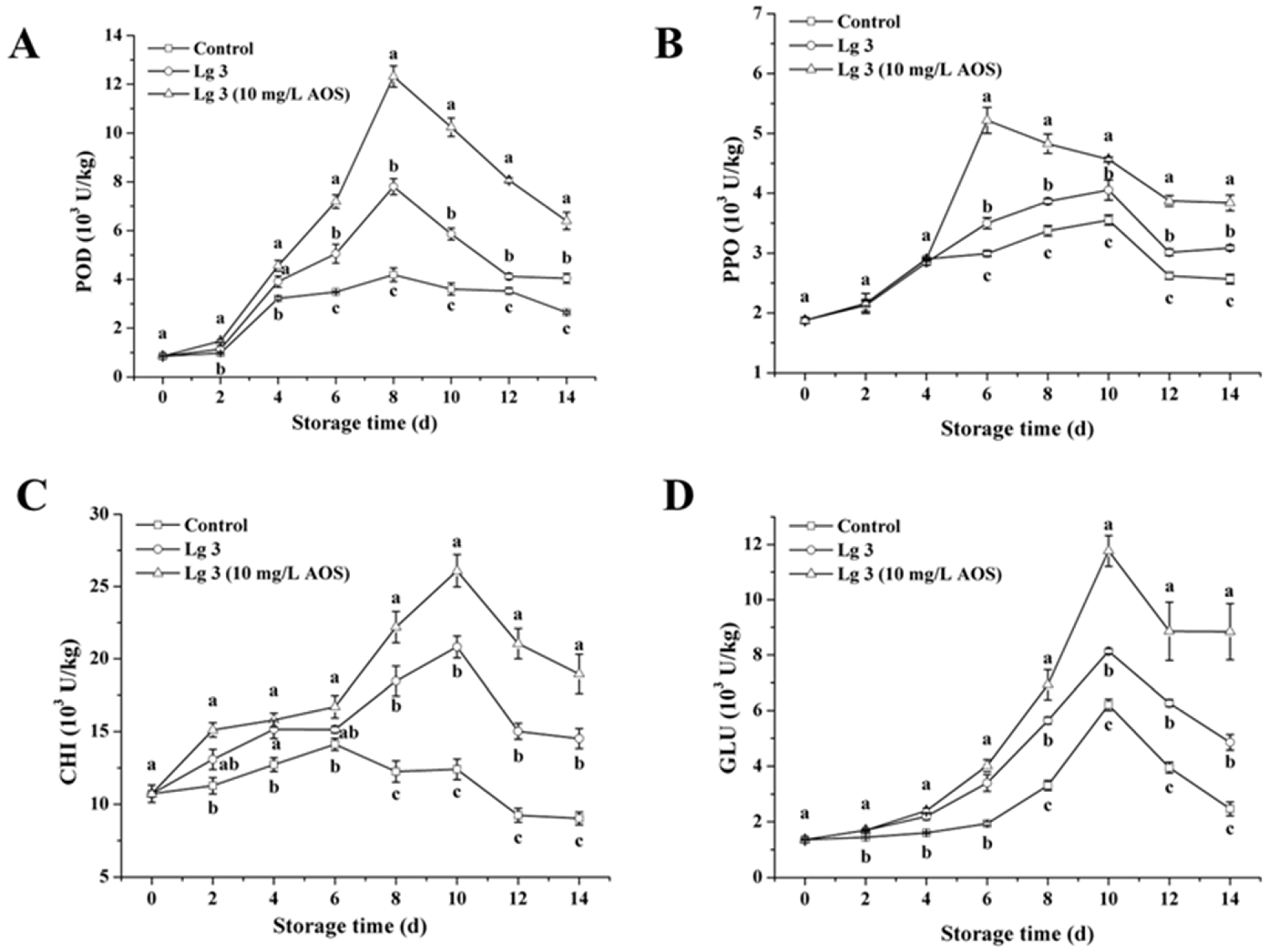
3.2. Effect of H. thailandica Lg 3 Enhanced with AOS on POD, PPO, CHI and GLU Activities in Banana Fruits
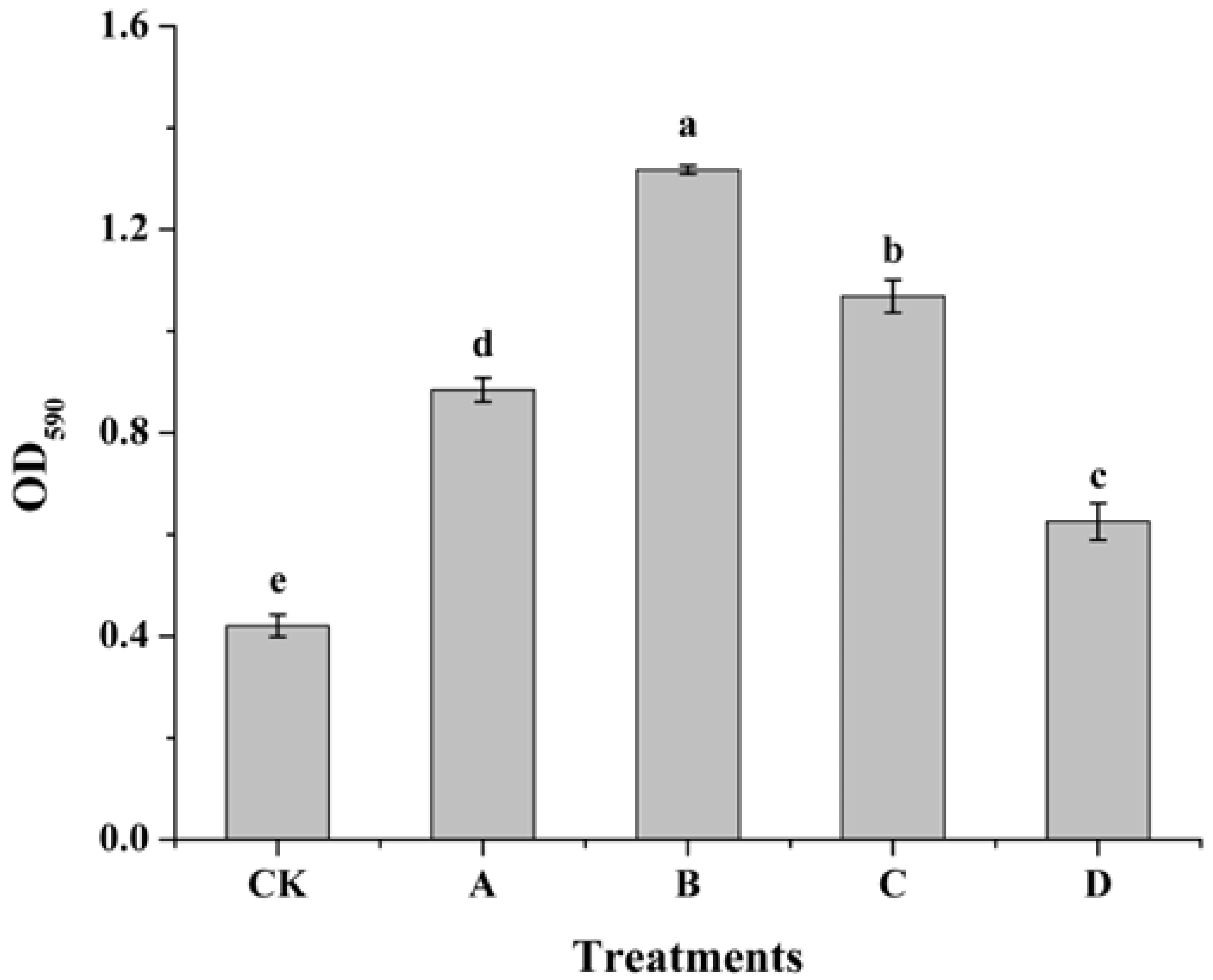
3.3. Effect of AOS on Biofilm Formation of H. thailandica Lg 3
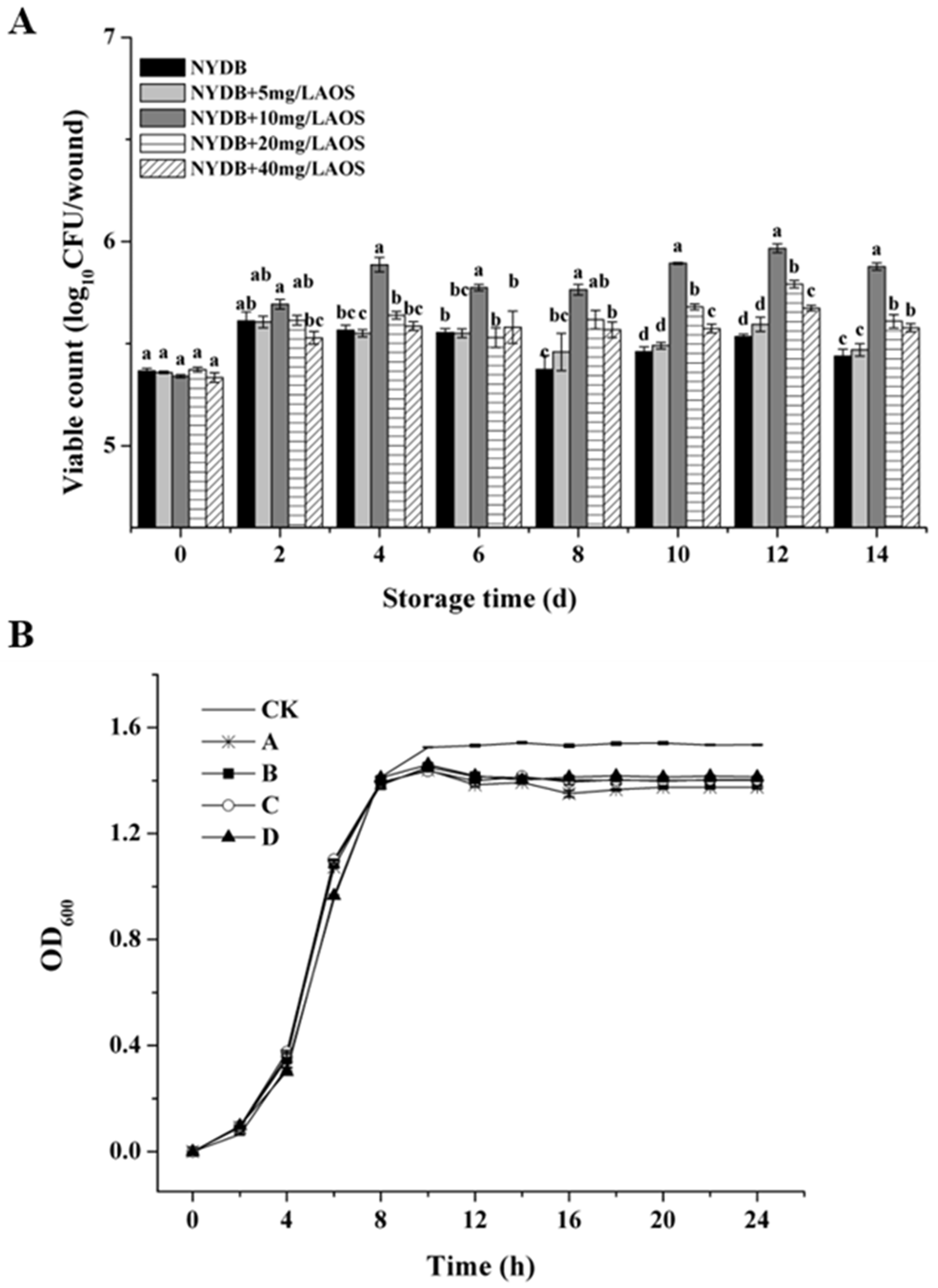
3.4. Effect of AOS on Population Dynamics of H. thailandica Lg 3 in NYDB and Banana Wound
3.5. Effects of AOS on ROS, MDA Content and Survival Rate of H. thailandica Lg 3 Under Oxidative Stress
3.6. Effect of AOS on the Activity of Antioxidant Enzymes and AOS Content of H. thailandica Lg 3
3.7. Effect of AOS on Inhibition of Spore Germination and Growth of H. thailandica Lg 3
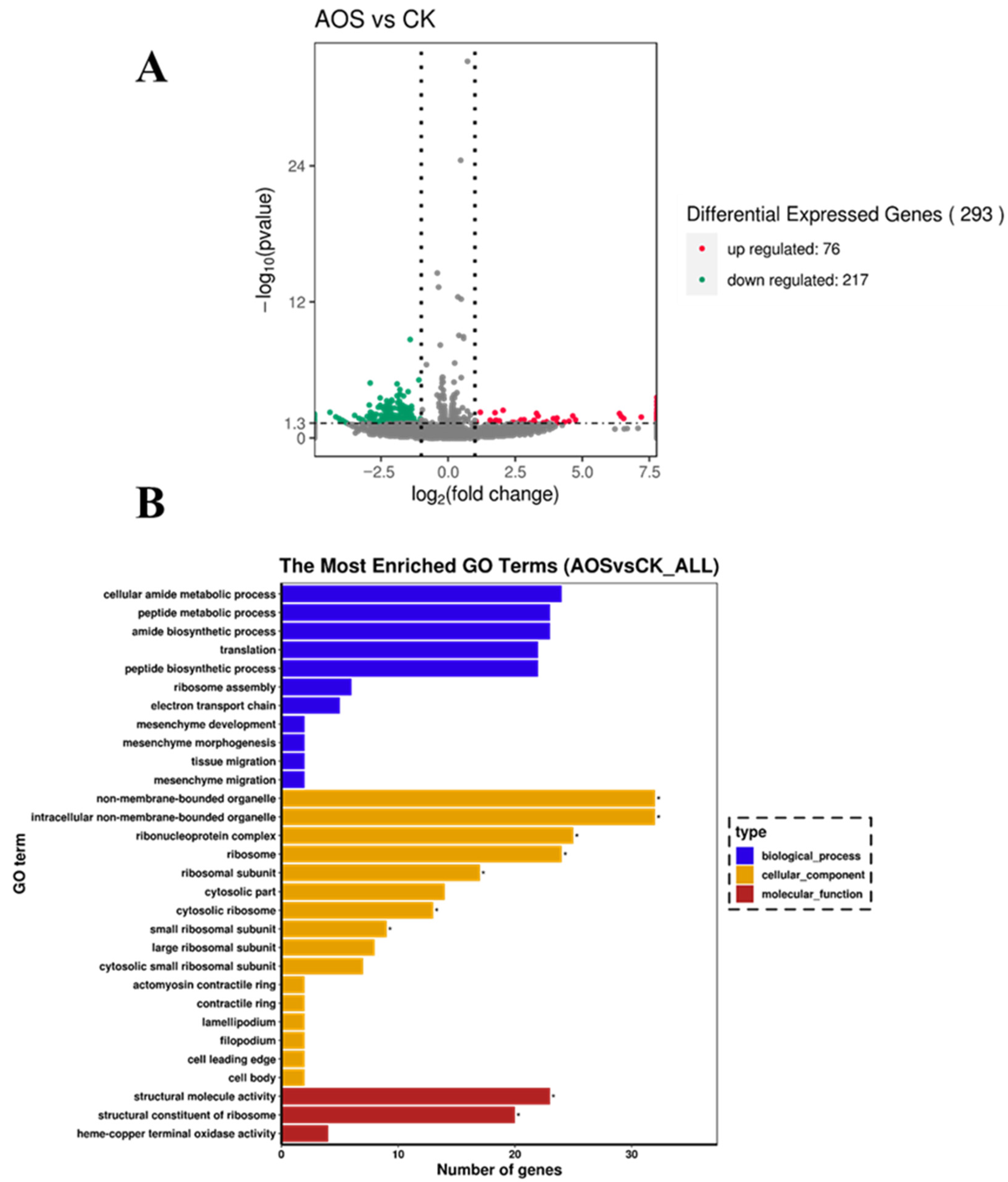
3.8. RNA-Seq Analysis
3.9. GO Enrichment
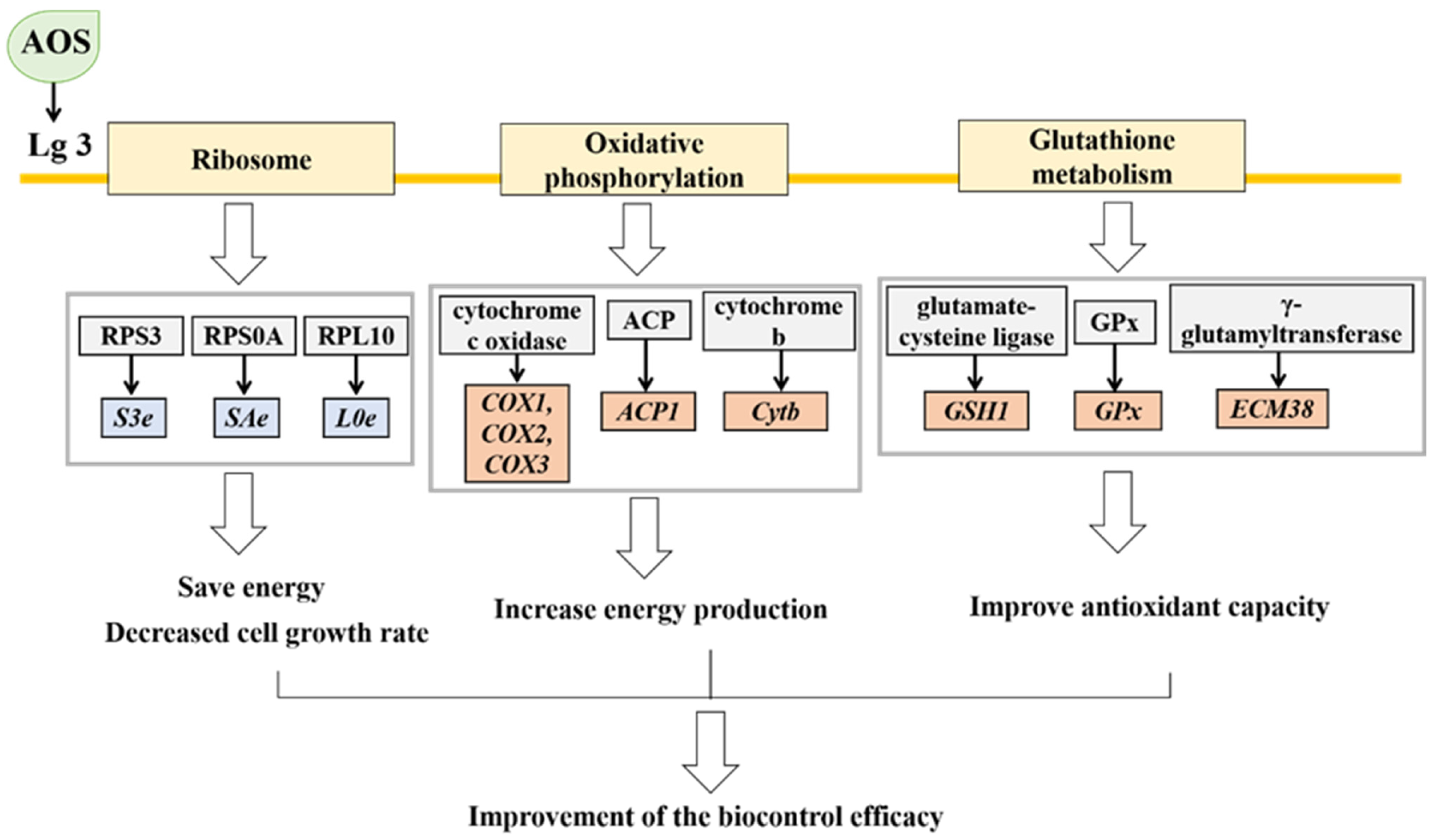
3.10. KEGG Enrichment
3.11. RT-qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seid, F.; Alemu, K.; Tsedaley, B. Effect of combined application of plant defense-inducing chemical, inorganic salt and hot water treatment for the management of postharvest anthracnose of banana (Colletotrichum musae). Discov. Food 2025, 5, 16. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum Species Associated with Anthracnose Disease in Tropical Fruit Crops—A Review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, M.; Qin, X.; Dong, Q.; Li, Z. Antimicrobial function of yeast against pathogenic and spoilage microorganisms via either antagonism or encapsulation: A review. Food Microbiol. 2023, 112, 104242. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, H.; Lin, Y.; Feng, S.; Li, X. Biocontrol mechanisms of antagonistic yeasts on postharvest fruits and vegetables and the approaches to enhance the biocontrol potential of antagonistic yeasts. Int. J. Food Microbiol. 2025, 430, 111038. [Google Scholar] [CrossRef]
- Edo, G.S.; Godana, E.A.; Ngea, G.L.N.; Wang, K.; Yang, Q.; Zhang, H. Improving Biocontrol Potential of Antagonistic Yeasts Against Fungal Pathogen in Postharvest Fruits and Vegetables Through Application of Organic Enhancing Agents. Foods 2025, 14, 3075. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-based biocontrol solutions for fruits and vegetables: Recent insight, patents, and innovative trends. Recent Patents Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Qi, T.; Deng, L.; Yi, L.; Zeng, K. Influence of arginine on the biocontrol efficiency of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 2022, 101, 103888. [Google Scholar] [CrossRef]
- Foku, J.M.; Godana, E.A.; Yang, Q.; Zhang, H. Phytic acid promotes the oxidative stress tolerance of Meyerozyma (Pichia) caribbica enhancing its efficacy against natural decay and retaining the quality of table grapes. Plant Physiol. Biochem. 2025, 219, 109463. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Y.; Zou, X.; Zhao, Z.; Jiang, S.; Chen, Y.; Xu, F.; Shao, X. β-Glucan Enhances the Biocontrol Efficacy of Marine Yeast Scheffersomyeces spartinae W9 against Botrytis cinerea in Strawberries. J. Fungi 2023, 9, 474. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Zheng, X.; Yu, T.; Yan, F. Enhancement of biocontrol efficacy of Kluyveromyces marxianus induced by N-acetylglucosamine against Penicillium expansum. Food Chem. 2023, 404, 134658. [Google Scholar] [CrossRef]
- Guo, S.; Godana, E.A.; Yang, Q.; Wang, K.; Chen, H.; Zhang, H. Culturing of Wickerhamomyces anomalus together with chitosan improves its oxidative stress tolerance and disease defence-related enzymes. N. Z. J. Crop Hortic. Sci. 2025, 53, 549–562. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, D.; Zhang, X.; Solairaj, D.; Xi, Y.; Chen, H.; Li, Y.; Zhang, H. Alginate oligosaccharide-driven resistance in Debaryomyces hansenii Y3: A dual omics perspective. N. Z. J. Crop Hortic. Sci. 2025, 53, 563–586. [Google Scholar] [CrossRef]
- Han, J.; Zhao, L.; Zhu, H.; Dhanasekaran, S.; Zhang, X.; Zhang, H. Study on the effect of alginate oligosaccharide combined with Meyerozyma guilliermondii against Penicillium expansum in pears and the possible mechanisms involved. Physiol. Mol. Plant Pathol. 2021, 115, 101654. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Chen, Y.; Ye, J.; Jiang, S.; Xu, F.; Shao, X. Combined application of alginate oligosaccharide and marine yeast Sporidiobolus pararoseus to control brown rot of peach fruit. Postharvest Biol. Technol. 2024, 208, 112677. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, J.; Tian, X.; Zhang, D.; Wang, Q. Management of blue mold (Penicillium italicum) on mandarin fruit with a combination of the yeast, Meyerozyma guilliermondii and an alginate oligosaccharide. Biol. Control 2021, 152, 104451. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Li, Y.; Han, X.; Luo, X.; Chen, L.; Zhang, T.; Wang, N.; Wang, W. Alginate Oligosaccharides Ameliorate DSS-Induced Colitis through Modulation of AMPK/NF-κB Pathway and Intestinal Microbiota. Nutrients 2022, 14, 2864. [Google Scholar] [CrossRef]
- Peng, C.; Xu, W.; Wang, X.; Meng, F.; Zhao, Y.; Wang, Q.; Wang, X.; Lodi, R.S.; Dong, X.; Zhu, C.; et al. Alginate oligosaccharides trigger multiple defense responses in tobacco and induce resistance to Phytophthora infestans. Front. Plant Sci. 2025, 16, 1506873. [Google Scholar] [CrossRef]
- Wang, W.; Bose, S.K.; Jia, X.; Howlader, P.; Yin, H. A combined analysis of transcriptome and proteome reveals the regulation mechanism of alginate oligosaccharides on alleviating energy deficit in postharvest strawberry. Postharvest Biol. Technol. 2025, 220, 113302. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Zheng, X.; Apaliya, M.T.; Yang, Q.; Zhao, L.; Gu, X.; Zhang, H. Control of postharvest blue mold decay in pears by Meyerozyma guilliermondii and it’s effects on the protein expression profile of pears. Postharvest Biol. Technol. 2018, 136, 124–131. [Google Scholar] [CrossRef]
- Yan, F.; Zhong, J.; Chen, J.; Liu, W.; Chen, X. Application of alginate oligosaccharide produced by enzymatic hydrolysis in the preservation of prawns. Food Biosci. 2022, 49, 101897. [Google Scholar] [CrossRef]
- Zhao, L.; Lan, C.; Tang, X.; Li, B.; Zhang, X.; Gu, X.; Zhang, H. Efficacy of Debaryomyce hansenii in the biocontrol for postharvest soft rot of strawberry and investigation of the physiological mechanisms involved. Biol. Control. 2022, 174, 105011. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Mangieri, N.; Maghradze, D.; Foschino, R.; Valdetara, F.; Cantoral, J.M.; Vigentini, I. Wild Grape-Associated Yeasts as Promising Biocontrol Agents against Vitis vinifera Fungal Pathogens. Front. Microbiol. 2017, 8, 2025. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Yang, Q.; Godana, E.A.; Liu, J.; Li, J.; Zhang, H. Trehalose supplementation enhanced the biocontrol efficiency of Sporidiobolus pararoseus Y16 through increased oxidative stress tolerance and altered transcriptome. Pest Manag. Sci. 2021, 77, 4425–4436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wisniewski, M.; Droby, S.; Vero, S.; Tian, S.; Hershkovitz, V. Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. Int. J. Food Microbiol. 2011, 146, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Godana, E.A.; Yang, Q.; Zhao, L.; Zhang, X.; Liu, J.; Zhang, H. Pichia anomala Induced with Chitosan Triggers Defense Response of Table Grapes Against Post-harvest Blue Mold Disease. Front. Microbiol. 2021, 12, 704519. [Google Scholar] [CrossRef]
- Han, P.; Ni, L.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Optimization of the freeze-drying of marine yeast Sporidiobolus pararoseus ZMY-1 for its application in biocontrol of fungal infections. Biol. Control. 2021, 161, 104707. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Li, Z.; Li, C.; Li, B.; Gu, X.; Zhang, X.; Zhang, H. Screening and identification of an antagonistic yeast controlling postharvest blue mold decay of pears and the possible mechanisms involved. Biol. Control. 2019, 133, 26–33. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Dilip, D.; Sten, J.; Ravat, V.K.; Bhutia, D.D.; Panja, B.; Saha, J. Antagonistic Yeasts for Biocontrol of the Banana Postharvest Anthracnose Pathogen Colletotrichum musae. J. Phytopathol. 2017, 165, 35–43. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Yi, L.; Zeng, K. Tryptophan enhances biocontrol efficacy of Metschnikowia citriensis FL01 against postharvest fungal diseases of citrus fruit by increasing pulcherriminic acid production. Int. J. Food Microbiol. 2023, 386, 110013. [Google Scholar] [CrossRef]
- Adhikari, S.; Ghosh, S.; Azahar, I.; Adhikari, A.; Shaw, A.K.; Konar, S.; Roy, S.; Hossain, Z. Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ. Exp. Bot. 2018, 153, 143–162. [Google Scholar] [CrossRef]
- Bai, X.; Bi, Y.; Li, Y.; Wang, Y.; Niu, L.; Wang, T.; Shang, Q. Mechanism of Latent Infection for Postharvest Diseases of Fruits and Vegetables. Food Sci. 2015, 36, 278–282. [Google Scholar]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.; Wang, J.; Liu, Y.; Deng, Y. Roles of High Osmolarity Glycerol and Cell Wall Integrity Pathways in Cadmium Toxicity in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Q.; Fang, T.; Shen, Y.; Jing, S.; Guo, N. Glycine Enhances Oxidative Stress Tolerance and Biocontrol Efficacy of Sporidiobolus pararoseus against Aspergillus niger Decay of Apples. Foods 2023, 12, 4121. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, S.-Y.; Wang, C.-X.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Antagonistic mechanisms of yeasts Meyerozyma guilliermondii and M. caribbica for the control of plant pathogens: A review. Biol. Control. 2023, 186, 105333. [Google Scholar] [CrossRef]
- He, F.; Zhao, L.; Zheng, X.; Abdelhai, M.H.; Boateng, N.S.; Zhang, X.; Zhang, H. Investigating the effect of methyl jasmonate on the biocontrol activity of Meyerozyma guilliermondii against blue mold decay of apples and the possible mechanisms involved. Physiol. Mol. Plant Pathol. 2020, 109, 101454. [Google Scholar] [CrossRef]
- Warner, J.R. The Economics of Ribosome Biosynthesis in Yeast; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Shah, A.N.; Cadinu, D.; Henke, R.M.; Xin, X.; Dastidar, R.G.; Zhang, L. Deletion of a subgroup of ribosome-related genes minimizes hypoxia-induced changes and confers hypoxia tolerance. Physiol. Genom. 2011, 43, 855–872. [Google Scholar] [CrossRef]
- Zhong, J.; Xiao, C.; Gu, W.; Du, G.; Sun, X.; He, Q.-Y.; Zhang, G. Transfer RNAs Mediate the Rapid Adaptation of Escherichia coli to Oxidative Stress. PLOS Genet. 2015, 11, e1005302. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Liang, S.-L.; Han, S.-Y.; Zheng, S.-P.; Ye, Y.-R.; Lin, Y. Quantitative iTRAQ LC–MS/MS proteomics reveals the cellular response to heterologous protein overexpression and the regulation of HAC1 in Pichia pastoris. J. Proteom. 2013, 91, 58–72. [Google Scholar] [CrossRef]
- Yadavilli, S.; Mayo, L.D.; Higgins, M.; Lain, S.; Hegde, V.; Deutsch, W.A. Ribosomal protein S3: A multi-functional protein that interacts with both p53 and MDM2 through its KH domain. DNA Repair 2009, 8, 1215–1224. [Google Scholar] [CrossRef]
- Seong, K.M.; Jung, S.-O.; Kim, H.D.; Kim, H.J.; Jung, Y.-J.; Choi, S.-Y.; Kim, J. Yeast ribosomal protein S3 possesses a β-lyase activity on damaged DNA. FEBS Lett. 2012, 586, 356–361. [Google Scholar] [CrossRef]
- Tabb-Massey, A.; Caffrey, J.M.; Logsden, P.; Taylor, S.; Trent, J.O.; Ellis, S.R. Ribosomal proteins Rps0 and Rps21 of Saccharomyces cerevisiae have overlapping functions in the maturation of the 3' end of 18S rRNA. Nucleic Acids Res. 2003, 31, 6798–6805. [Google Scholar] [CrossRef]
- Pollutri, D.; Penzo, M. Ribosomal Protein L10: From Function to Dysfunction. Cells 2020, 9, 2503. [Google Scholar] [CrossRef]
- Farre, J.-C.; Carolino, K.; Devanneaux, L.; Subramani, S. OXPHOS deficiencies affect peroxisome proliferation by downregulating genes controlled by the SNF1 signaling pathway. eLife 2022, 11, e75143. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Ishimori, K. Regulation of electron transfer in the terminal step of the respiratory chain. Biochem. Soc. Trans. 2023, 51, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chang, X.; Li, W. Differential analysis of the transcriptome of Cryptococcus laurentii under carboxymethyl cellulose induced culture. Food Ferment. Ind. 2022, 48, 55–63. [Google Scholar]
- Song, Z.; Ding, X.; Tang, X.; Zhu, M.; Hou, Y. Transcriptome analysis of fruiting bodies of Lactarius deliciosus at two developmental stages. Acta Agric. Zhejiangensis 2020, 32, 337–347. [Google Scholar]
- Brody, S.; Oh, C.; Hoja, U.; Schweizer, E. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 1997, 408, 217–220. [Google Scholar] [CrossRef]
- Flores-Mireles, D.; Camacho-Villasana, Y.; Lutikurti, M.; García-Gurrerio, A.E.; Lozano-Rosas, G.; Chagoya, V.; Gutiérrez-Cirlos, E.B.; Brandt, U.; Cabrera-Orefice, A.; Pérez-Martínez, X. The cytochrome b carboxyl terminal region is necessary for mitochondrial complex III assembly. Life Sci. Alliance 2023, 6, 15. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Gavriliuk, L.A. Glutathione Synthesis in Cancer Cells. Biochem. Mosc. 2020, 85, 895–907. [Google Scholar] [CrossRef]
- Kong, M.; Wang, F.; Tian, L.; Tang, H.; Zhang, L. Functional identification of glutamate cysteine ligase and glutathione synthetase in the marine yeast Rhodosporidium diobovatum. Sci. Nat. 2017, 105, 4. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Tran, L.-T.; Kamakura, M.; Izawa, S.; Miki, T.; Tsujimoto, Y.; Kimura, A. Oxidative stress response in yeast: Glutathione peroxidase of Hansenula mrakii is bound to the membrane of both mitochondria and cytoplasm. Biochim. Biophys. Acta (BBA) Gen. Subj. 1995, 1245, 325–330. [Google Scholar] [CrossRef]
- Tsuzi, D.; Maeta, K.; Takatsume, Y.; Izawa, S.; Inoue, Y. Distinct regulatory mechanism of yeast GPX2 encoding phospholipid hydroperoxide glutathione peroxidase by oxidative stress and a calcineurin/Crz1-mediated Ca2+ signaling pathway. FEBS Lett. 2004, 569, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, K.; Thierie, J.; Penninckx, M.J. γ-glutamyl transpeptidase in the yeast Saccharomyces cerevisiae and its role in the vacuolar transport and metabolism of glutathione. Biochem. Soc. 2001, 359, 631–637. [Google Scholar] [CrossRef]
- Jaspers, C.J.; Gigot, D.; Penninckx, M.J. Pathways of glutathione degradation in the yeast Saccharomyces cerevisiae. Phytochemistry 1985, 24, 703–707. [Google Scholar] [CrossRef]
- Minden, S.; Aniolek, M.; Noorman, H.; Takors, R. Performing in spite of starvation: How Saccharomyces cerevisiae maintains robust growth when facing famine zones in industrial bioreactors. Microb. Biotechnol. 2023, 16, 148–168. [Google Scholar] [CrossRef]
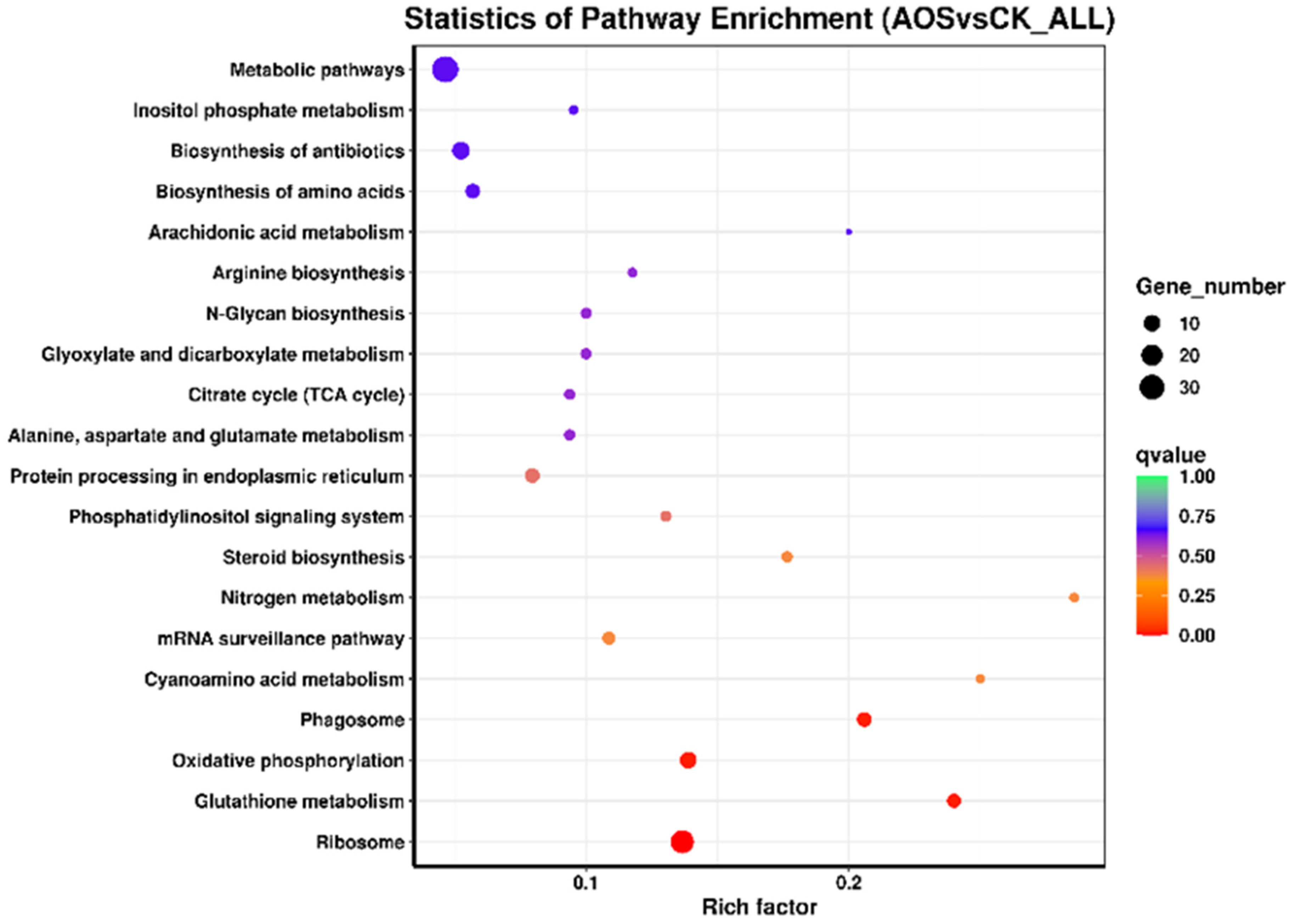
| Sample Name | Clean Reads | Clean Bases | Q30 (%) | GC Content (%) |
|---|---|---|---|---|
| CK_1 | 41,776,868 | 6.31 G | 93.69% | 39.04% |
| CK_2 | 37,422,730 | 5.65 G | 93.87% | 39.19% |
| CK_3 | 42,329,510 | 6.39 G | 93.79% | 38.81% |
| AOS_1 | 42,189,562 | 6.37 G | 94.02% | 38.78% |
| AOS_2 | 41,830,758 | 6.32 G | 93.65% | 39.01% |
| AOS_3 | 40,589,372 | 6.13 G | 94.09% | 38.98% |
| Gene | RNA-Seq (Log2FC) | RT-qPCR(Log2FC) |
|---|---|---|
| L-lactate dehydrogenase | −1.8812 | −1.8600 ± 0.0043 |
| beta-1 tubulin | −1.8262 | −1.7100 ± 0.0030 |
| ribosomal protein P0 | −1.3298 | −1.2033 ± 0.0014 |
| 40S ribosomal protein S6-B | −2.0875 | −2.2433 ± 0.0018 |
| 60S ribosomal protein L8 | −2.0982 | −2.5733 ± 0.0026 |
| 60S ribosomal protein L16 | −1.5576 | −1.5000 ± 0.0043 |
| 60S ribosomal protein L15 | −1.6818 | −1.6967 ± 0.0008 |
| 60S ribosomal protein L10 | −1.4456 | −1.5367 ± 0.0058 |
| glutamate–cysteine ligase | 6.4516 | 5.8167 ± 0.0004 |
| glutathione peroxidase | 7.1974 | 7.3033 ± 0.0029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Chen, X.; Lai, X.; Ren, X.; Hong, J.; Yan, F. Improvement of Biocontrol Efficiency of Hanseniaspora thailandica Induced by Alginate Oligosaccharide Against Banana Anthracnose Caused by Colletotrichum musae. J. Fungi 2025, 11, 824. https://doi.org/10.3390/jof11120824
Wu Y, Chen X, Lai X, Ren X, Hong J, Yan F. Improvement of Biocontrol Efficiency of Hanseniaspora thailandica Induced by Alginate Oligosaccharide Against Banana Anthracnose Caused by Colletotrichum musae. Journal of Fungi. 2025; 11(12):824. https://doi.org/10.3390/jof11120824
Chicago/Turabian StyleWu, Yinfeng, Xiaojie Chen, Xiaoting Lai, Xiaomin Ren, Jianqu Hong, and Fen Yan. 2025. "Improvement of Biocontrol Efficiency of Hanseniaspora thailandica Induced by Alginate Oligosaccharide Against Banana Anthracnose Caused by Colletotrichum musae" Journal of Fungi 11, no. 12: 824. https://doi.org/10.3390/jof11120824
APA StyleWu, Y., Chen, X., Lai, X., Ren, X., Hong, J., & Yan, F. (2025). Improvement of Biocontrol Efficiency of Hanseniaspora thailandica Induced by Alginate Oligosaccharide Against Banana Anthracnose Caused by Colletotrichum musae. Journal of Fungi, 11(12), 824. https://doi.org/10.3390/jof11120824





