Cryptic Diversity and Ecological Overlap in Sporothrix schenckii: Insights from Multilocus Phylogenetics of Clinical and Environmental Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
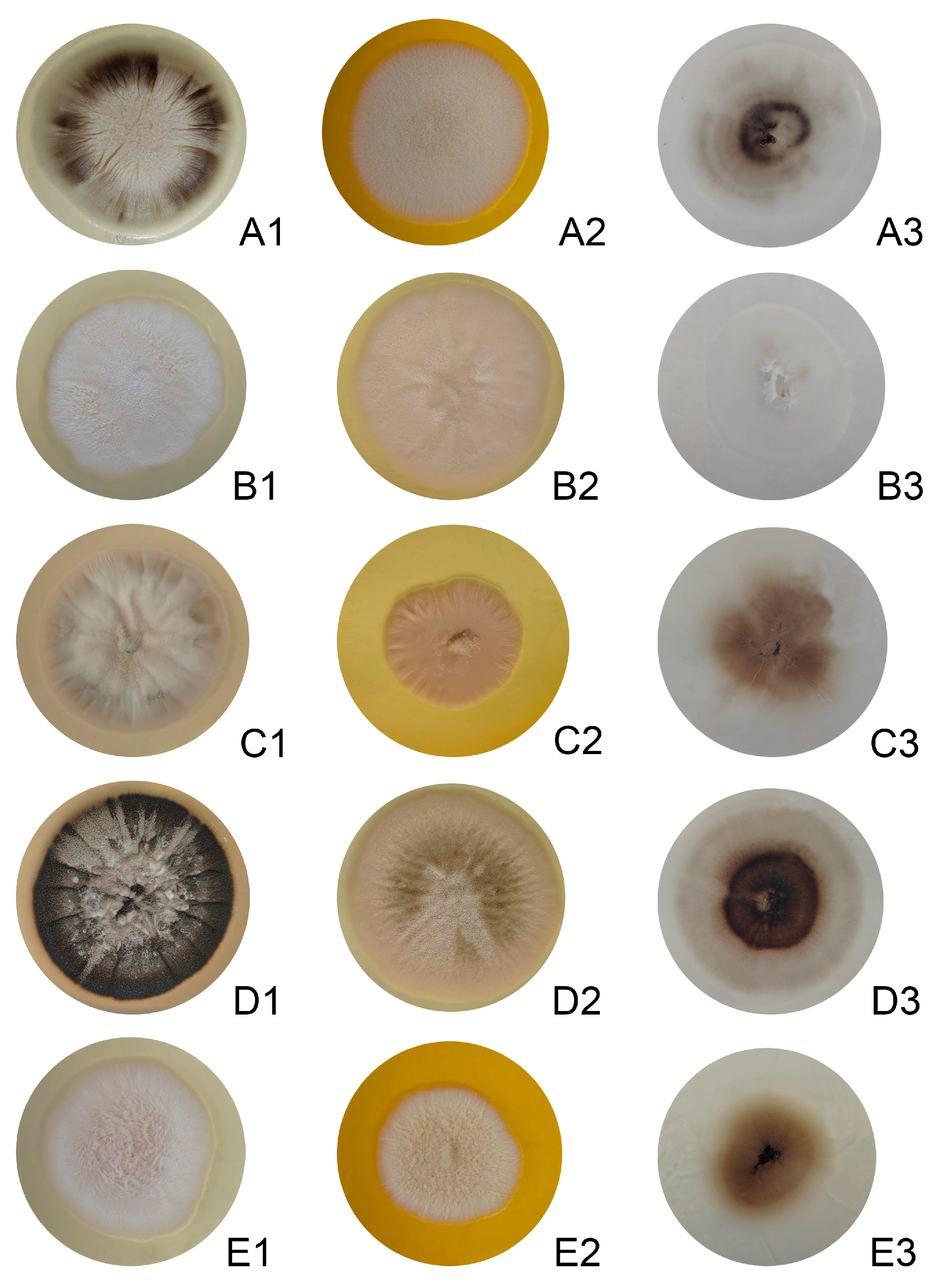
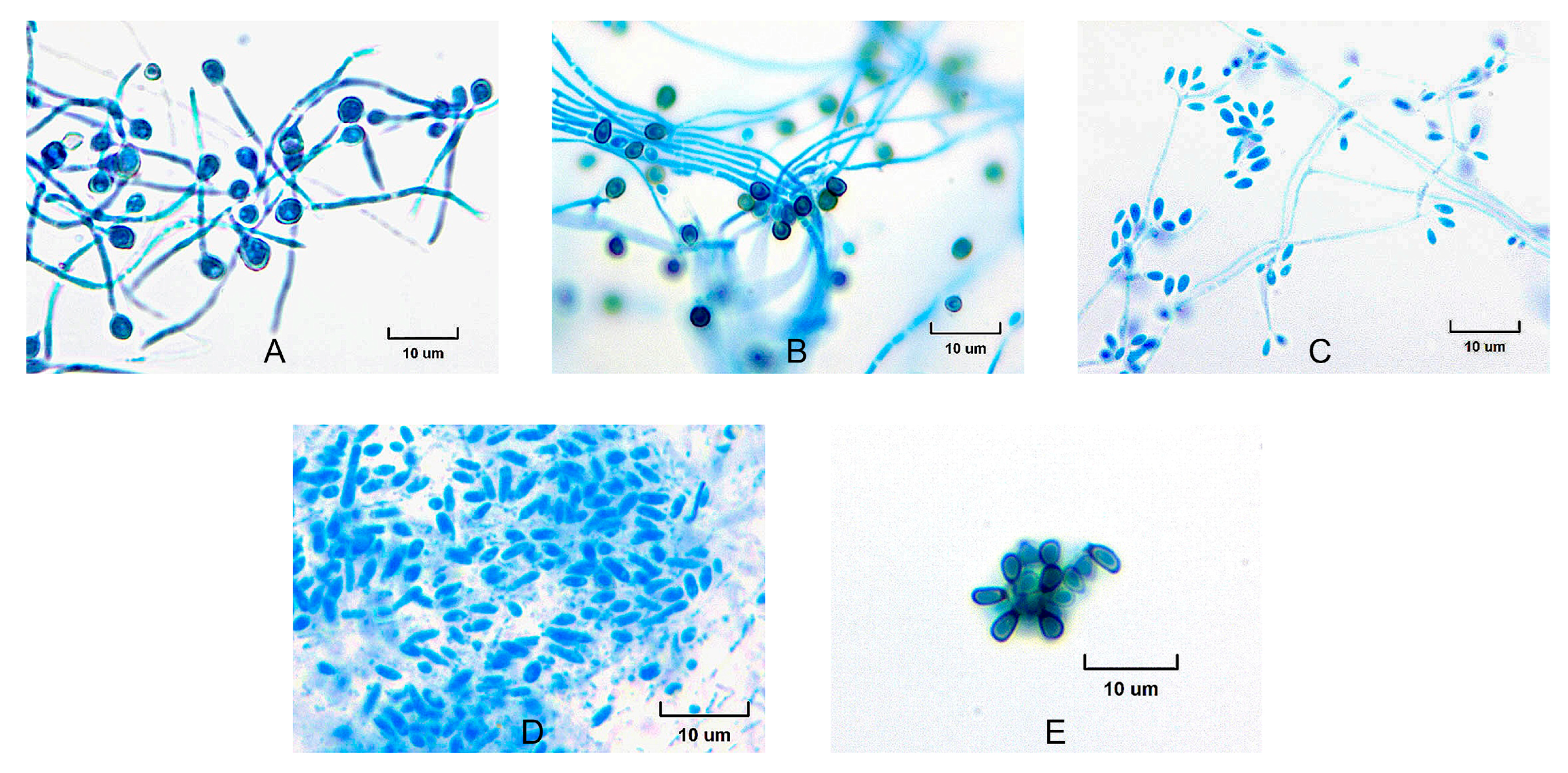
2.2. Morphological and Physiological Studies
2.3. DNA Extraction
2.4. PCR Amplification
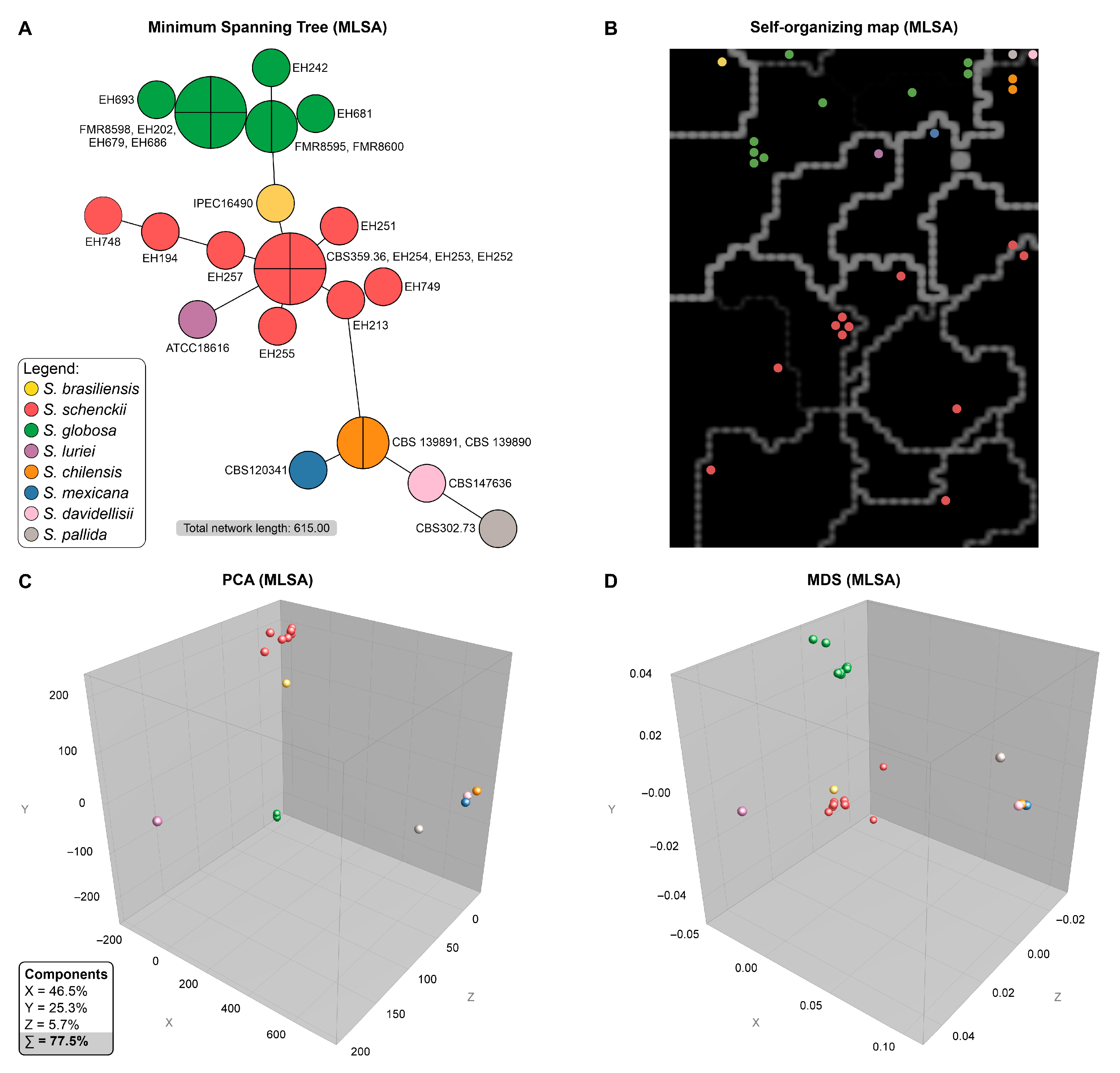
2.5. Phylogenetic Analysis
2.6. Bioinformatic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marimon, R.; Gené, J.; Cano, J.; Trilles, L.; Dos Santos Lazéra, M.; Guarro, J. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 2006, 44, 3251–3256. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Chen, Y.J.; Phukhamsakda, C.; Boekhout, T.; Groenewald, J.Z.; McKenzi, E.H.C.; Francisco, E.C.; Frisvad, J.C.; Groenewald, M.; Hurdeal, V.G.; et al. What are the 100 most cited fungal genera? Stud. Mycol. 2024, 108, 1–411. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Macedo, P.M.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- de Beer, Z.W.; Procter, M.; Wingfield, M.J.; Marincowitz, S.; Duong, T.A. Generic boundaries in the Ophiostomatales reconsidered and revised. Stud. Mycol. 2022, 101, 57–120. [Google Scholar] [CrossRef]
- Kidd, S.E.; Sandoval-Denis, M.; Malik, R.; Hagen, F.; Rodrigues, A.M. Sporothrix davidellisii: A new pathogenic species belonging to the Sporothrix pallida complex. Med. Mycol. 2025, 63, myaf034. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.C.; Gonçalves, S.S.; de Carvalho, J.A.; Bonifaz, A.; Brilhante, R.S.N.; de Camargo, Z.P.; Rodrigues, A.M. Insights from cutting-edge diagnostics and epidemiology of sporotrichosis and taxonomic shifts in Sporothrix. Curr. Fungal Infect. Rep. 2025, 19, 3. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Cruz Choappa, R.; Fernandes, G.F.; De Hoog, G.S.; Camargo, Z.P. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016, 120, 246–264. [Google Scholar] [CrossRef] [PubMed]
- do Monte Alves, M.; Pipolo Milan, E.; da Silva-Rocha, W.P.; Soares de Sena da Costa, A.; Araujo Maciel, B.; Cavalcante Vale, P.H.; de Albuquerque, P.R.; Lopes Lima, S.; Salles de Azevedo Melo, A.; Messias Rodrigues, A.; et al. Fatal pulmonary sporotrichosis caused by Sporothrix brasiliensis in Northeast Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008141. [Google Scholar] [CrossRef] [PubMed]
- Toriello, C.; Brunner-Mendoza, C.; Ruiz-Baca, E.; Duarte-Escalante, E.; Pérez-Mejía, A.; Del Rocío Reyes-Montes, M. Sporotrichosis in Mexico. Braz. J. Microbiol. 2021, 52, 49–62. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; del Rocío Reyes-Montes, M.; Pérez-Mejía, A.; Navarro-Barranco, H.; Souza, V.; Zúñiga, G.; Toriello, C. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J. Clin. Microbiol. 2002, 40, 3004–3011. [Google Scholar] [CrossRef]
- Rangel-Gamboa, L.; Martinez-Hernandez, F.; Maravilla, P.; Flisser, A. A population genetics analysis in clinical isolates of Sporothrix schenckii based on calmodulin and calcium/calmodulin-dependent kinase partial gene sequences. Mycoses 2018, 61, 383–392. [Google Scholar] [CrossRef]
- Dixon, D.M.; Salkin, I.F.; Duncan, R.A.; Hurd, N.J.; Haines, J.H.; Kemna, M.E.; Coles, F.B. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 1991, 29, 1106–1113. [Google Scholar] [CrossRef]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Nirenberg, H.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Teixeira, M.M.; de Hoog, G.S.; Schubach, T.M.P.; Pereira, S.A.; Fernandes, G.F.; Bezerra, L.M.L.; Felipe, M.S.; de Camargo, Z.P. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013, 7, e2281. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolution confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Prim, R.C. Shortest connection networks and some generalizations. Bell Syst. Tech. J. 1957, 36, 1389–1401. [Google Scholar] [CrossRef]
- Patterson, N.; Price, A.L.; Reich, D. Population structure and eigenanalysis. PLoS Genet. 2006, 2, e190. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Mardia, K.V.; Kent, J.T.; Taylor, C.C. Multivariate Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2024; p. 592. [Google Scholar]
- Kohonen, T. Self-Organizing Maps, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001; p. 502. [Google Scholar]
- Vesanto, J.; Himberg, J.; Alhoniemi, E.; Parhankangas, J. Self-organizing map in Matlab: The SOM Toolbox. In Proceedings of the Matlab DSP Conference, Espoo, Finland, 16–17 November 1999; pp. 16–17. [Google Scholar]
- Rodrigues, A.M.; Goncalves, S.S.; de Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; Camargo, Z.P. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J. Fungi 2022, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Kano, R.; Bonifaz, A.; Toriello, C.; Negroni, R.; Rego, R.S.M.; Gremiao, I.D.F.; et al. Trends in the molecular epidemiology and population genetics of emerging Sporothrix species. Stud. Mycol. 2021, 100, 100129. [Google Scholar] [CrossRef]
- Santos, M.T.; Nascimento, L.F.J.; Barbosa, A.A.T.; Martins, M.P.; Tunon, G.I.L.; Santos, P.O.M.; Dantas-Torres, F.; Dolabella, S.S. The rising incidence of feline and cat-transmitted sporotrichosis in Latin America. Zoonoses Public Health 2024, 71, 609–619. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, S.; Zhong, M.; Gyawali, K.R.; Pan, W.; Xu, M.; Huang, H.; Huang, X. Current situation of sporotrichosis in China. Future Microbiol. 2024, 19, 1097–1106. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Xu, J. Fungal species concepts in the genomics era. Genome 2020, 63, 459–468. [Google Scholar] [CrossRef]
- Sepúlveda, V.E.; Márquez, R.; Turissini, D.A.; Goldman, W.E.; Matute, D.R. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. mBio 2017, 8, e01339-17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Terra, P.P.D.; de Hoog, S.; Brilhante, R.S.N.; de Aguiar Cordeiro, R.; de Souza Collares Maia Castelo-Branco, D.; Rocha, M.F.G.; et al. The global epidemiology of emerging Histoplasma species in recent years. Stud. Mycol. 2020, 97, 100095. [Google Scholar] [CrossRef]
- Valero, C.; Gago, S.; Monteiro, M.C.; Alastruey-Izquierdo, A.; Buitrago, M.J. African histoplasmosis: New clinical and microbiological insights. Med. Mycol. 2018, 56, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mapengo, R.E.; Maphanga, T.G.; Jofre, G.I.; Rader, J.A.; Turissini, D.A.; Birkhead, M.; Kwabia, S.A.; Sepúlveda, V.E.; Buitrago, M.J.; Teixeira Md, M.; et al. Genomic epidemiology of Histoplasma in Africa. mBio 2025, 16, e00564-25. [Google Scholar] [CrossRef]
- Roberto, T.N.; De Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Exploring genetic diversity, population structure, and phylogeography in Paracoccidioides species using AFLP markers. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hagen, F.; de Camargo, Z.P. A spotlight on Sporothrix and sporotrichosis. Mycopathologia 2022, 187, 407–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 2015, 35, 1–20. [Google Scholar] [CrossRef]
- Zhou, X.; Rodrigues, A.M.; Feng, P.; Hoog, G.S. Global ITS diversity in the Sporothrix schenckii complex. Fungal Divers. 2014, 66, 153–165. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, S.; de Camargo, Z.P. Emergence of pathogenicity in the Sporothrix schenckii complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef]
- de Beer, Z.W.; Harrington, T.C.; Vismer, H.F.; Wingfield, B.D.; Wingfield, M.J. Phylogeny of the Ophiostoma stenoceras–Sporothrix schenckii complex. Mycologia 2003, 95, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.A.; de Carvalho, J.A.; Bicalho, G.C.; de Magalhães Soares, D.F.; de Oliveira, C.S.F.; Tavares, G.C.; Rodrigues, A.M.; de Azevedo, M.I. The emergence of new Sporothrix brasiliensis genotypes in current epidemic of sporotrichosis in Southeastern Brazil. Mycoses 2024, 67, e13792. [Google Scholar] [CrossRef]
- Losada, L.C.M.L.; Monteiro, R.C.; de Carvalho, J.A.; Hagen, F.; Fisher, M.C.; Spruijtenburg, B.; Meis, J.F.; de Groot, T.; Goncalves, S.S.; Negroni, R.; et al. High-throughput microsatellite markers development for genetic characterization of emerging Sporothrix species. J. Fungi 2023, 9, 354. [Google Scholar] [CrossRef]
- Gonçalves, S.S.; da Cruz Bahiense Rocha, I.; Rediguieri, B.C.; de Carvalho, J.A.; Maifrede, S.B.; Kruschewsky, W.L.L.; Falqueto, A.; Rodrigues, A.M. Human and feline sporotrichosis in a reference center of Southeastern Brazil: Genetic differentiation, diversity, and antifungal susceptibility of Sporothrix species. J. Fungi 2023, 9, 831. [Google Scholar] [CrossRef]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A one health approach to combatting Sporothrix brasiliensis: Narrative review of an emerging zoonotic fungal pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Castro, R.; Pinto-Almazan, R.; Arenas, R.; Sanchez-Cardenas, C.D.; Espinosa-Hernandez, V.M.; Sierra-Maeda, K.Y.; Conde-Cuevas, E.; Juarez-Duran, E.R.; Xicohtencatl-Cortes, J.; Carrillo-Casas, E.M.; et al. Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years. J. Fungi 2022, 8, 588. [Google Scholar] [CrossRef]
- Florez-Munoz, S.V.; Alzate, J.F.; Mesa-Arango, A.C. Molecular identification and antifungal susceptibility of clinical isolates of Sporothrix schenckii complex in Medellin, Colombia. Mycopathologia 2019, 184, 53–63. [Google Scholar] [CrossRef]
- Rojas, O.C.; Bonifaz, A.; Campos, C.; Trevino-Rangel, R.J.; Gonzalez-Alvarez, R.; Gonzalez, G.M. Molecular identification, antifungal susceptibility, and geographic origin of clinical strains of Sporothrix schenckii complex in Mexico. J. Fungi 2018, 4, 86. [Google Scholar] [CrossRef]
- Martínez-Duncker, I.; Mayorga-Rodríguez, J.; Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Baruch-Martínez, D.A.; López-Ramírez, L.A.; Mora-Montes, H.M. Phenotypic immunological profiling and antifungal susceptibility of Sporothrix schenckii clinical isolates from a hyperendemic region in western Mexico. Med. Mycol. 2025, 63, myaf073. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Hu, X.; Liu, Z.; Lin, Y.; Wu, H.; Li, F. Clinical epidemiology of sporotrichosis in Jilin province, China (1990–2019): A series of 4969 cases. Infect. Drug Resist. 2022, 15, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Hemashetter, B.M.; Verma, S.; Chauhan, S.; Nath, R.; Savio, J.; Capoor, M.; Kaur, H.; Ghosh, A.K.; et al. Phenotypic and molecular characterisation of Sporothrix globosa of diverse origin from India. Braz. J. Microbiol. 2021, 52, 91–100. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, M.; Wang, Y.; Li, R.; He, L.; Wan, Z.; Li, F.; Zhang, J. Population structure and genetic diversity of Sporothrix globosa in China according to 10 novel microsatellite loci. J. Med. Microbiol. 2019, 68, 248–254. [Google Scholar] [CrossRef]
- Madrid, H.; Cano, J.; Gene, J.; Bonifaz, A.; Toriello, C.; Guarro, J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev. Iberoam. Micol. 2009, 26, 218–222. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Kadasa, N.M.S.; Al Zahrani, H.S.; Ahmed, S.A.; Feng, P.; Gerrits van den Ende, A.H.G.; Zhang, Y.; Kano, R.; Li, F.; Li, S.; et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017, 66, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Aguilar-Ancori, E.; Tirado-Sánchez, A.; Bonifaz, A. Ecological determinants of sporotrichosis etiological agents. J. Fungi 2018, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Martínez-Álvarez, J.A. Virulence factors of Sporothrix schenckii. J. Fungi 2022, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.A.; Machado, T.C.; Sasaki, A.A.; Glaser, F.; Alvarado, P.; Bonifaz, A.; Gonçalves, S.S.; Gremião, I.D.F.; Pereira, S.A.; Gompertz, O.F.; et al. Exploring molecular evolution and genetic diversity in 3-carboxymuconate cyclase (Gp60–70), the major antigen of pathogenic Sporothrix species. Mycology 2025, 16, 1–27. [Google Scholar] [CrossRef]
- de Carvalho, J.A.; Hagen, F.; Fisher, M.C.; de Camargo, Z.P.; Rodrigues, A.M. Genome-wide mapping using new AFLP markers to explore intraspecific variation among pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2020, 14, e0008330. [Google Scholar] [CrossRef]
- Spruijtenburg, B.; Bombassaro, A.; Meijer, E.F.J.; Rodrigues, A.M.; Grisolia, M.E.; Vicente, V.A.; de Queiroz-Telles, F.; Meis, J.F.; de Groot, T. Sporothrix brasiliensis genotyping reveals numerous independent zoonotic introductions in Brazil. J. Infect. 2023, 86, 610–613. [Google Scholar] [CrossRef]
- dos Santos, A.R.; Misas, E.; Min, B.; Le, N.; Bagal, U.R.; Parnell, L.A.; Sexton, D.J.; Lockhart, S.R.; de Souza Carvalho Melhem, M.; Takahashi, J.P.F.; et al. Emergence of zoonotic sporotrichosis in Brazil: A genomic epidemiology study. Lancet Microbe 2024, 5, e282–e290. [Google Scholar] [CrossRef]
- Mackinnon, J.E.; Conti-Diaz, I.A.; Gezuele, E.; Civila, E.; da Luz, S. Isolation of Sporothrix schenckii from nature and considerations on its pathogenicity and ecology. Sabouraudia 1969, 7, 38–45. [Google Scholar] [CrossRef]
- Du, W.; Giosa, D.; Wei, J.; Giuffrè, L.; Shi, G.; El Aamri, L.; D’Alessandro, E.; Hafidi, M.; de Hoog, S.; Romeo, O.; et al. Long-read PacBio genome sequencing of four environmental saprophytic Sporothrix species spanning the pathogenic clade. BMC Genom. 2022, 23, 506. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Zhou, X. Comparative genomic data provide new insight on the evolution of pathogenicity in Sporothrix species. Front. Microbiol. 2020, 11, 565439. [Google Scholar] [CrossRef] [PubMed]
- Gomez, O.M.; Alvarez, L.C.; Muñoz, J.F.; Misas, E.; Gallo, J.E.; Jimenez, M.D.P.; Arango, M.; McEwen, J.G.; Hernandez, O.; Clay, O.K. Draft genome sequences of two Sporothrix schenckii clinical isolates associated with human sporotrichosis in Colombia. Genome Announc. 2018, 6, e00495-18. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, W.; Giosa, D.; Criseo, G.; Zhang, J.; He, T.; Huang, X.; Sun, J.; Sun, Y.; Huang, J.; et al. Whole-genome sequencing and in silico analysis of two strains of Sporothrix globosa. Genome Biol. Evol. 2016, 8, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- New, D.; Beukers, A.G.; Kidd, S.E.; Merritt, A.J.; Weeks, K.; van Hal, S.J.; Arthur, I. Identification of multiple species and subpopulations among Australian clinical Sporothrix isolates using whole genome sequencing. Med. Mycol. 2019, 57, 905–908. [Google Scholar] [CrossRef]
- Fernandes, G.F.; dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; de Camargo, Z.P. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; de Camargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med. Mycol. 2015, 53, 178–188. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Cordoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter and international study of MIC/MEC distributions for definition of epidemiological cutoff values (ECVs) for species of Sporothrix identified by molecular methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef]

| Genbank Accession Code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Origin | Source | Clinical Form | Sex | Age | ITS | BT2 | CAL | TEF | Donated by |
| EH-194 | Puebla, México | Soil | NA | NA | NA | PX275390 | Universidad Autónoma de Puebla | |||
| EH-202 | México | Human | L | M | 28 | PX275391 | Centro Dermatológico Dr. Ladislao de la Pascua | |||
| EH-213 | México | Human | F | M | 47 | PX275392 | Centro Dermatológico Dr. Ladislao de la Pascua | |||
| EH-242 | Guatemala | Human | U | U | U | PX275393 | Universidad de San Carlos de Guatemala | |||
| EH-251 | Puebla, México | Soil | NA | NA | NA | PX275394 | Universidad Autónoma de Puebla | |||
| EH-252 | Puebla, México | Soil | NA | NA | NA | PX275395 | Universidad Autónoma de Puebla | |||
| EH-253 | Puebla, México | Soil | NA | NA | NA | PX275396 | Universidad Autónoma de Puebla | |||
| EH-254 | Puebla, México | Soil | NA | NA | NA | PX275397 | Universidad Autónoma de Puebla | |||
| EH-255 | Puebla, México | Soil | NA | NA | NA | PX275398 | Universidad Autónoma de Puebla | |||
| EH-257 | Puebla, México | Soil | NA | NA | NA | PX275399 | Universidad Autónoma de Puebla | |||
| EH-679 | Colombia | Human | F | M | 52 | PX275400 | Universidad Autónoma de Colombia | |||
| EH-681 | Colombia | Human | L | M | 17 | PX275401 | Universidad Autónoma de Colombia | |||
| EH-686 | Colombia | Human | F | F | 51 | PX275402 | Universidad Autónoma de Colombia | |||
| EH-693 | Colombia | Human | L | F | 18 | PX275403 | Universidad Autónoma de Colombia | |||
| EH-748 | Guadalajara, México | Human | F | F | 70 | PX275404 | Inst Dermatológico de Jalisco Dr. José Barba Rubio | |||
| EH-749 | Puebla, México | Human | D | M | 20 | PX275405 | Hospital General Dr. Manuel Gea Gonzalez | |||
| Clade | Isolates (n) | No. of Sites | C | V | Pi | S | π | H | Hd | Eta |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 5 | 2149 | 1924 | 9 | 0 | 9 | 0.00186 | 4 | 0.900 | 9 |
| B | 3 | 2149 | 1859 | 100 | 0 | 91 | 0.03556 | 3 | 1.0 | 109 |
| C | 2 | 2149 | 1958 | 14 | 0 | 14 | 0.00710 | 2 | 1.0 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner-Mendoza, C.; Messias Rodrigues, A.; Duarte-Escalante, E.; Reyes-Montes, M.d.R.; Pérez-Mejía, A.; Navarro-Barranco, H.; Calderón-Ezquerro, M.d.C.; Toriello, C. Cryptic Diversity and Ecological Overlap in Sporothrix schenckii: Insights from Multilocus Phylogenetics of Clinical and Environmental Isolates. J. Fungi 2025, 11, 759. https://doi.org/10.3390/jof11110759
Brunner-Mendoza C, Messias Rodrigues A, Duarte-Escalante E, Reyes-Montes MdR, Pérez-Mejía A, Navarro-Barranco H, Calderón-Ezquerro MdC, Toriello C. Cryptic Diversity and Ecological Overlap in Sporothrix schenckii: Insights from Multilocus Phylogenetics of Clinical and Environmental Isolates. Journal of Fungi. 2025; 11(11):759. https://doi.org/10.3390/jof11110759
Chicago/Turabian StyleBrunner-Mendoza, Carolina, Anderson Messias Rodrigues, Esperanza Duarte-Escalante, María del Rocío Reyes-Montes, Amelia Pérez-Mejía, Hortensia Navarro-Barranco, María del Carmen Calderón-Ezquerro, and Conchita Toriello. 2025. "Cryptic Diversity and Ecological Overlap in Sporothrix schenckii: Insights from Multilocus Phylogenetics of Clinical and Environmental Isolates" Journal of Fungi 11, no. 11: 759. https://doi.org/10.3390/jof11110759
APA StyleBrunner-Mendoza, C., Messias Rodrigues, A., Duarte-Escalante, E., Reyes-Montes, M. d. R., Pérez-Mejía, A., Navarro-Barranco, H., Calderón-Ezquerro, M. d. C., & Toriello, C. (2025). Cryptic Diversity and Ecological Overlap in Sporothrix schenckii: Insights from Multilocus Phylogenetics of Clinical and Environmental Isolates. Journal of Fungi, 11(11), 759. https://doi.org/10.3390/jof11110759








