Candida krusei Empyema: A Lung Transplant Case and Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
3. Case Report
4. Results
5. Discussion
5.1. Treatment Challenges and Lacunae in Guidelines
5.2. Recent Pharmacological Developments
5.3. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAC | Non-albicans Candida |
| AST | American Society of Transplantation |
| ESCMID | European Society of Clinical Microbiology and Infectious Diseases |
| AFST | Antifungal Susceptibility Testing |
| ECMM | European Confederation of Medical Mycology |
| ISHAM | International Society for Human and Animal Mycology |
| ASM | American Society for Microbiology |
| SoR | Strength of Recommendation |
| QoE | Quality of Evidence |
| IPF | Idiopathic pulmonary fibrosis |
| BAL | Bronchoalveolar lavage |
References
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Kollef, M.H. Secular trends in candidemia-related hospitalization in the United States, 2000–2005. Infect. Control Hosp. Epidemiol. 2008, 29, 978–980. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Kim, H.Y.; Stocker, S.; Kidd, S.; Alastruey-Izquierdo, A.; Dao, A.; Harrison, T.; Wahyuningsih, R.; Rickerts, V.; Perfect, J.; et al. Pichia kudriavzevii (Candida krusei): A systematic review to inform the World Health Organisation priority list of fungal pathogens. Med. Mycol. 2024, 62, myad132. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.E.; January, S.E.; Vazquez Guillamet, R. Diagnosis and Treatment of Fungal Infections in Lung Transplant Recipients. Pathogens 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.M.; Kennedy, C.C.; Razonable, R.R.; Beam, E. Fungal Infection and Prevention in Lung Transplant. Curr. Fungal Infect. Rep. 2021, 15, 136–142. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Jain, K.; Wang, Y.; Jain, P.; Kalita, B.; Shivarathri, R.; Chauhan, M.; Kaur, H.; Chauhan, N.; Xu, J.; Chowdhary, A. Genomic analyses reveal high diversity and rapid evolution of Pichia kudriavzevii within a neonatal intensive care unit in Delhi, India. Antimicrob. Agents Chemother. 2025, 69, e0170924. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Samaranayake, L.P. Candida krusei: Biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J. Med. Microbiol. 1994, 41, 295–310. [Google Scholar] [CrossRef]
- Sachivkina, N.; Podoprigora, I.; Bokov, D. Morphological characteristics of Candida albicans, Candida krusei, Candida guilliermondii, and Candida glabrata biofilms, and response to farnesol. Vet. World. 2021, 14, 1608–1614. [Google Scholar] [CrossRef]
- Bonatti, H.; Stelzmueller, I.; Berger, N.; Lechner, M.; Grif, K.; Geltner, C.; Margreiter, R.; Lass-Flörl, C. Infections caused by Candida krusei in five transplant and two surgical patients. Surg. Infect. 2009, 10, 265–271. [Google Scholar] [CrossRef]
- Cascio, A.; Barone, M.; Micali, V.; Iaria, C.; Delfino, D.; David, A.; Monaco, M.; Monaco, F. On a fatal case of Candida krusei pleural empyema in a pregnant woman with spontaneous esophagus perforation. Mycopathologia 2010, 169, 451–455. [Google Scholar] [CrossRef]
- Srinivasnakshatri, V.K.; Subramani, P.; Venkateshwaraprasad, K.N.; Varma, P. A fatal case of fungal empyema due to Candida krusei and Candida tropicalis: A rare occurrence with an atypical presentation. J. Clin. Diagn. Res. 2014, 8, DD01–DD02. [Google Scholar] [CrossRef] [PubMed]
- Bukamur, H.; Ahmed, W.; Numan, Y.; Shahoub, I.; Zeid, F. Candida krusei Empyema Thoracis: A Community-Acquired Infection Requiring a High Index of Suspicion. Case Rep. Infect. Dis. 2018, 2018, 8039803. [Google Scholar] [CrossRef] [PubMed]
- Vrba, R.; Lubuska, L.; Spicka, P. Hybrid transthoracic oesophagectomy due to carcinoma with complications after COVID-19 pneumonia—A case report. Int. J. Surg. Case Rep. 2022, 90, 106749. [Google Scholar] [CrossRef]
- Baker, A.W.; Maziarz, E.K.; Arnold, C.J.; Johnson, M.D.; Workman, A.D.; Reynolds, J.M.; Perfect, J.R.; Alexander, B.D. Invasive Fungal Infection After Lung Transplantation: Epidemiology in the Setting of Antifungal Prophylaxis. Clin. Infect. Dis. 2020, 70, 30–39. [Google Scholar] [CrossRef]
- Senger, S.S.; Thompson, G.R., III; Samanta, P.; Ahrens, J.; Clancy, C.J.; Nguyen, M.H. Candida Empyema Thoracis at Two Academic Medical Centers: New Insights Into Treatment and Outcomes. Open Forum Infect. Dis. 2021, 8, ofaa656. [Google Scholar] [CrossRef] [PubMed]
- Abbas, J.; Bodey, G.P.; Hanna, H.A.; Mardani, M.; Girgawy, E.; Abi-Said, D.; Whimbey, E.; Hachem, R.; Raad, I. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch. Intern. Med. 2000, 160, 2659–2664. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Aslam, S.; Rotstein, C.; AST Infectious Disease Community of Practice. Candida infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13623. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Guisso, L.P.; Ribeiro, N.P.; Carmona, W.R.; Roseno, A.C.B.; Pessan, J.P.; Monteiro, D.R. Novel Therapeutic Approaches to Control Fungal Biofilms. In Fungal Biofilms and Related Infections; Ouyang, J., Andes, D., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Sutar, Y.; Nabeela, S.; Singh, S.; Alqarihi, A.; Solis, N.; Ghebremariam, T.; Filler, S.; Ibrahim, A.S.; Date, A.; Uppuluri, P. Niclosamide-loaded nanoparticles disrupt Candida biofilms and protect mice from mucosal candidiasis. PLoS Biol. 2022, 20, e3001762. [Google Scholar] [CrossRef] [PubMed]
- Niño-Vega, G.A.; Padró-Villegas, L.; López-Romero, E. New Ground in Antifungal Discovery and Therapy for Invasive Fungal Infections: Innovations, Challenges, and Future Directions. J. Fungi 2024, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, Y.; He, W.; Chen, T.; Liu, N.; Ma, L.; Qiu, Z.; Shang, Z.; Wang, Z. A polyene macrolide targeting phospholipids in the fungal cell membrane. Nature 2025, 640, 743–751. [Google Scholar] [CrossRef] [PubMed]
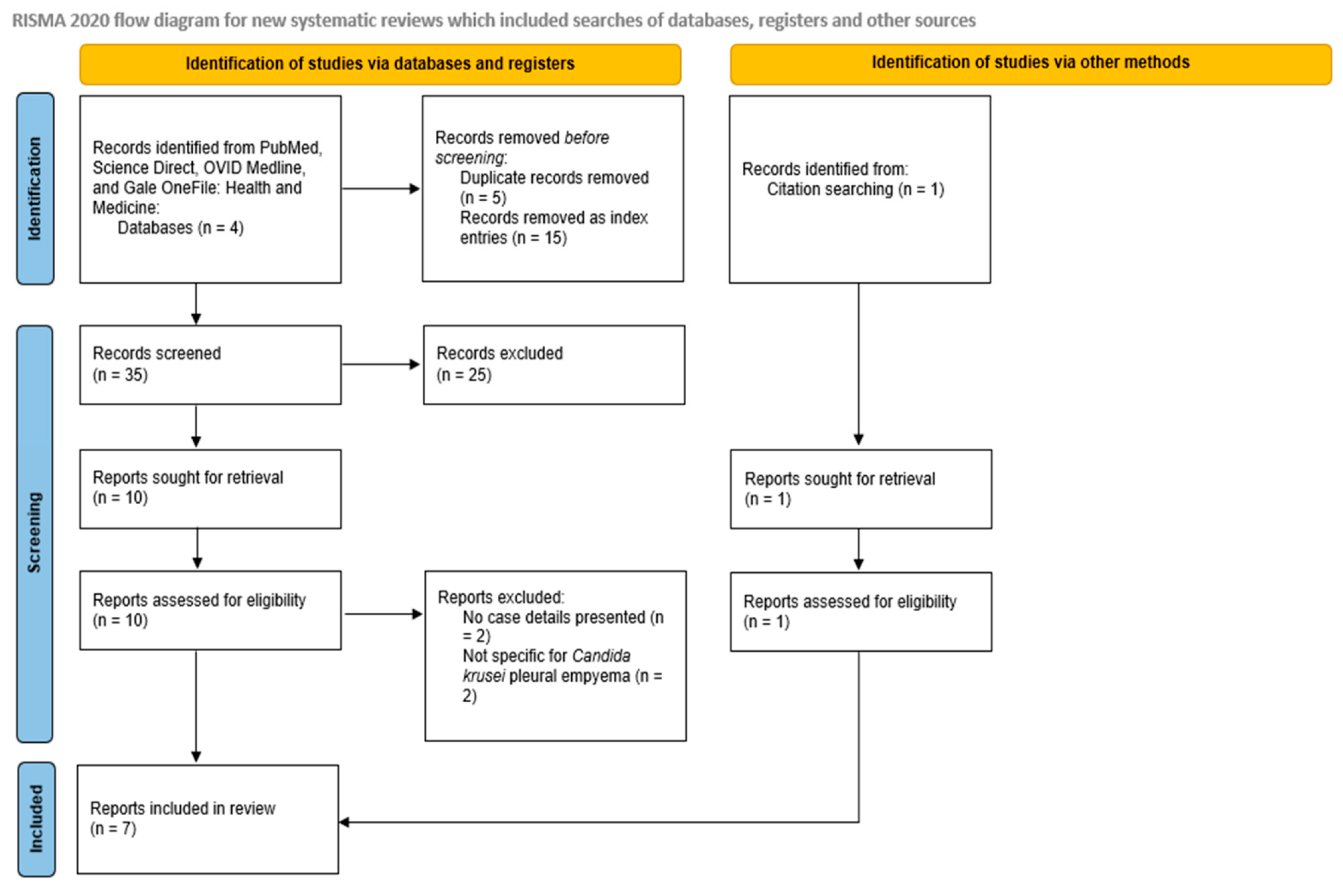
| Database | Date of Search | Records Retrieved | Included Studies |
|---|---|---|---|
| PubMed | 7 June 2025 | 5 | 2 |
| ScienceDirect | 7 June 2025 | 45 | 2 |
| OVID MEDLINE | 7 June 2025 | 2 | 2 |
| Gale OneFile: Health and Medicine | 7 June 2025 | 3 | 2 |
| First Author, Year, Country | Age/Sex | Underlying Condition/Risk Factor/Source | Time from Event to Empyema (Days) | Clinical Presentation | Comorbidities | Management Strategy | Antifungal Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
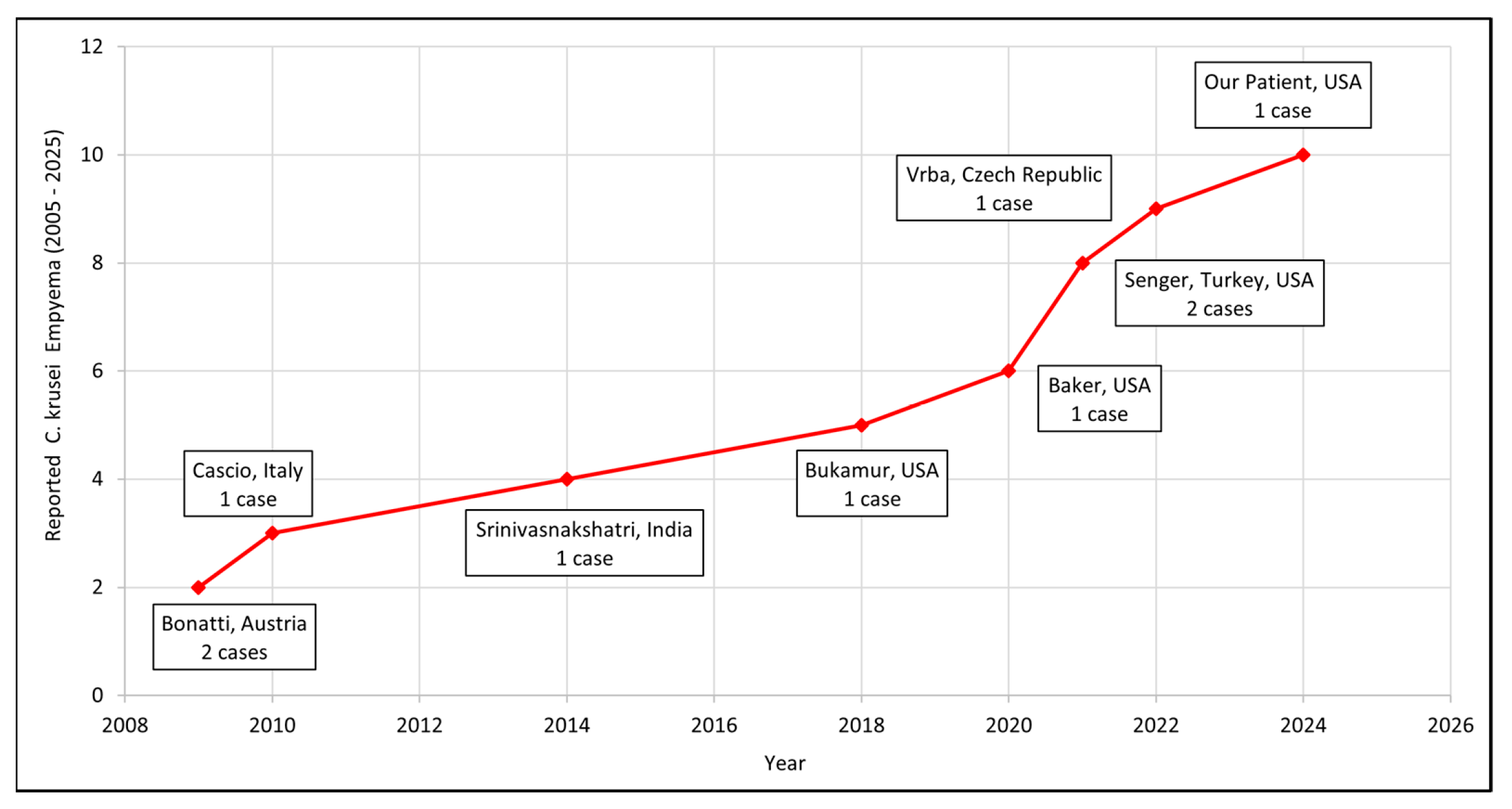
| Bonatti (2009), Austria [11] | 58/F | Post-op hemorrhage (Lung Tx) | 105 | - | - | Percutaneous drainage | Fluconazole (P) * → Caspofungin, Voriconazole | Cured. Died of graft failure at 5 mos. |
| 59/M | Eso. perf./ Boerhaave syndrome | 7 | Sepsis | - | Eso. leak closure + decortication + CT- guided drainage | Fluconazole (P) * → Caspofungin | Cured. | |
| Cascio (2010), Italy [12] | 45/F | Eso. perf. | - | Fever, fatigue, dyspnea, constipation, nausea | 7 months pregnant, heavy drinker | Chest tube + Dx Thoracotomy | Fluconazole → Voriconazole → Caspofungin | Cured. Died of MOF on the 42nd day |
| Srinivasnakshatri (2014), India [13] | 11/F | PDA device closure/community-acquired | - | Abdominal pain, nausea | PDA (closed) | Thoracentesis (Dx) + ICD | Amphotericin B | Died. |
| Bukamur (2018), USA [14] | 74/F | Eso. perf. | - | Nausea, vomiting, dysphagia | Schizophrenia, dementia, COPD, CVA | Chest tube, decortication + extensive pleural peel | Micafungin → Voriconazole | Cured. |
| Vrba (2022) [15], Czech Republic | 69/M | Anastomosis dehiscence post-esophagectomy | 11 | Respiratory failure, septic shock | SCC esophagus, alcohol and tobacco abuse | Surgery + CT-guided drainage | Fluconazole | Cured. Discharged after 56 days. |
| Our Patient (2024), USA | 70/M | B/L Lung Tx | 81 | Shortness of breath on exertion | IPF, OSA, HTN, DM, CAD (S/P PCI 2019) | Thoracocenteses (multiple) | Caspofungin → Anidulafungin → Voriconazole → Voriconazole + Micafungin | Cured. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatela, S.; Nair-Collins, S.; Godart, G.; Peacock, M.A.; Larimore, K.; Cuthbert, K.; Munipalli, B.; Chitale, R.; Durvasula, R.; Oring, J. Candida krusei Empyema: A Lung Transplant Case and Systematic Review of the Literature. J. Fungi 2025, 11, 735. https://doi.org/10.3390/jof11100735
Karatela S, Nair-Collins S, Godart G, Peacock MA, Larimore K, Cuthbert K, Munipalli B, Chitale R, Durvasula R, Oring J. Candida krusei Empyema: A Lung Transplant Case and Systematic Review of the Literature. Journal of Fungi. 2025; 11(10):735. https://doi.org/10.3390/jof11100735
Chicago/Turabian StyleKaratela, Shifa, Sangeeta Nair-Collins, Gabriel Godart, Mary Ann Peacock, Kelly Larimore, Kristin Cuthbert, Bala Munipalli, Rohit Chitale, Ravi Durvasula, and Justin Oring. 2025. "Candida krusei Empyema: A Lung Transplant Case and Systematic Review of the Literature" Journal of Fungi 11, no. 10: 735. https://doi.org/10.3390/jof11100735
APA StyleKaratela, S., Nair-Collins, S., Godart, G., Peacock, M. A., Larimore, K., Cuthbert, K., Munipalli, B., Chitale, R., Durvasula, R., & Oring, J. (2025). Candida krusei Empyema: A Lung Transplant Case and Systematic Review of the Literature. Journal of Fungi, 11(10), 735. https://doi.org/10.3390/jof11100735






