The Cytochrome P450 Enzyme SsCyp64 Mediates γ-linolenyl Alcohol in Regulating Sexual Mating/Filamentation and Pathogenicity of Sporisorium scitamineum
Abstract
1. Introduction
2. Materials and Methods
2.1. Domain Prediction and Phylogenetic Analysis
2.2. Strains and Culture Conditions
2.3. Strain Construction and Identification
2.4. Extraction of Total DNA and RNA
2.5. RT-qPCR Analysis
2.6. Analysis of H2O2 and SDS Tolerance
2.7. Mating/Filamentous Growth Assay
2.8. Transcriptome Analysis
2.9. Metabolomic Analysis
2.10. Pathogenicity Analysis
2.11. Statistical Analysis
3. Results
3.1. SsCyp64 Positively Regulates Oxidative Stress in S. scitamineum, but Negatively Affects the Stability of the Cell Membrane
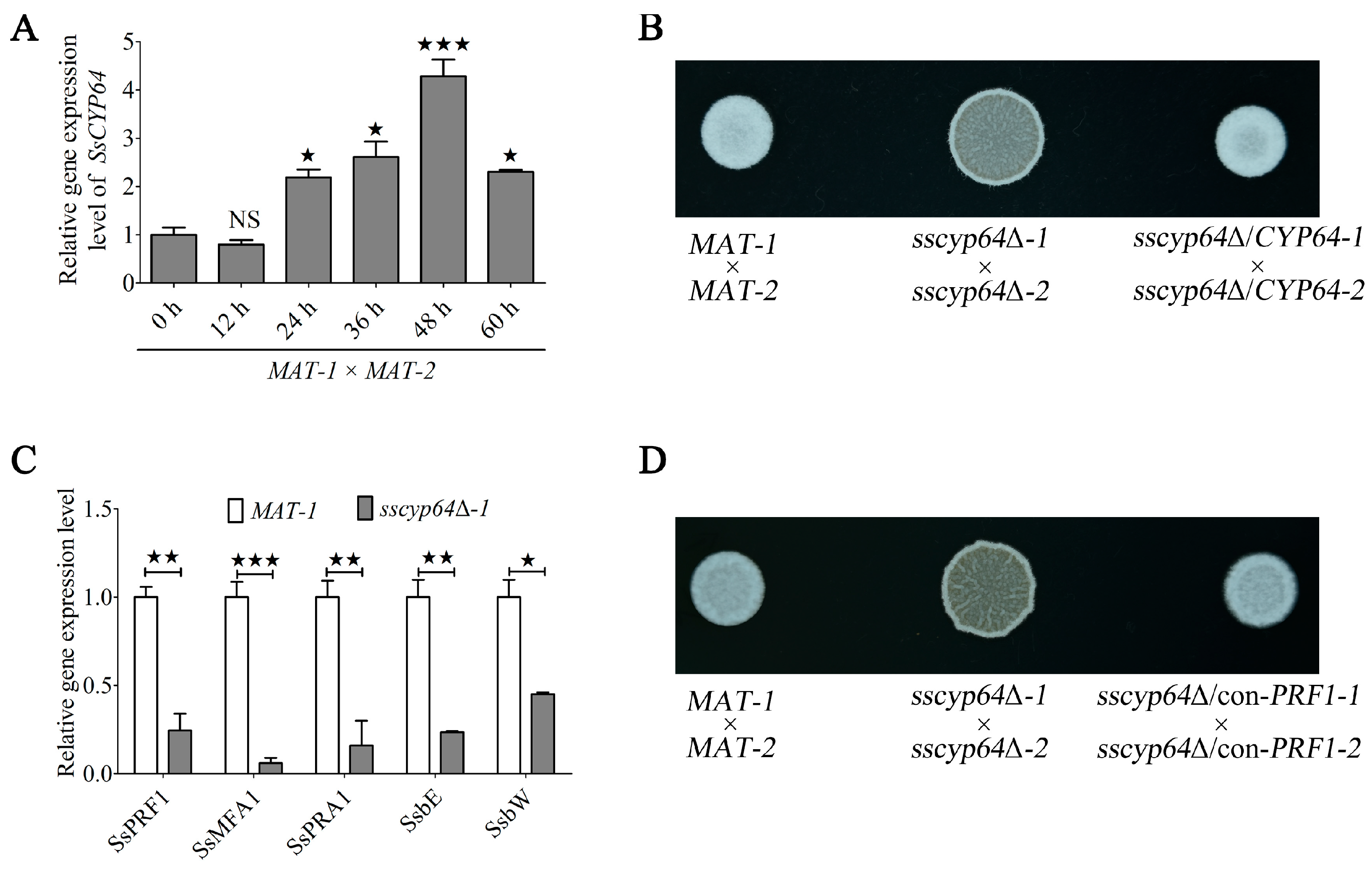
3.2. SsCyp64 Regulates the Mating/Filamentation of S. scitamineum by Mediating SsPRF1 Transcription
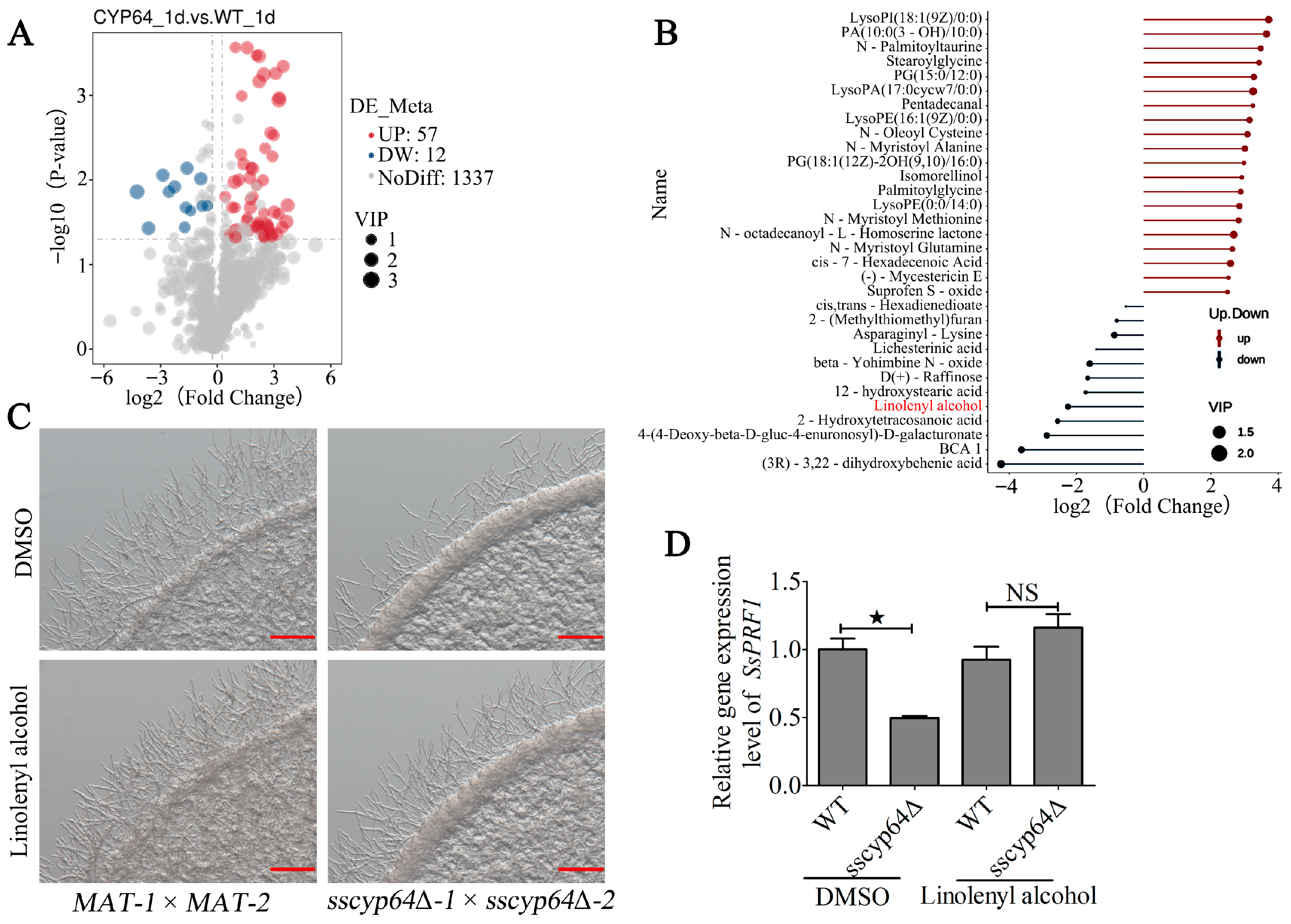
3.3. SsCyp64 Mediates γ-Linolenyl Alcohol Synthesis to Regulate the Formation of Dikaryotic Hyphae in S. scitamineum
3.4. SsCyp64 Is Essential for the Pathogenicity of S. scitamineum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Gou, Y.; Yi, C.; Li, Z.; Wang, W.; Lin, P.; Wang, W.; Sun, T.; Wang, T.; Zhao, W.; et al. ScWRKY2: A key regulator for smut resistance in sugarcane. Plant Biotechnol. J. 2025, 23, 3667–3681. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Wu, Q.; Wang, D.; Li, Z.; Sun, T.; Sun, X.; Cui, T.; Su, Y.; Wang, H.; Que, Y. Cellular heterogeneity and immune responses to smut pathogen in sugarcane. Plant Biotechnol. J. 2025, 90, 635–650. [Google Scholar] [CrossRef]
- Taniguti, L.M.; Schaker, P.D.; Benevenuto, J.; Peters, L.P.; Carvalho, G.; Palhares, A.; Quecine, M.C.; Nunes, F.R.; Kmit, M.C.; Wai, A.; et al. Complete genome sequence of Sporisorium scitamineum and biotrophic interaction transcriptome with sugarcane. PLoS ONE 2015, 10, e0129318. [Google Scholar] [CrossRef]
- Yan, M.; Cai, E.; Zhou, J.; Chang, C.; Xi, P.; Shen, W.; Li, L.; Jiang, Z.; Deng, Y.Z.; Zhang, L.H. A dual-color imaging system for sugarcane smut fungus Sporisorium scitamineum. Plant Dis. 2016, 100, 2357–2362. [Google Scholar] [CrossRef]
- Zhu, G.; Deng, Y.; Cai, E.; Yan, M.; Cui, G.; Wang, Z.; Zou, C.; Zhang, B.; Xi, P.; Chang, C.; et al. Identification and functional analysis of the pheromone response factor gene of Sporisorium scitamineum. Front. Microbiol. 2019, 10, 2115. [Google Scholar] [CrossRef]
- Cai, E.; Sun, S.; Deng, Y.; Huang, P.; Sun, X.; Wang, Y.; Chang, C.; Jiang, Z. Histidine kinase Sln1 and cAMP/PKA signaling pathways antagonistically regulate Sporisorium scitamineum mating and virulence via transcription factor Prf1. J. Fungi 2021, 7, 610. [Google Scholar] [CrossRef]
- Liu, B.; Song, Z.; Qi, X. Plant cytochrome P450 enzymes for bioactive metabolites biosynthesis, growth regulation and stress adaptation. Plant Physiol. 2025, 199, kiaf297. [Google Scholar] [CrossRef]
- Yu, J.; Chang, P.K.; Bhatnagar, D.; Cleveland, T.E. Genes encoding cytochrome P450 and monooxygenase enzymes define one end of the aflatoxin pathway gene cluster in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2000, 53, 583–590. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Chang, P.K.; Scharfenstein, L.L., Jr.; Cary, J.W.; Crawford, J.M.; Townsend, C.A. Absence of the aflatoxin biosynthesis gene, norA, allows accumulation of deoxyaflatoxin B1 in Aspergillus flavus cultures. FEMS Microbiol. Lett. 2010, 305, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.; Woloshuk, C.P. ord1, an oxidoreductase gene responsible for conversion of O-methylsterigmatocystin to aflatoxin in Aspergillus flavus. Appl. Environ. Microbiol. 1997, 63, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Montalbano, B.; Dyer, J.M.; Bhatnagar, D.; Cleveland, T.E. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Environ. Microbiol. 1998, 64, 4834–4841. [Google Scholar] [CrossRef]
- Zeng, H.; Hatabayashi, H.; Nakagawa, H.; Cai, J.; Suzuki, R.; Sakuno, E.; Tanaka, T.; Ito, Y.; Ehrlich, K.C.; Nakajima, H.; et al. Conversion of 11-hydroxy-O-methylsterigmatocystin to aflatoxin G1 in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 90, 635–650. [Google Scholar] [CrossRef]
- Li, S.; He, J.; Wu, Q.; Gou, J.; Wang, D.; Niu, X. Gene fusion and functional diversification of P450 genes facilitate thermophilic fungal adaptation to temperature change. Mycology 2024, 15, 485–505. [Google Scholar] [CrossRef]
- Cordova, L.T.; Butler, J.; Alper, H.S. Direct production of fatty alcohols from glucose using engineered strains of Yarrowia lipolytica. Metab. Eng. Commun. 2020, 10, e00105. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Md Toha, Z.; Muhamad, M.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Arsad, H. Antioxidant effects, antiproliferative effects, and molecular docking of Clinacanthus nutans leaf extracts. Molecules 2020, 25, 2067. [Google Scholar] [CrossRef] [PubMed]
- Crout, R.J.; Gilbertson, J.R.; Gilbertson, J.D.; Platt, D.; Langkamp, H.H.; Connamacher, R.H. Effect of linolenyl alcohol on the in-vitro growth of the oral bacterium Streptococcus mutans. Arch. Oral. Biol. 1982, 27, 1033–1037. [Google Scholar] [CrossRef]
- Gilbertson, J.R.; Langkamp, H.H.; Connamacher, R.; Platt, D. Use of lipids to potentiate the antibacterial activity of aminoglycosides. Antimicrob. Agents Chemother. 1984, 26, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Rodríguez, S.; García Gancedo, A.; Vilas, P.; Gil-Fernández, C. Inactivation and inhibition of African swine fever virus by monoolein, monolinolein, and gamma-linolenyl alcohol. Brief report. Arch. Virol. 1986, 88, 285–292. [Google Scholar] [CrossRef]
- Cai, E.; Deng, J.; Feng, R.; Zheng, W.; Wang, Y.; Yan, M.; Chang, C. SsCyp86 modulates Sporisorium scitamineum mating/filamentation and pathogenicity through regulating fatty acid metabolism. Virulence 2024, 15, 2395833. [Google Scholar] [CrossRef]
- Aristóteles, G.-N.; Clarice, L.L.; Guerrero, R.T. DNA extraction from frozen field-collected and dehydrated herbarium fungal basidiomata: Performance of SDS and CTAB-based methods. Biotemas 2005, 18, 19–32. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xu, L.; Wu, Q.; Liu, Y.; Ling, H.; Liu, Y.; Zhang, Y.; Guo, J.; Su, Y.; Chen, J.; et al. Erratum: Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 2015, 16, 244. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Renal Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.; Li, L.; Deng, Y.; Sun, S.; Jia, H.; Wu, R.; Zhang, L.; Jiang, Z.; Chang, C. MAP kinase Hog1 mediates a cytochrome P450 oxidoreductase to promote the Sporisorium scitamineum cell survival under oxidative stress. Environ. Microbiol. 2021, 23, 3306–3317. [Google Scholar] [CrossRef]
- Kiyota, R.; Arakawa, M.; Yamakawa, R.; Yasmin, A.; Ando, T. Biosynthetic pathways of the sex pheromone components and substrate selectivity of the oxidation enzymes working in pheromone glands of the fall webworm, Hyphantria cunea. Insect Biochem. Mol. Biol. 2011, 41, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yan, J.; Liu, C.; Xing, J.; Ren, Z.; Hendy, A.; Zheng, L.; Huang, J.; Chen, X.L. Perilipin LDP1 coordinates lipid droplets formation and utilization for appressorium-mediated infection in Magnaporthe oryzae. Environ. Microbiol. 2020, 22, 2843–2857. [Google Scholar] [CrossRef]
- Chen, D.; Cai, X.; Xing, J.; Chen, S.; Zhao, J.; Qu, Z.; Li, G.; Liu, H.; Zheng, L.; Huang, J.; et al. A lipid droplet-associated protein Nem1 regulates appressorium function for infection of Magnaporthe oryzae. aBIOTECH 2023, 4, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Z.; Zhang, B.; Chang, C.; Wang, Y.; Lu, S.; Sun, S.; Zhang, X.; Chen, B.; Jiang, Z. The MAP Kinase SsKpp2 Is Required for Mating/Filamentation in Sporisorium scitamineum. Front. Microbiol. 2018, 9, 2555. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ghith, A.; Bruning, J.B.; Bell, S.G. The catalytic activity and structure of the lipid metabolizing CYP124 cytochrome P450 enzyme from Mycobacterium marinum. Arch. Biochem. Biophys. 2023, 737, 109554. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Gao, X.; Luo, J.; et al. Silencing of cytochrome P450 gene CYP321A1 effects tannin detoxification and metabolism in Spodoptera litura. Int. J. Biol. Macromol. 2022, 194, 895–902. [Google Scholar] [CrossRef]
- Cai, E.; Jia, H.; Feng, R.; Zheng, W.; Li, L.; Zhang, L.; Jiang, Z.; Chang, C. Cytochrome c-peroxidase modulates ROS homeostasis to regulate the sexual mating of Sporisorium scitamineum. Microbiol. Spectr. 2023, 11, e0205723. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, E.; Xiong, B.; Ling, Q.; Li, X.; Chen, X.; Chang, C.; Wu, J.; Zhang, N. The Cytochrome P450 Enzyme SsCyp64 Mediates γ-linolenyl Alcohol in Regulating Sexual Mating/Filamentation and Pathogenicity of Sporisorium scitamineum. J. Fungi 2025, 11, 729. https://doi.org/10.3390/jof11100729
Cai E, Xiong B, Ling Q, Li X, Chen X, Chang C, Wu J, Zhang N. The Cytochrome P450 Enzyme SsCyp64 Mediates γ-linolenyl Alcohol in Regulating Sexual Mating/Filamentation and Pathogenicity of Sporisorium scitamineum. Journal of Fungi. 2025; 11(10):729. https://doi.org/10.3390/jof11100729
Chicago/Turabian StyleCai, Enping, Bo Xiong, Qiuping Ling, Xueting Li, Xinglong Chen, Changqing Chang, Jiayun Wu, and Nannan Zhang. 2025. "The Cytochrome P450 Enzyme SsCyp64 Mediates γ-linolenyl Alcohol in Regulating Sexual Mating/Filamentation and Pathogenicity of Sporisorium scitamineum" Journal of Fungi 11, no. 10: 729. https://doi.org/10.3390/jof11100729
APA StyleCai, E., Xiong, B., Ling, Q., Li, X., Chen, X., Chang, C., Wu, J., & Zhang, N. (2025). The Cytochrome P450 Enzyme SsCyp64 Mediates γ-linolenyl Alcohol in Regulating Sexual Mating/Filamentation and Pathogenicity of Sporisorium scitamineum. Journal of Fungi, 11(10), 729. https://doi.org/10.3390/jof11100729





