Biocontrol Efficiency of Leuconostoc mesenteroides GY-2 Against Postharvest Black Rot Caused by Alternaria alternata and the Mechanisms of Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit and Pathogens
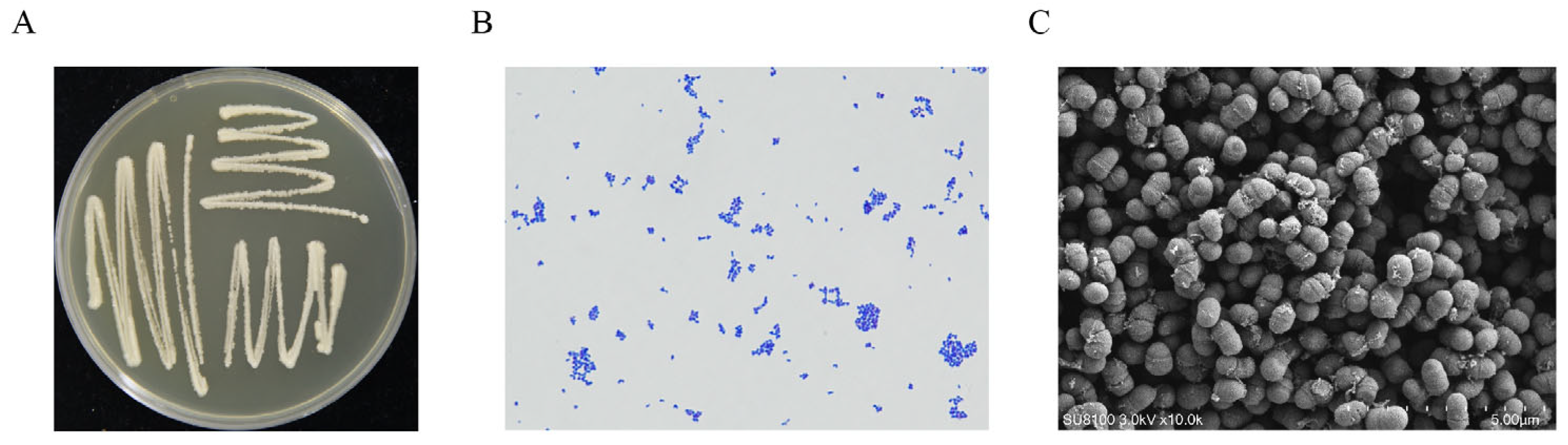
2.2. Isolation Candidate Biocontrol Strains
2.3. Assessment of the Inhibitory Activity of Strain GY-2 Against Apple Pathogens In Vitro
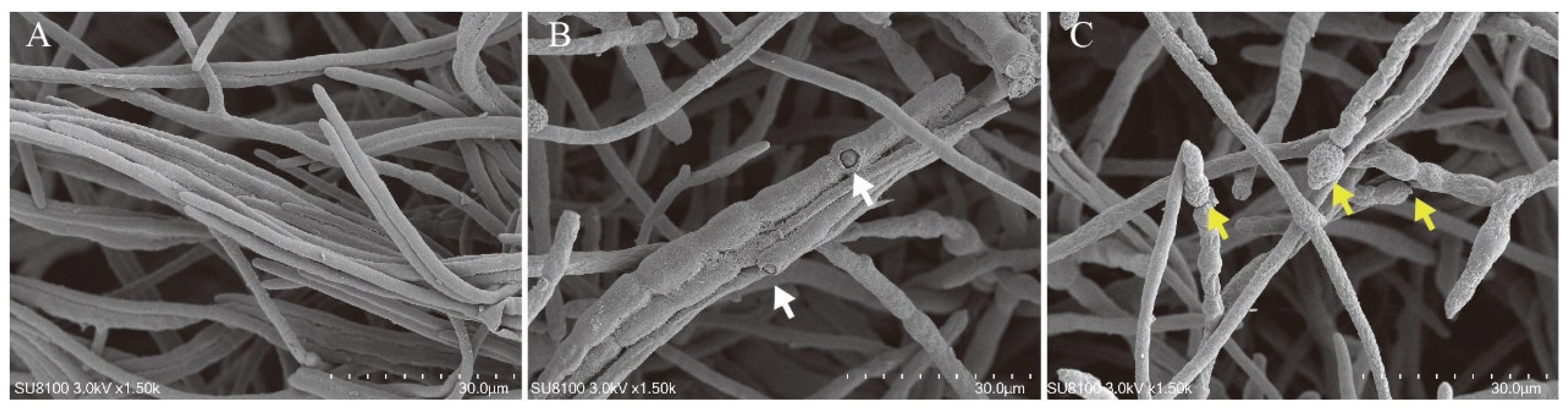
2.4. Scanning Electron Microscopy
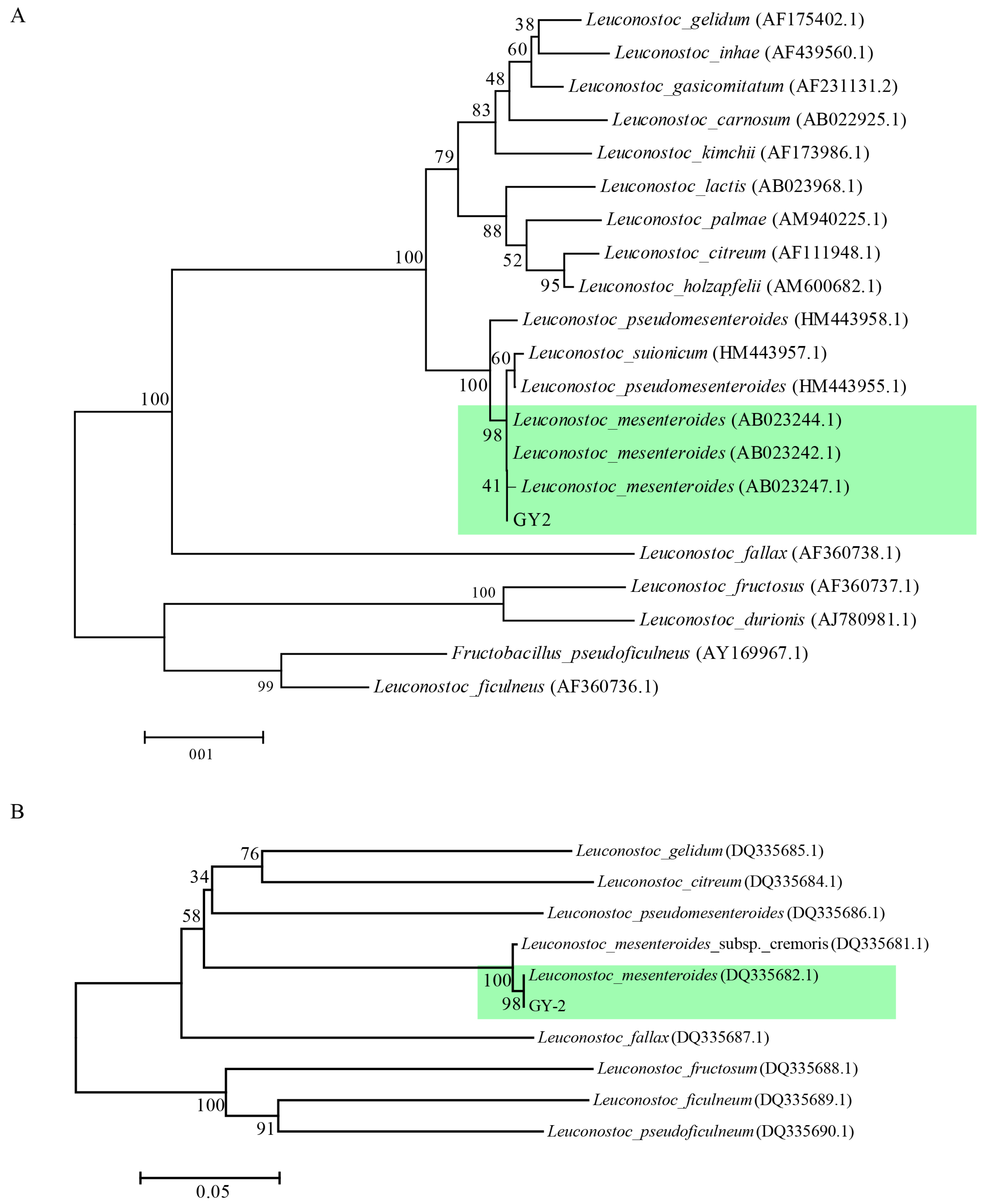
2.5. Identification of the Candidate Strain GY-2
2.6. Analysis of Organic Compounds by Solid Phase Microextraction–Gas Chromatography–Mass Spectrometer (SPME-GC–MS)
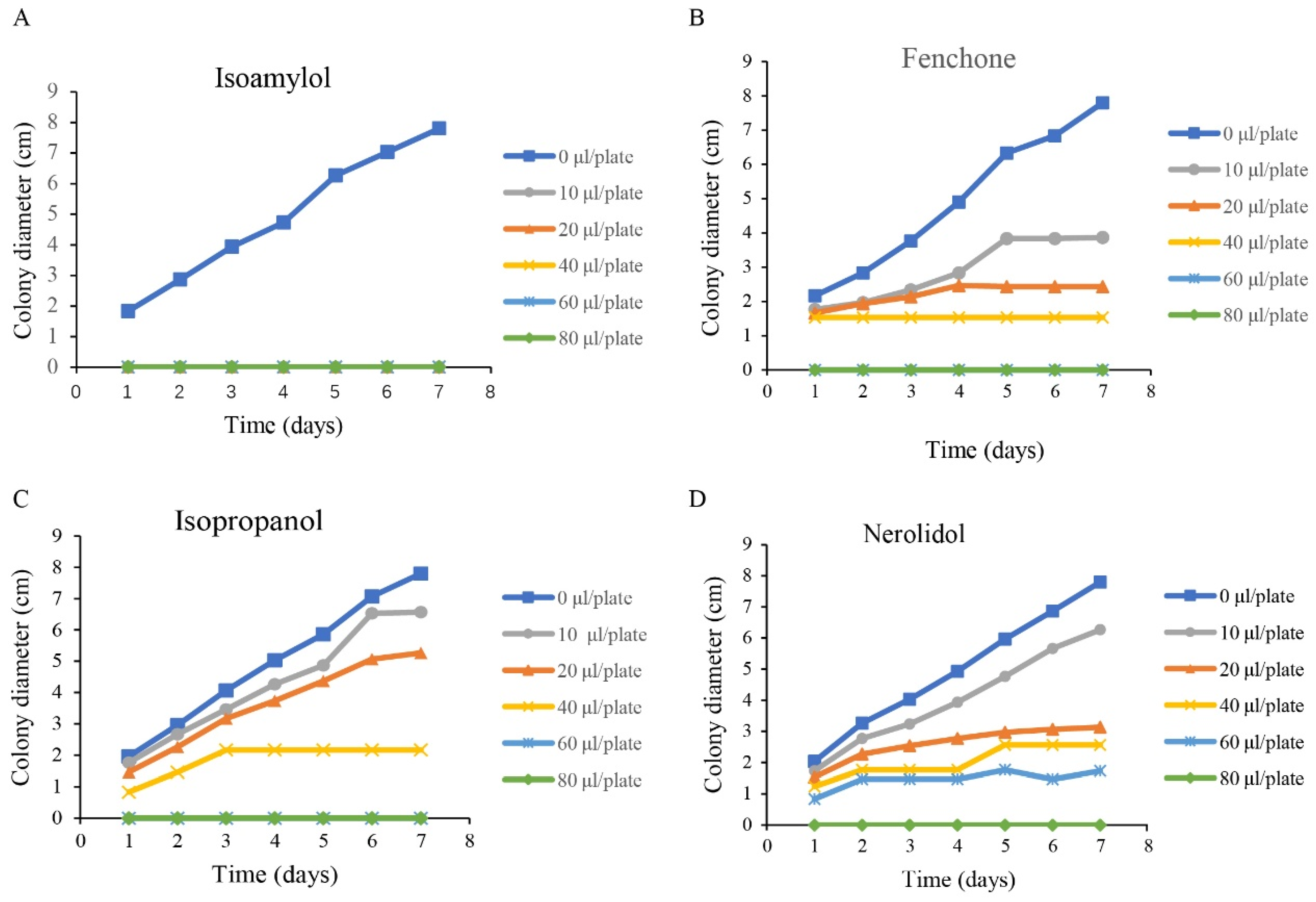
2.7. Analysis of Inhibitory Effect of Compounds on Mycelial Growth of A. alternata
2.8. Effect of Strain GY-2 on Apple Black Rot
2.8.1. Wound Inoculations
2.8.2. Inoculations Without Wounds
2.9. Colonization of Strain GY-2 on Apple Fruit
2.10. Effect of Strain GY-2 on Defense-Related Enzyme Activities
2.11. Effect of Strain GY-2 on the Postharvest Apple Quality
2.12. Statistical Analysis
3. Result
3.1. Evaluation of Biocontrol Effects of Strain GY-2 In Vitro
3.2. Identification of Strain GY-2
3.3. Identification of VOCs Emitted from GY-2 and Its Antifungal Activity
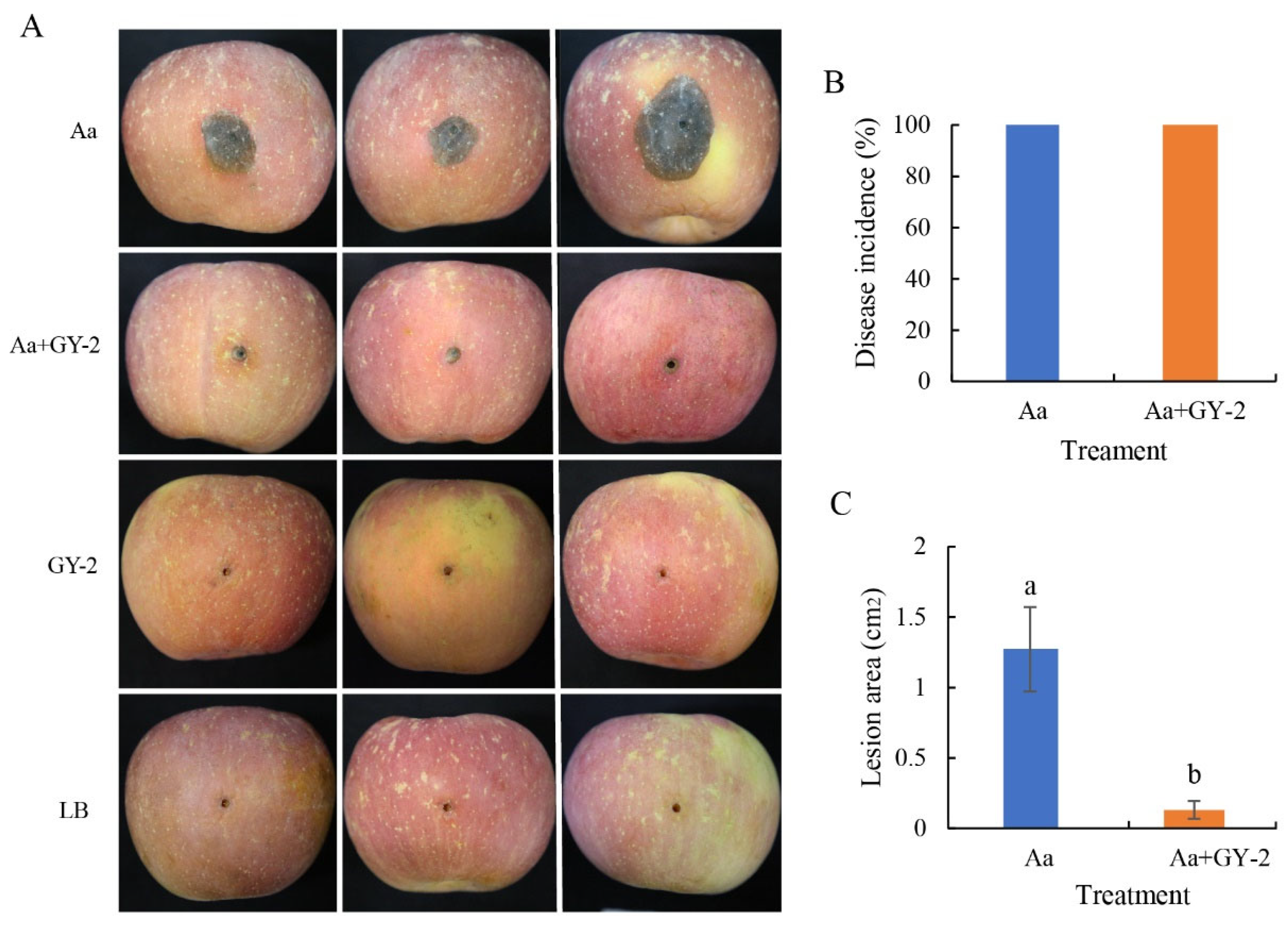
3.4. Biocontrol Activity of L. mesenteroides GY-2 Against A. alternata in Apple Fruit
3.5. Colonization of L. mesenteroides GY-2 on Apple Fruit
3.6. Effect of L. mesenteroides GY-2 on Antioxidant Enzymes and Defense-Related Enzymes in Apple
3.7. Effects of L. mesenteroides GY-2 on Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Li, Q.; Cao, J.; Qu, G.; Jiang, W. Comparative transcriptomic analysis provides novel insights into the difference in textural alteration between mealy and crisp apple patterns. Food Res. Int. 2023, 169, 112941. [Google Scholar] [CrossRef]
- Xiao, C.L.; Boal, R.J. Residual activity of fludioxonil and pyrimethanil against Penicillium expansum on apple fruit. Plant Dis. 2009, 93, 1003–1008. [Google Scholar] [CrossRef]
- Köhl, J.; Wenneker, M.; Groenenboom-de Haas, B.H.; Anbergen, R.; Goossen-van de Geijn, H.M.; Lombaers-van der Plas, C.H.; Pinto, F.A.M.F.; Kastelein, P. Dynamics of postharvest pathogens Neofabraea spp. and Cadophora spp. in plant residues in Dutch apple and pear orchards. Plant Pathol. 2018, 67, 1264–1277. [Google Scholar] [CrossRef]
- Jijakli, M.H.; Lepoivre, P. State of the Art and Challenges of Post-Harvest Disease Management in Apples; BT–fruit and Vegetable Diseases; Mukerji, K.G., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 59–94. [Google Scholar] [CrossRef]
- Jurick, W.M.; Kou, L.P.; Gaskins, V.L.; Luo, Y.G. First report of Alternaria alternata causing postharvest decay on apple fruit during cold storage in Pennsylvania. Plant Dis. 2014, 98, 690. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.B.; Walgenbach, J.F.; Aldwinckle, H.S.; Agnello, A. Compendium of Apple and Pear Diseases and Pests; APS Publications: St. Paul, MN, USA, 2014. [Google Scholar] [CrossRef]
- Wang, H.; Fu, L.; Li, C.; Zhang, X.; Narcisse, K.E.; Qi, H.; Han, C.; Wang, X.; Ma, H.; Zhu, C.; et al. Tannic acid exerts antifungal activity in vitro and in vivo against Alternaria alternata causing postharvest rot on apple fruit. Physiol. Mol. Plant Pathol. 2023, 125, 102012. [Google Scholar] [CrossRef]
- Gotthardt, M.; Kanawati, B.; Schmidt, F.; Asam, S.; Hammerl, R.; Frank, O.; Hofmann, T.; Schmitt-Kopplin, P.; Rychlik, M. Comprehensive analysis of the Alternaria mycobolome using mass spectrometry-based metabolomics. Mol. Nutr. Food Res. 2020, 64, 1900558. [Google Scholar] [CrossRef]
- Puntscher, H.; Cobankovic, I.; Marko, D.; Warth, B. Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS. Food Control 2019, 102, 157–165. [Google Scholar] [CrossRef]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal efficacy of ursolic acid in control of Alternaria alternata causing black spot rot on apple fruit and possible mechanisms involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Shu, C.; Cui, K.; Li, Q.; Cao, J.; Jiang, W. Epsilon-poly-l-lysine (ε-PL) exhibits multifaceted antifungal mechanisms of action that control postharvest Alternaria rot. Int. J. Food Microbiol. 2021, 348, 109224. [Google Scholar] [CrossRef]
- Gur, L.; Reuveni, M.; Cohen, Y. β-Aminobutyric acid induced resistance against Alternaria fruit rot in apple fruits. J. Fungi 2021, 7, 564. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Y.N.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Chen, D.; Chen, T.; Chen, Y.; Zhang, Z.; Li, B.; Tian, S. Bio-source substances against postharvest diseases of fruits: Mechanisms, applications and perspectives. Postharvest Biol. Technol. 2023, 198, 112240. [Google Scholar] [CrossRef]
- Jabbari, V.; Khiabani, M.S.; Mokarram, R.R.; Hassanzadeh, A.M.; Ahmadi, E.; Gharenaghadeh, S.; Karimi, N.; Kafil, H.S. Lactobacillus plantarum as a probiotic potential from Kouzeh cheese (traditional Iranian cheese) and its antimicrobial activity. Probiotics Antimicrob. Proteins 2017, 9, 189–193. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef]
- Nuraida, L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci. Hum. Well. 2015, 4, 47–55. [Google Scholar] [CrossRef]
- Kwak, M.K.; Liu, R.; Kim, M.K.; Moon, D.; Kim, A.H.; Song, S.H.; Kang, S.O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. F. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotech. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Ngea, G.L.N.; Yang, Q.; Tchabo, W.; Castoria, R.; Zhang, X.; Zhang, H. Leuconostoc mesenteroides subsp. mesenteroides LB7 isolated from apple surface inhibits P. expansum in vitro and reduces patulin in fruit juices. Int. J. Food Microbiol. 2021, 339, 109025. [Google Scholar] [CrossRef]
- Tang, J.; Yi, L.; Zeng, K. Isolation of antagonistic lactic acid bacteria from traditional fermented foods of China and biocontrol mode of Leuconostoc mesenteroides SNP037 against green pepper soft rot caused by Pectobacterium carotovorum. Postharvest Biol. Technol. 2024, 212, 112872. [Google Scholar] [CrossRef]
- Chen, O.; Hong, Y.; Ma, J.; Deng, L.; Yi, L.; Zeng, K. Screening lactic acid bacteria from pickle and cured meat as biocontrol agents of Penicillium digitatum on citrus fruit. Biol. Control 2021, 158, 104606. [Google Scholar] [CrossRef]
- Dai, P.; Li, N.; Li, B.; Wang, S.; Wang, Y.; Meng, X.; Li, B.; Cao, K.; Hu, T. An endophytic strain Bacillus velezensis JZ51 controlled pink mold rot of postharvest apple fruit via antagonistic action and disease resistance induction. Postharvest Biol. Technol. 2024, 210, 112793. [Google Scholar] [CrossRef]
- Delgado, N.; Olivera, M.; Cadiz, F.; Bravo, G.; Montenegro, I.; Madrid, A.; Fuentealba, C.; Pedreschi, R.; Salgado, E.; Besoain, X. Volatile organic compounds (VOCs) produced by Gluconobacter cerinus and Hanseniaspora osmophila displaying control effect against table grape-rot pathogens. Antibiotics 2021, 10, 663. [Google Scholar] [CrossRef]
- Moyes, R.B.; Reynolds, J.; Breakwell, D.P. Differential staining of bacteria: Gram stain. Appendix 3C Curr. Protoc. Microbiol. 2009, 15. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kurowski, T.P.; Damszel, M.; Krawczyk, K.; Karwowska, A. Effectiveness of the Bacillus sp, Sp-a9 strain as a biological control agent for spring wheat (Triticum aestivum L.). J. Agr. Sci. Technol. 2018, 20, 609–619. [Google Scholar]
- Wen, Y.; Zhang, G.S.; Bahadur, A.; Xu, Y.T.; Liu, Y.; Tian, M.; Ding, W.; Chen, T.; Zhang, W.; Liu, G.X. Genomic investigation of desert Streptomyces huasconensis D23 reveals its environmental adaptability and antimicrobial activity. Microorganisms 2022, 10, 2408. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wei, S.; Xu, C.; Chen, M.; Sang, J.; Han, Y.; Yan, H.; Li, Z.; Cui, Z. Antifungal activity and mechanisms of 2-ethylhexanol, a volatile organic compound produced by Stenotrophomonas sp. NAU1697, against Fusarium oxysporum f. sp. cucumerinum. J. Agric. Food Chem. 2024, 72, 15213–15227. [Google Scholar] [CrossRef]
- Hong, P.; Hao, W.; Luo, J.; Chen, S.; Hu, M.; Zhong, G. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Tulukoğlu-Kunt, K.S.; Özden, M.; Di Francesco, A. Exploring wild and local fruits as sources of promising biocontrol agents against Alternaria spp. in Apples. Horticulturae 2023, 9, 1156. [Google Scholar] [CrossRef]
- Bektas, I.; Yazdıc, F.C.; Kusek, M. Molecular characterization of apple endophytic bacteria and biological effect on Alternaria rot on apple. J. Phytopathol. 2024, 172, e13323. [Google Scholar] [CrossRef]
- Trias, R.; Badosa, E.; Montesinos, E.; Baneras, L. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Habbadi, K.; Achbani, E.H.; Benkirane, R.; El Handi, K.; Ou-Zine, M.; Benali, T.; Elbeaino, T. Antagonistic effect of Leuconostoc mesenteroides on grapevine crown gall and fire blight. J. Crop Improv. 2023, 37, 431–446. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhao, Y.; Feng, J.; Chen, Y.; Li, K.; Zhang, M.; Qi, D.; Zhou, D.; Wei, Y.; et al. Biocontrol potential of volatile organic compounds produced by Streptomyces corchorusii CG-G2 to strawberry anthracnose caused by Colletotrichum gloeosporioides. Food Chem. 2024, 437, 137938. [Google Scholar] [CrossRef]
- Xing, S.; Gao, Y.; Li, X.; Ren, H.; Gao, Y.; Yang, H.; Liu, Y.; He, S.; Huang, Q. Antifungal activity of volatile components from Ceratocystis fimbriata and its potential biocontrol mechanism on Alternaria alternata in postharvest cherry tomato fruit. Microbiol. Spectr. 2023, 11, e02713–e02722. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Cernava, T.; Gómez-Lama Cabanás, C.; Berg, G.; Mercado-Blanco, J. Identification of volatile organic compounds emitted by two beneficial endophytic Pseudomonas strains from olive roots. Plants 2022, 11, 318. [Google Scholar] [CrossRef]
- de Paiva, E.; Serradilla, M.J.; Ruiz-Moyano, S.; Cordoba, M.G.; Villalobos, M.C.; Casquete, R.; Hernandez, A. Combined effect of antagonistic yeast and modified atmosphere to control Penicillium expansum infection in sweet cherries cv. Ambrunes. Int. J. Food Microbiol. 2017, 241, 276–282. [Google Scholar] [CrossRef]
- Manso, T.; Nunes, C. Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol. Technol. 2011, 61, 64–71. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Ye, W.Q.; Sun, Y.F.; Tang, Y.J.; Zhou, W.W. Biocontrol potential of a broad-spectrum antifungal strain Bacillus amyloliquefaciens B4 for postharvest loquat fruit storage. Postharvest Biol. Technol. 2021, 174, 111439. [Google Scholar] [CrossRef]
- Zhang, Q.; Yong, D.; Zhang, Y.; Shi, X.; Li, B.; Li, G.; Liang, W.; Wang, C. Streptomyces rochei A-1 induces resistance and defense-related responses against Botryosphaeria dothidea in apple fruit during storage. Postharvest Biol. Technol. 2016, 115, 30–37. [Google Scholar] [CrossRef]
- Ji, Z.L.; Peng, S.; Zhu, W.; Dong, J.P.; Zhu, F. Induced resistance in nectarine fruit by Bacillus licheniformis W10 for the control of brown rot caused by Monilinia fructicola. Food Microbiol. 2020, 92, 103558. [Google Scholar] [CrossRef]
- Han, Z.; Li, B.; Gong, D.; Xie, P.; Yu, L.; Wang, Y.; Han, Y.; Li, Y.; Prusky, D.; Romanazzi, G.; et al. Preharvest chitooligosaccharide spray alleviates chilling injury in harvested muskmelon fruit by regulating membrane lipid metabolism and activating antioxidant enzyme activity. Postharvest Biol. Technol. 2023, 204, 112452. [Google Scholar] [CrossRef]
- Li, X.; Xiong, T.; Zhu, Q.; Zhou, Y.; Lei, Q.; Lu, H.; Chen, W.; Li, X.; Zhu, X. Combination of 1-MCP and modified atmosphere packaging (MAP) maintains banana fruit quality under high temperature storage by improving antioxidant system and cell wall structure. Postharvest Biol. Technol. 2023, 198, 112265. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Cui, J.; Jia, X.; Wang, W. Isolation and characterization of Bacillus amyloliquefaciens L-1 for biocontrol of pear ring rot. Hortic. Plant J. 2017, 3, 183–189. [Google Scholar] [CrossRef]
- Yang, W.; Wang, M.; Wang, H.; Zhang, C.; Zhang, Q.; Xiao, H. Exploitation of the biocontrol potential of a marine-derived Bacillus velezensis and its application on postharvest strawberry. Food Control 2024, 161, 110311. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during Kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef]
- Jeon, H.H.; Kim, K.H.; Chun, B.H.; Ryu, B.H.; Han, N.S.; Jeon, C.O. A proposal of Leuconostoc mesenteroides subsp. Jonggajibkimchii subsp. nov. and reclassification of Leuconostoc mesenteroides subsp. suionicum (Gu Et Al., 2012) as Leuconostoc suionicum sp. nov. based on complete genome sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2225–2230. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Zannini, E.; Coffey, A.; Arendt, E.K. “Green preservatives”: Combating fungi in the food and feed industry by applying antifungal lactic acid bacteria. Adv. Food Nutr. Res. 2012, 66, 217–238. [Google Scholar] [CrossRef] [PubMed]




| Pathogens | Inhibition Rates of Confrontation Assay (%) | Inhibition Rates of VOCs (%) |
|---|---|---|
| Alternaria alternata | 0 | 70.8 |
| Trichothecium roseum | 40.4 | 52.1 |
| Botryosphaeria dothidea | 0 | 11.1 |
| Colletotrichum fructicola | 29.8 | 39.1 |
| Valsa mali | 16.7 | 51.0 |
| Name | Retention Time | CAS No. | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| Isopropanol | 2.08 | 67-63-0 | C3H8O | 60.1 |
| Isoamylol | 3.95 | 123-51-3 | C5H12O | 88.15 |
| Fenchone | 5.15 | 1195-79-5 | C10H16O | 152.23 |
| Nerolidol | 16.24 | 142-50-7 | C15H26O | 222.37 |
| Treatment | Disease Incidence (%) | Lesion Number | Total of Lesion Area (mm2) |
|---|---|---|---|
| Aa | 63.3 | 13 | 290.7 |
| Aa+GY-2 | 26.7 | 9 | 115.3 |
| Treatment | Acidity (%) | Soluble Solid Content (%) | Firmness (N) | Weight Loss Rate (%) |
|---|---|---|---|---|
| CK | 0.90 ± 0.12 | 13.57 ± 1.69 | 6.58 ± 0.72 | 3.43 ± 1.29 |
| GY-2 | 0.92 ± 0.02 | 13.87 ± 1.16 | 7.12 ± 1.06 | 4.13 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Li, B.; Li, Y.; Wang, L.; Hu, T.; Wang, Y.; Meng, X.; Li, B.; Cao, K.; Wang, S.; et al. Biocontrol Efficiency of Leuconostoc mesenteroides GY-2 Against Postharvest Black Rot Caused by Alternaria alternata and the Mechanisms of Action. J. Fungi 2025, 11, 705. https://doi.org/10.3390/jof11100705
Dai P, Li B, Li Y, Wang L, Hu T, Wang Y, Meng X, Li B, Cao K, Wang S, et al. Biocontrol Efficiency of Leuconostoc mesenteroides GY-2 Against Postharvest Black Rot Caused by Alternaria alternata and the Mechanisms of Action. Journal of Fungi. 2025; 11(10):705. https://doi.org/10.3390/jof11100705
Chicago/Turabian StyleDai, Pengbo, Bing Li, Yanan Li, Li Wang, Tongle Hu, Yanan Wang, Xianglong Meng, Bo Li, Keqiang Cao, Shutong Wang, and et al. 2025. "Biocontrol Efficiency of Leuconostoc mesenteroides GY-2 Against Postharvest Black Rot Caused by Alternaria alternata and the Mechanisms of Action" Journal of Fungi 11, no. 10: 705. https://doi.org/10.3390/jof11100705
APA StyleDai, P., Li, B., Li, Y., Wang, L., Hu, T., Wang, Y., Meng, X., Li, B., Cao, K., Wang, S., & Sun, M. (2025). Biocontrol Efficiency of Leuconostoc mesenteroides GY-2 Against Postharvest Black Rot Caused by Alternaria alternata and the Mechanisms of Action. Journal of Fungi, 11(10), 705. https://doi.org/10.3390/jof11100705





