Endophytic Fungal Diversity in Carpesium lipskyi from the Gaoligong Mountains, Yunnan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling Site Information
2.2. Genomic DNA Extraction and PCR Amplification
2.3. Endophytic Fungal Diversity Analysis
3. Results
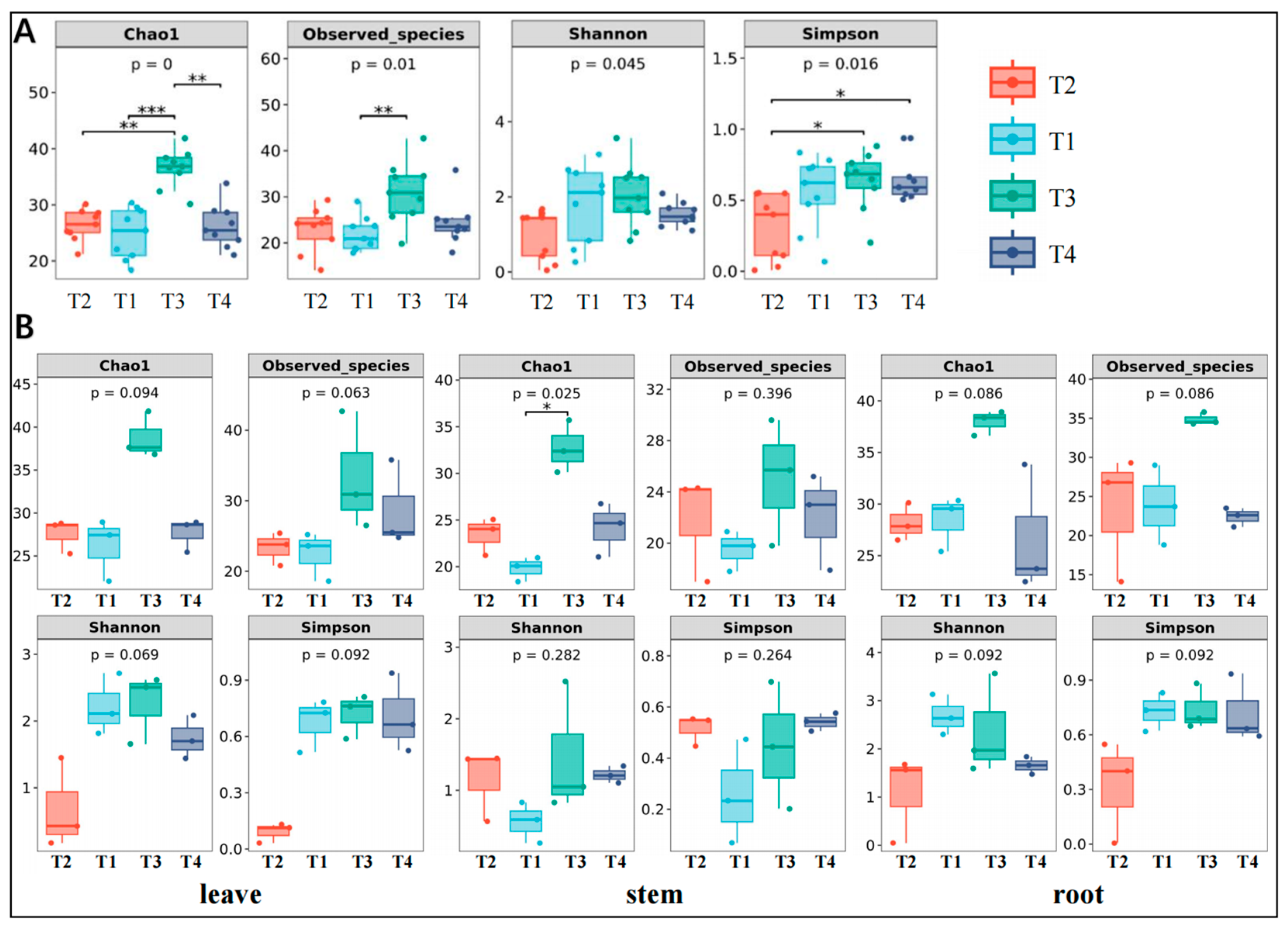
3.1. Analysis of Endophytic Fungal Diversity
3.2. Comparison of the Composition of Endophytic Fungal Communities
3.3. Comparison of Species Composition Variability
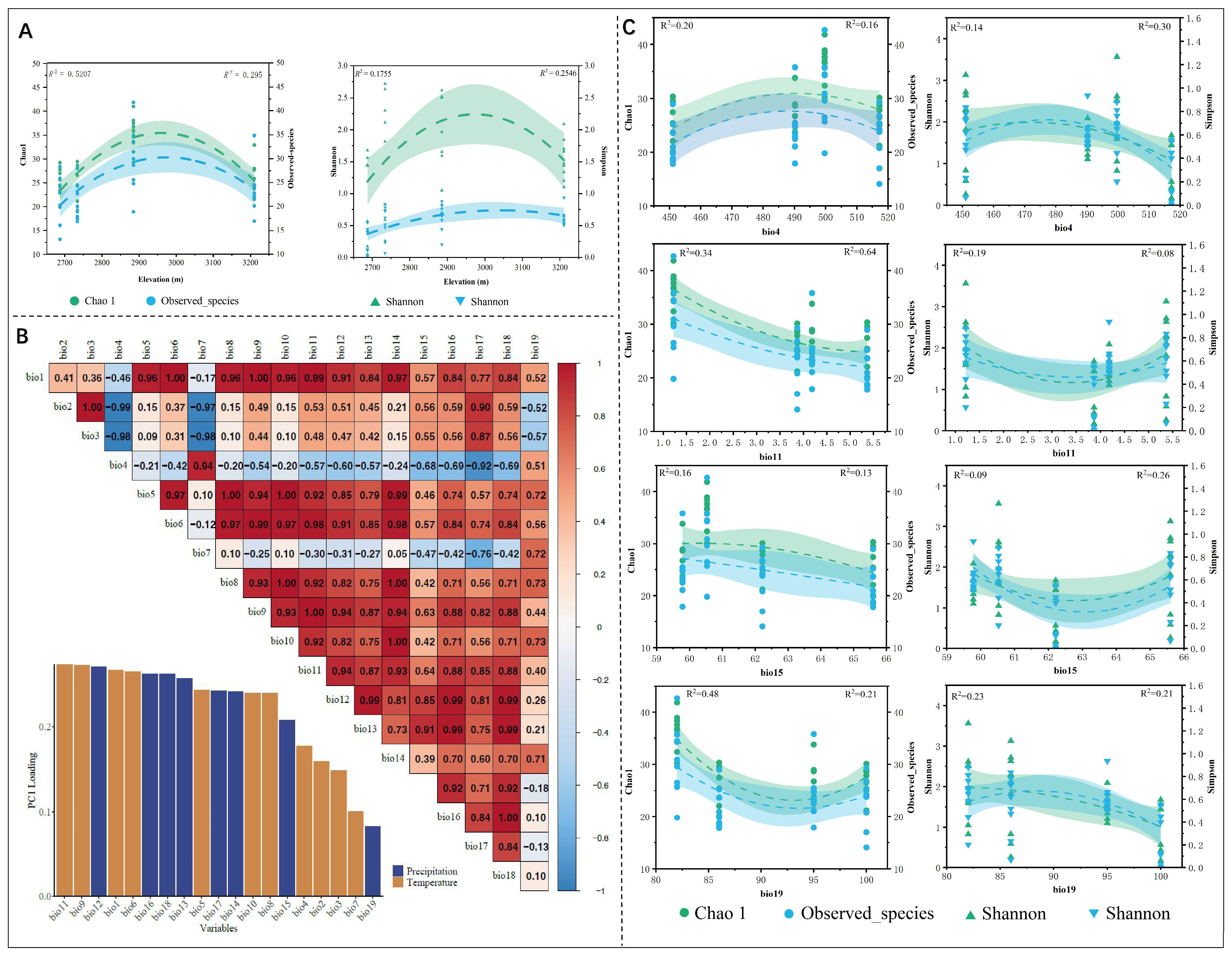
3.4. The Correlation Between the Diversity of Endophytic Fungi in Plants and Elevation
4. Discussion
4.1. Analysis of Species Composition in Taxonomy
4.2. Analysis of Variability in Species Composition and Its Potential Medicinal Value
4.3. Mechanisms of Environmental Factors Influencing the Diversity of Plant Endophytic Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.; Doilom, M.; Jeewon, R.; Hyde, K.D.; Manawasinghe, I.S.; Chethana, K.W.T.; Balasuriya, A.; Thakshila, S.A.D.; Luo, M.; Mapook, A.; et al. Challenges and Update on Fungal Endophytes: Classification, Definition, Diversity, Ecology, Evolution and Functions. Fungal Divers. 2025, 131, 301–367. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal Endophytes as Prolific Source of Phytochemicals and Other Bioactive Natural Products: A Review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Zuo, Y.; Hu, Q.; Zhang, K.; He, X. Host and Tissue Affiliations of Culturable Endophytic Fungi Associated with Xerophytic Plants in the Desert Region of Northwest China. Agronomy 2022, 12, 727. [Google Scholar] [CrossRef]
- Belbahri, L. Potentials of Endophytic Fungi in the Biosynthesis of Versatile Secondary Metabolites and Enzymes. Forests 2021, 12, 1784. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doering, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Doroteia Campos, M.; Rei, F.; do Rosário Félix, M. Spatial and Temporal Variation of Fungal Endophytic Richness and Diversity Associated to the Phyllosphere of Olive Cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Segaran, G.; Sathiavelu, M. Fungal Endophytes: A Potent Biocontrol Agent and a Bioactive Metabolites Reservoir. Biocatal. Agric. Biotechnol. 2019, 21, 101284. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Khan, N. Endophytic Fungal Diversity and Their Interaction with Plants for Agriculture Sustainability Under Stressful Condition. Recent. Pat. Food Nutr. Agric. 2020, 11, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Vetrovsky, T.; Kohout, P.; Kopecky, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcova, V.; Choi, J.; Meszarosova, L.; Human, Z.R.; et al. A Meta-Analysis of Global Fungal Distribution Reveals Climate-Driven Patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Geml, J.; Timling, I.; Robinson, C.H.; Lennon, N.; Nusbaum, H.C.; Brochmann, C.; Noordeloos, M.E.; Taylor, D.L. An Arctic Community of Symbiotic Fungi Assembled by Long-Distance Dispersers: Phylogenetic Diversity of Ectomycorrhizal Basidiomycetes in Svalbard Based on Soil and Sporocarp DNA. J. Biogeogr. 2012, 39, 74–88. [Google Scholar] [CrossRef]
- Cordier, T.; Robin, C.; Capdevielle, X.; Fabreguettes, O.; Desprez-Loustau, M.-L.; Vacher, C. The Composition of Phyllosphere Fungal Assemblages of European Beech (Fagus Sylvatica) Varies Significantly along an Elevation Gradient. New Phytol. 2012, 196, 510–519. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Chen, X.; Qu, T.; Zheng, X.; Pang, L.; Gu, X.; Fu, Z. Characterization and Phylogenetic Analysis of the Complete Chloroplast Genome of Carpesium Lipskyi (Asteraceae, Inuleae). Mitochondrial DNA Part B 2024, 9, 924–928. [Google Scholar] [CrossRef]
- Park, Y.J.; Cheon, S.Y.; Lee, D.S.; Cominguez, D.C.; An, H.J. Anti-Inflammatory and Antioxidant Effects of Carpesium cernuum L. Methanolic Extract in LPS-Stimulated RAW 264.7 Macrophages. Mediat. Inflamm. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, L.; Tan, D.; Wu, D.; Wu, X.; Fan, Q.; Bai, C.; Yang, J.; Xie, J.; He, Y. Fatty Acid Metabolites of Dendrobium Nobile Were Positively Correlated with Representative Endophytic Fungi at Altitude. Front. Microbiol. 2023, 14, 1128956. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Li, L.; Asem, M.D.; Zhang, Y.; Mohamad, O.A.; Salam, N.; Li, W. Endophytic Bacteria Associated with Endangered Plant Ferula sinkiangensis K. M. Shen in an Arid Land: Diversity and Plant Growth-Promoting Traits. J. Arid. Land. 2017, 9, 432–445. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. Embnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Chao, A. Non-Parametric Estimation of the Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Alban, R. Multivariate Analyses in Microbial Ecology. Fems Microbiol. Ecol. 2010, 62, 142–160. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the Healthy “Core Microbiome” of Oral Microbial Communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Zhang, Y.; Woodward, N.T.; Unger, D.; Hung, I.-K.; Oswald, B.P.; Farrish, K.W. A GIS Tool for Plant Spatial Pattern Analysis. Environ. Modell. Softw. 2011, 26, 1251–1254. [Google Scholar] [CrossRef][Green Version]
- Mahandran, V.; Murugan, C.M.; Anisha, P.S.; Wang, G.; Chen, J.; Nathan, P.T. Chemical Components Change along the Ontogeny of a Bat Fruit (Neolamarckia cadamba) with Ripening Asynchrony in Favour of Its Fruit Selection and Seed Dispersal. Sci. Nat. 2021, 108, 46. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A. Endophytic Fungal Communities and Their Biotechnological Implications for Agro-Environmental Sustainability. Folia Microbiol. 2022, 67, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Hyde, K.D.; Corke, H.; Sun, M. Biodiversity of Endophytic Fungi Associated with 29 Traditional Chinese Medicinal Plants. Fungal Divers. 2008, 33, 61–75. [Google Scholar]
- Chowdhary, K.; Kaushik, N.; Coloma, A.G.; Raimundo, C.M. Endophytic Fungi and Their Metabolites Isolated from Indian Medicinal Plant. Phytochem. Rev. 2012, 11, 467–485. [Google Scholar] [CrossRef]
- Banerjee, D.; Manna, S.; Mahapatra, S.; Pati, B.R. Fungal Endophytes in Three Medicinal Plants of Lamiaceae. Acta Microbiol. Immunol. Hung. 2009, 56, 243–250. [Google Scholar] [CrossRef]
- Velez-Martinez, G.A.; Reyes-Ardila, W.L.; Duque-Zapata, J.D.; Rugeles-Silva, P.A.; Munoz Florez, J.E.; Lopez-alvarez, D. Soil Bacteria and Fungi Communities Are Shaped by Elevation Influences in Colombian Forest and Paramo Natural Ecosystems. Int. Microbiol. 2024, 27, 377–391. [Google Scholar] [CrossRef]
- Liu, X.; Jia, P.; Cadotte, M.W.; Zhu, C.; Si, X.; Wang, Y.; Chen, F.; Wu, J.; Zhou, S. Host Plant Environmental Filtering Drives Foliar Fungal Community Assembly in Symptomatic Leaves. Oecologia 2021, 195, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, C.; Lin, L.; Keyhani, N.O.; Zhu, M.; Zhao, Z.; Zhang, W.; Yang, C.; Su, H.; Liu, P.; et al. Assessing the Structure and Diversity of Fungal Community in Plant Soil under Different Climatic and Vegetation Conditions. Front. Microbiol. 2023, 14, 1288066. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Nagahama, T. Fungal Diversity in Deep-Sea Extreme Environments. Fungal Ecol. 2012, 5, 463–471. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, S.; Hu, Y.; Zhang, Y.; Song, Y.; Chu, J.; Liu, C.; Rao, Y. Enhanced Cercosporin Production by Co-Culturing Cercospora sp. JNU001 with Leaf-Spot-Disease-Related Endophytic Bacteria. Microb. Cell Fact. 2021, 20, 100. [Google Scholar] [CrossRef]
- Xie, F.; Sun, Y.; Zi, Z.-F.; Wang, W.-J.; Wan, D.-Y.; Zhou, H.; Ding, Z.-T. Discovery of Pyranonaphthoquinones and an Eighteen-Membered Ring Macrolide from the Rhizospheric Soil-Derived Fungus Phialocephala sp. YUD18001 by OSMAC Strategy. Fitoterapia 2023, 171, 105690. [Google Scholar] [CrossRef]
- Xie, F.; Chang, W.; Zhang, M.; Li, Y.; Li, W.; Shi, H.; Zheng, S.; Lou, H. Quinone Derivatives Isolated from the Endolichenic Fungus Phialocephala Fortinii Are Mdr1 Modulators That Combat Azole Resistance in Candida Albicans. Sci. Rep. 2016, 6, 33687. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula Mucilaginosa-Alternative Sources of Natural Carotenoids, Lipids, and Enzymes for Industrial Use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Sohrab, M.H.; Rony, S.R.; Tareq, F.S.; Hasan, C.M.; Mazid, M.A. Cytotoxic and Antibacterial Naphthoquinones from an Endophytic Fungus, Cladosporium sp. Toxicol. Rep. 2016, 3, 861–865. [Google Scholar] [CrossRef]
- Cao, F.; Yang, Q.; Shao, C.-L.; Kong, C.-J.; Zheng, J.-J.; Liu, Y.-F.; Wang, C.-Y. Bioactive 7-Oxabicyclic[6.3.0]Lactam and 12-Membered Macrolides from a Gorgonian-Derived Cladosporium sp. Fungus. Mar. Drugs 2015, 13, 4171–4178. [Google Scholar] [CrossRef]
- Isaq, M.; Somu, P.; Acharya, D.; Gomez, L.A.; Thathapudi, J.J.; Ramachandra, Y.L.; Rudraiah, S.B.; Ravi, P.; Rai, P.S.; Rosalin, R.; et al. Phytochemical Screening and Bioactivity Studies of Endophytes Cladosporium sp. Isolated from the Endangered Plant Vateria Indica Using In Silico and In Vitro Analysis. Appl. Biochem. Biotechnol. 2022, 194, 4546–4569. [Google Scholar] [CrossRef]
- Lim, S.J.; Muhd Noor, N.D.; Sabri, S.; Ali, M.S.M.; Salleh, A.B.; Oslan, S.N. Features of the Rare Pathogen Meyerozyma guilliermondii Strain SO and Comprehensive in Silico Analyses of Its Adherence-Contributing Virulence Factor Agglutinin-like Sequences. J. Biomol. Struct. Dyn. 2023, 43, 3728–3748. [Google Scholar] [CrossRef]
- Cheng, T.; Lin, P.; Zhou, D.; Wang, H.; Zheng, K.; Shen, J.; Shi, S.; Hu, X.; Ye, X.; Cao, X. Distribution and Diversity of Cultured Endophytic Fungi in Gentiana straminea Maxim. at Different Altitudes on the Northeastern Qinghai-Tibetan Plateau. Front. Microbiol. 2024, 15, 1466613. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2010, 182, 314–330. [Google Scholar] [CrossRef]
- Sun, Z.H.; Peng, S.J.; Ou, X.K. Rapid Assessment and Explanation of Tree Species Abundance along the Elevation Gradient in Gaoligong Mountains, Yunnan, China. Chin. Sci. Bull. 2007, 52, 225–231. [Google Scholar] [CrossRef]
- Bhople, P.; Djukic, I.; Keiblinger, K.; Zehetnere, F.; Liu, D.; Bierbaumer, M.; Zechmeister-Boltenstern, S.; Joergensen, R.G.; Murugan, R. Variations in Soil and Microbial Biomass C, N and Fungal Biomass Ergosterol along Elevation and Depth Gradients in Alpine Ecosystems. Geoderma 2019, 345, 93–103. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Xiao, Y.; Chen, K.; Wang, J.; Zhou, G. Temperature Thresholds Drive the Biogeographic Pattern of Root Endophytic Fungal Diversity in the Qinghai-Tibet Plateau. Sci. Total Environ. 2023, 889, 164270. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Hernandez, M.; Blanco, J.; Diego Vargas, L.; Guillermo Acosta-Vargas, L.; Tamayo, G. Richness of Cultivable Endophytic Fungi along an Altitudinal Gradient in Wet Forests of Costa Rica. Fungal Ecol. 2016, 20, 124–131. [Google Scholar] [CrossRef]
- Laliberte, E.; Zemunik, G.; Turner, B.L. Environmental Filtering Explains Variation in Plant Diversity along Resource Gradients. Science 2014, 345, 1602–1605. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. Responses of the Morphological and Anatomical Structure of the Plant Leaf to Environmental Change. Chin. Bull. Bot. 2005, 22 (Suppl. S1), 118–127. [Google Scholar]
- Wenndt, A.J.; Evans, S.E.; van Diepeningen, A.D.; Logan, J.R.; Jacobson, P.J.; Seely, M.K.; Jacobson, K.M. Why Plants Harbor Complex Endophytic Fungal Communities: Insights From Perennial Bunchgrass Stipagrostis sabulicola in the Namib Sand Sea. Front. Microbiol. 2021, 12, 691584. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, R.; Li, W. Elevation Determines Fungal Diversity, and Land Use Governs Community Composition: A Dual Perspective from Gaoligong Mountains. Microorganisms 2024, 12, 2378. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Zhang, Y.; Liao, J.; Guan, K.; Zhai, J.; Meng, P.; Tang, X.; Dong, T.; Song, Y. Root Hair Developmental Regulators Orchestrate Drought Triggered Microbiome Changes and the Interaction with Beneficial Rhizobiaceae. Nat. Commun. 2024, 15, 10068. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and Host Range of Foliar Fungal Endophytes: Are Tropical Leaves Biodiversity Hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Condon, B.; Kinkel, L.; Komatsu, K.J.; Lumibao, C.Y.; May, G.; McCulley, R.L.; Borer, E.T. Effects of Nutrient Supply, Herbivory, and Host Community on Fungal Endophyte Diversity. Ecology 2019, 100, e02758. [Google Scholar] [CrossRef]
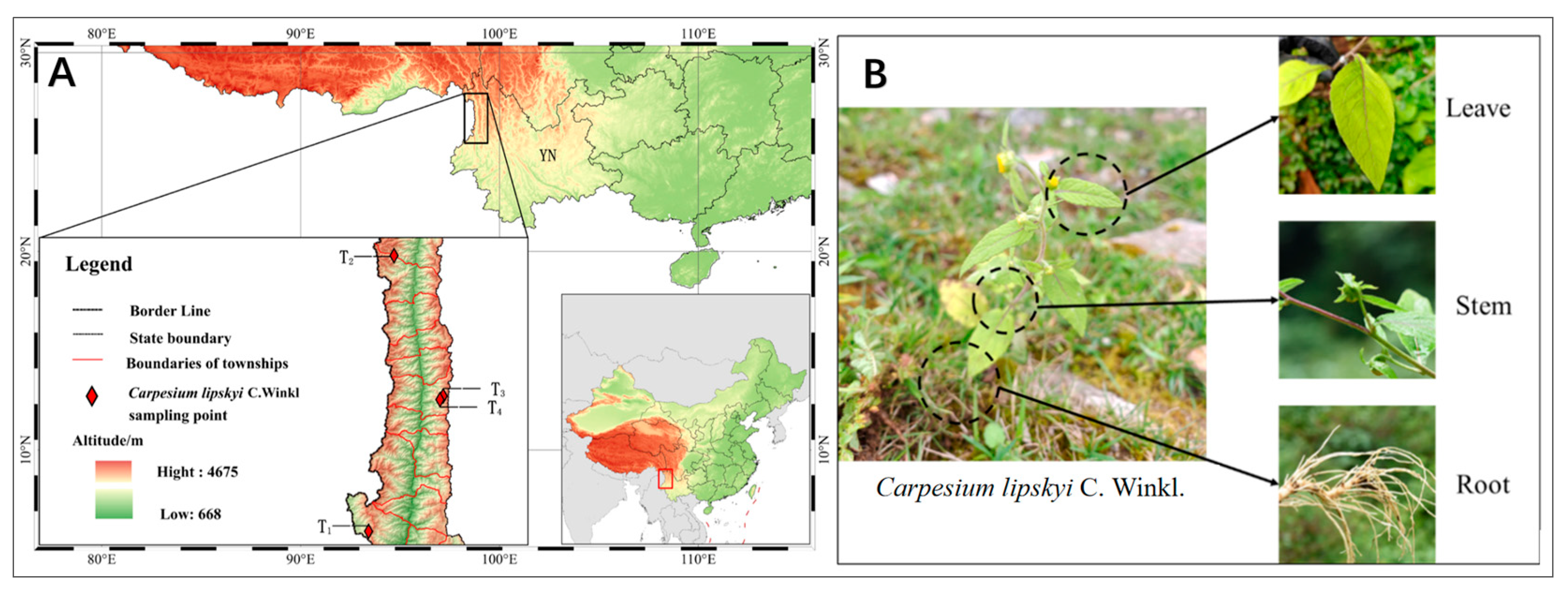


| ID | Collection Site | Longitude | Latitude | Altitude (m) | Growth Stage | Plant Height (cm) | Bio4 (standard Deviation × 100) | Bio11 (°C) | Bio15 (mm) | Bio19 (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| T2 | Shiyueliang Township, Fugong, Nujiang Prefecture, Yunnan Province | 98°46′49.82” | 27°9′55.02” | 2687.00 | florescence | 57 | 451.05 | 3.87 | 62.22 | 100.00 |
| T1 | Panma Town, Lushui, Nujiang Prefecture, Yunnan Province | 98°40′17.11” | 25°59′0.78” | 2734.00 | florescence | 54 | 517.21 | 5.37 | 65.60 | 86.00 |
| T3 | PiheNuZu Township, Fugong, Nujiang Prefecture, Yunnan Province | 98°59′27.06” | 26°33′48.34” | 2885.10 | florescence | 61 | 499.68 | 1.22 | 60.53 | 82.00 |
| T4 | PiheNuZu Township, Fugong, Nujiang Prefecture, Yunnan Province | 98°58′37.73” | 26°32′52.17” | 3210.37 | florescence | 57 | 490.17 | 4.18 | 59.78 | 95.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Liu, Q.; Di, Y.; Liu, T.; Su, Y.; He, Y.; Chen, J.; Peng, J.; Lee, S.Y.; Hoa, I.T.; et al. Endophytic Fungal Diversity in Carpesium lipskyi from the Gaoligong Mountains, Yunnan, China. J. Fungi 2025, 11, 704. https://doi.org/10.3390/jof11100704
Zheng H, Liu Q, Di Y, Liu T, Su Y, He Y, Chen J, Peng J, Lee SY, Hoa IT, et al. Endophytic Fungal Diversity in Carpesium lipskyi from the Gaoligong Mountains, Yunnan, China. Journal of Fungi. 2025; 11(10):704. https://doi.org/10.3390/jof11100704
Chicago/Turabian StyleZheng, Hancaiyuan, Qun Liu, Yining Di, Tao Liu, Yu Su, Yuqin He, Juntong Chen, Jingyi Peng, Shiou Yih Lee, Inh Thkim Hoa, and et al. 2025. "Endophytic Fungal Diversity in Carpesium lipskyi from the Gaoligong Mountains, Yunnan, China" Journal of Fungi 11, no. 10: 704. https://doi.org/10.3390/jof11100704
APA StyleZheng, H., Liu, Q., Di, Y., Liu, T., Su, Y., He, Y., Chen, J., Peng, J., Lee, S. Y., Hoa, I. T., Huang, X., & Liu, L. (2025). Endophytic Fungal Diversity in Carpesium lipskyi from the Gaoligong Mountains, Yunnan, China. Journal of Fungi, 11(10), 704. https://doi.org/10.3390/jof11100704





