Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma
Abstract
1. Introduction
2. Materials and Methods
2.1. Trichoderma Strains and Plant Pathogens
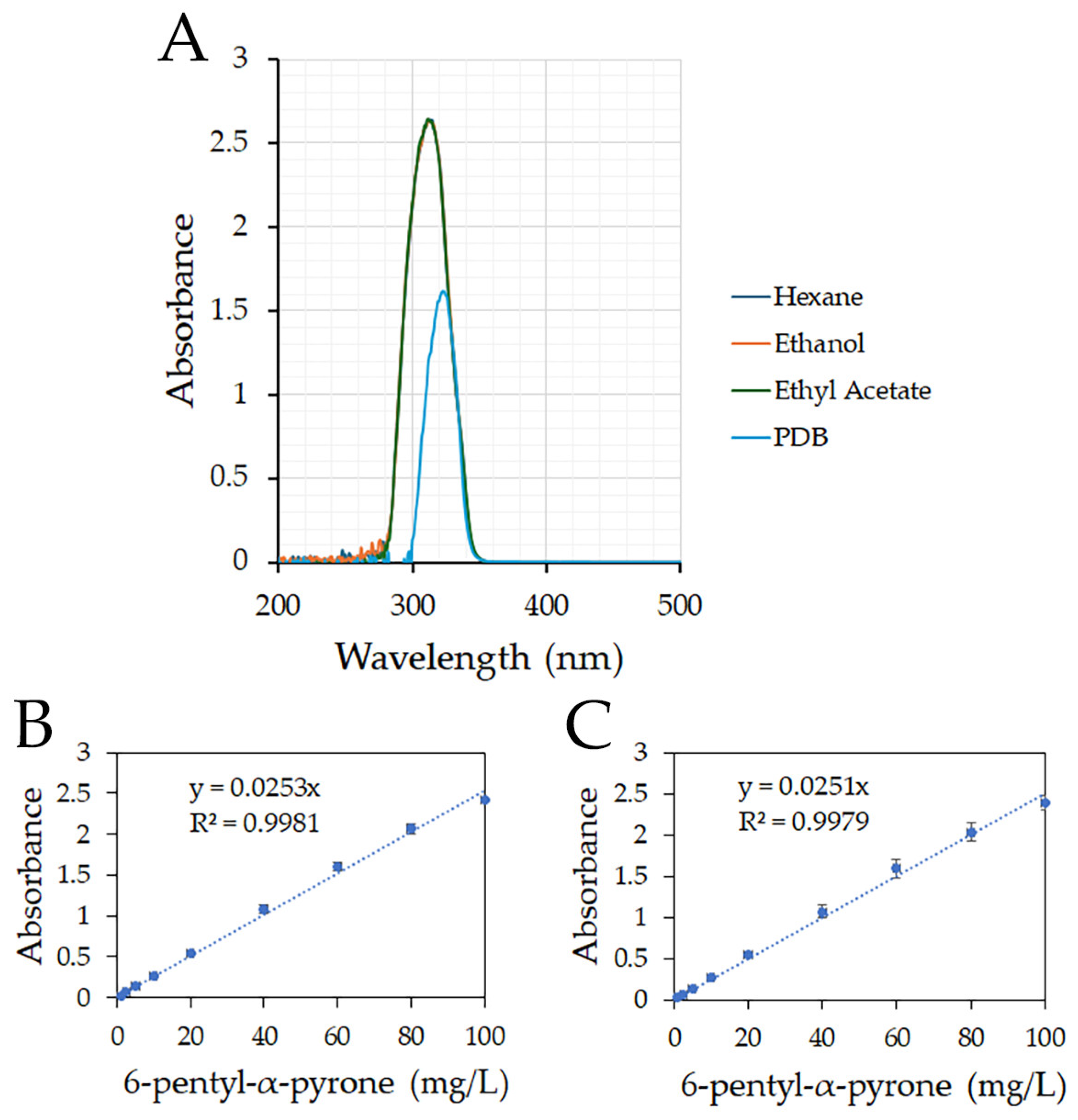
2.2. UV–VIS Analysis of 6-Pentyl-α-pyrone in Different Solvents
2.3. Dynamic Linear Range to Quantify 6-Pentyl-α-pyrone
2.4. Extraction of 6-Pentyl-α-pyrone from PDB
2.5. Extraction of Metabolites from Liquid Media
2.6. Thin Layer Chromatography Assay
2.7. Isolation and Characterization of 6-Pentyl-α-pyrone from the Ethyl Acetate Extract
2.8. Antagonistic Activity Under Light and Dark Conditions
2.9. Extraction of Metabolites from PDA
2.10. Statistical Analysis
3. Results
3.1. Dynamic Linear Range to Quantify 6-Pentyl-α-pyrone
3.2. Extraction of 6-Pentyl-α-pyrone from PDB
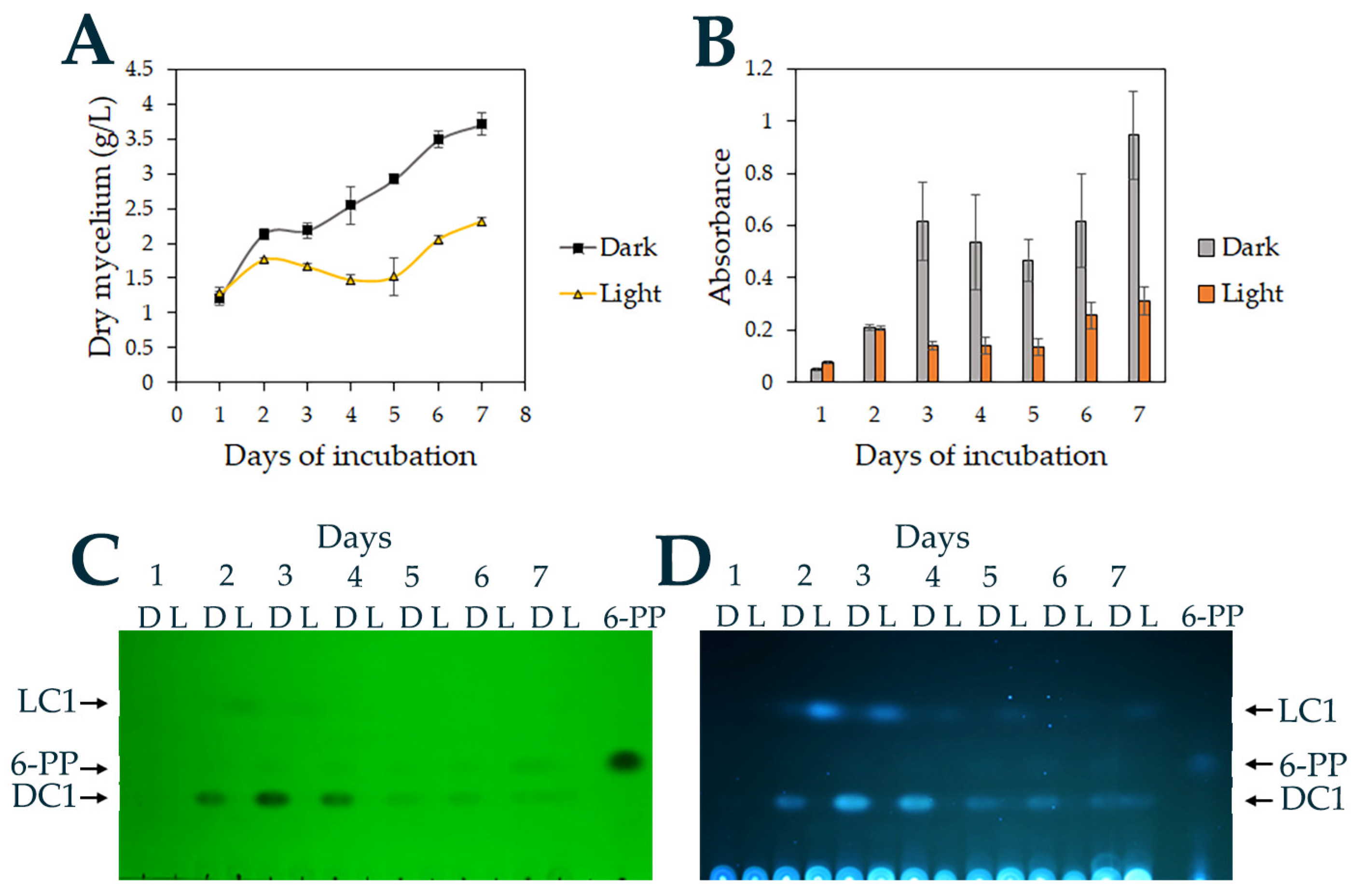
3.3. Light Regulates Growth and 6-Pentyl-α-pyrone Production in T. atroviride
3.4. Isolation and Characterization of 6-PP from the Ethyl Acetate Extract
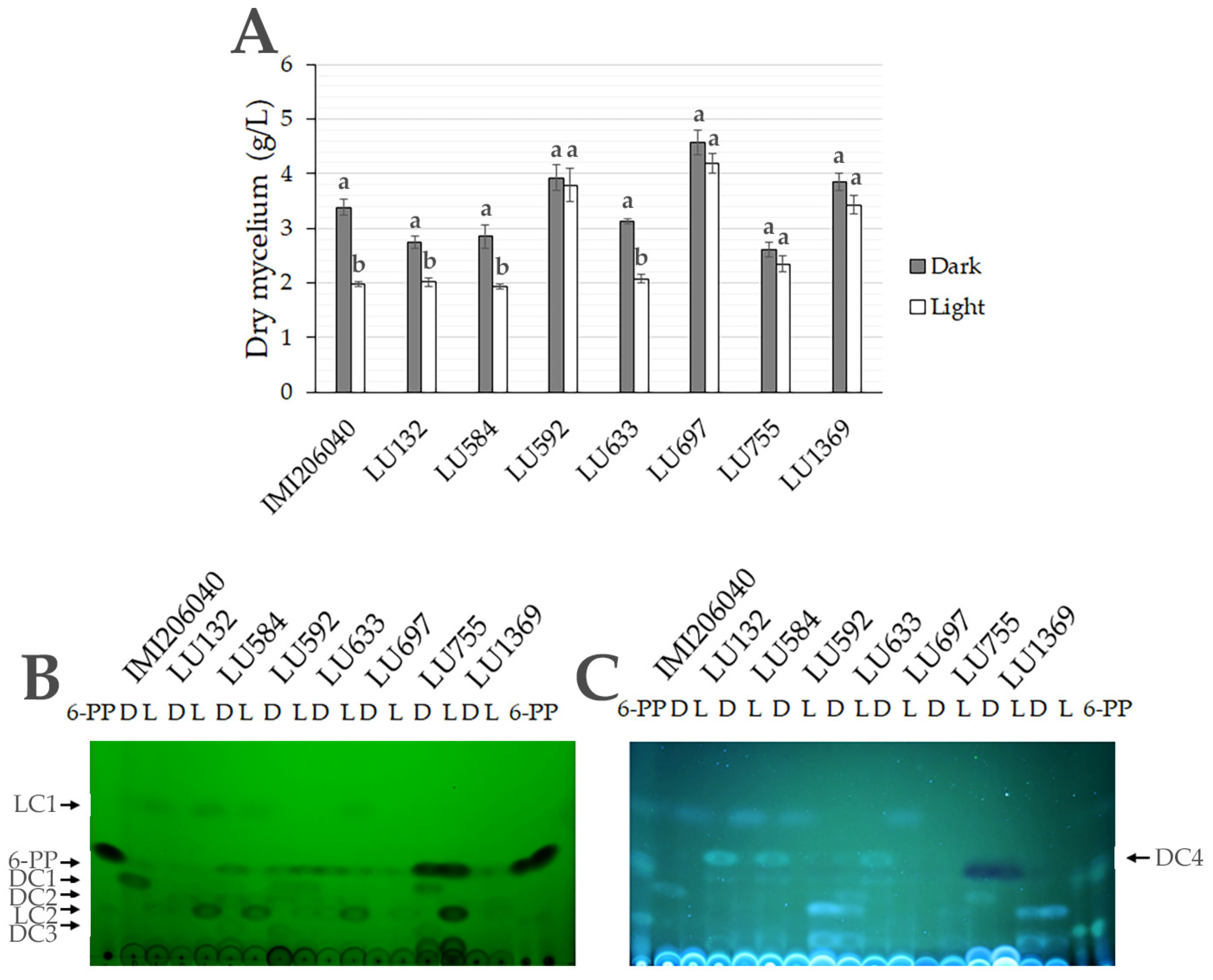
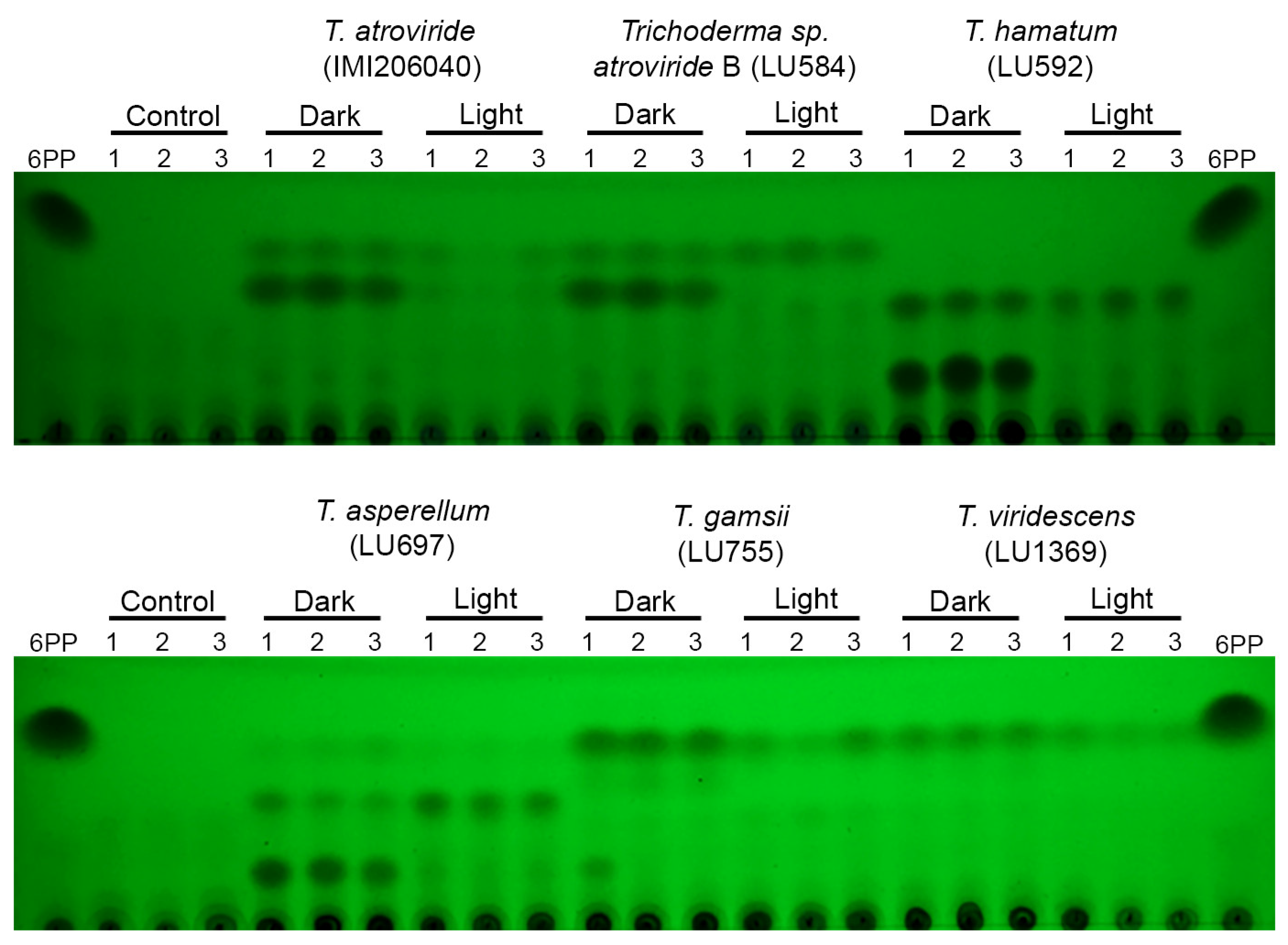
3.5. Metabolite Production Is Regulated by Light in Submerged Cultures of Trichoderma spp.
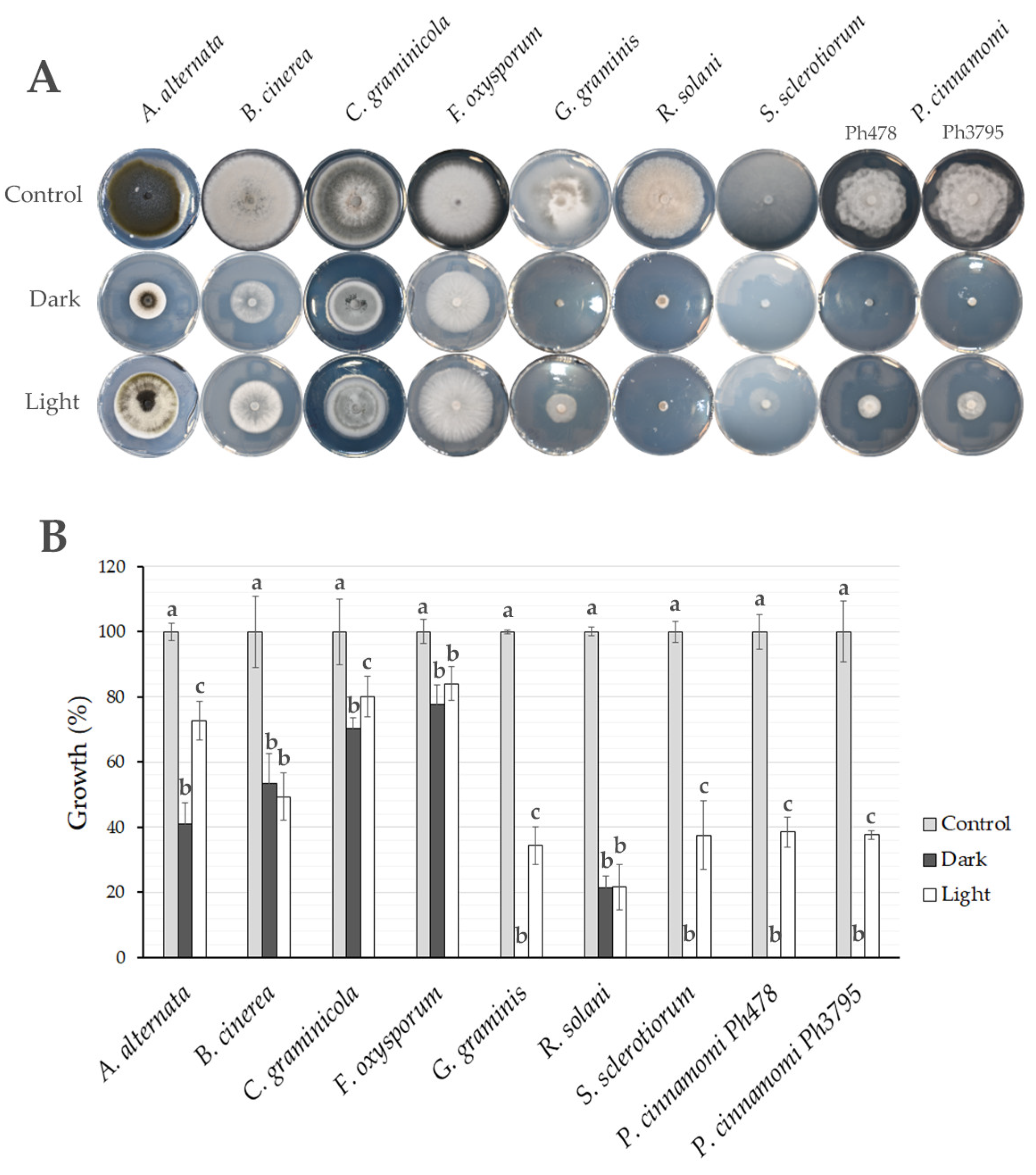
3.6. Light Influences the Antagonistic Capacity of Trichoderma spp.
3.7. Light Regulates Secreted Metabolites in Trichoderma spp. Growing on PDA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Schmoll, M.; Dattenböck, C.; Carreras-Villaseñor, N.; Mendoza-Mendoza, A.; Tisch, D.; Alemán, M.I.; Baker, S.E.; Brown, C.; Cervantes-Badillo, M.G.; Cetz-Chel, J.; et al. The Genomes of Three Uneven Siblings: Footprints of the Lifestyles of Three Trichoderma Species. Microbiol. Mol. Biol. Rev. 2016, 80, 205–327. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.; Wright, A.D. Trichopyrone and other constituents from the marine sponge-derived fungus Trichoderma sp. Z. Naturforsch. C J. Biosci. 2009, 64, 186–192. [Google Scholar] [CrossRef]
- Cruz-Magalhães, V.; Nieto-Jacobo, M.F.; van Zijll de Jong, E.; Rostás, M.; Padilla-Arizmendi, F.; Kandula, D.; Kandula, J.; Hampton, J.; Herrera-Estrella, A.; Steyaert, J.M. The NADPH oxidases Nox1 and Nox2 differentially regulate volatile organic compounds, fungistatic activity, plant growth promotion and nutrient assimilation in Trichoderma atroviride. Front. Microbiol. 2019, 9, 3271. [Google Scholar] [CrossRef]
- van Zijll de Jong, E.; Kandula, J.; Rostás, M.; Kandula, D.; Hampton, J.; Mendoza-Mendoza, A. Fungistatic Activity Mediated by Volatile Organic Compounds Is Isolate-Dependent in Trichoderma sp. “atroviride B”. J. Fungi 2023, 9, 238. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma Volatile Organic Compounds as a Biofumigation Tool against Late Blight Pathogen Phytophthora infestans in Postharvest Potato Tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef]
- Bello, F.; Montironi, I.D.; Medina, M.B.; Munitz, M.S.; Ferreira, F.V.; Williman, C.; Vázquez, D.; Cariddi, L.N.; Musumeci, M.A. Mycofumigation of postharvest blueberries with volatile compounds from Trichoderma atroviride IC-11 is a promising tool to control rots caused by Botrytis cinerea. Food Microbiol. 2022, 106, 104040. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Y.; Xia, Y.; Miao, Y.; Shao, J.; Xuan, W.; Liu, Y.; Xun, W.; Yan, Q.; Shen, Q.; et al. Trichoderma-secreted anthranilic acid promotes lateral root development via auxin signaling and RBOHF-induced endodermal cell wall remodeling. Cell Rep. 2024, 43, 114030. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1934578X1200701133. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Esquivel-Naranjo, E.U.; Soth, S.; Whelan, H.; Alizadeh, H.; Echaide-Aquino, J.F.; Kandula, D.; Hampton, J.G. Uncovering the multifaceted properties of 6-pentyl-alpha-pyrone for control of plant pathogens. Front. Plant Sci. 2024, 15, 1420068. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; del-Val, E.; Macías-Rodríguez, L.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol. Biochem. 2018, 122, 196–202. [Google Scholar] [CrossRef]
- Torres-Ortega, R.; Guillén-Alonso, H.; Alcalde-Vázquez, R.; Ramírez-Chávez, E.; Molina-Torres, J.; Winkler, R. In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction. Metabolites 2022, 12, 1231. [Google Scholar] [CrossRef]
- Henríquez-Urrutia, M.; Spanner, R.; Olivares-Yánez, C.; Seguel-Avello, A.; Pérez-Lara, R.; Guillén-Alonso, H.; Winkler, R.; Herrera-Estrella, A.; Canessa, P.; Larrondo, L.F. Circadian oscillations in Trichoderma atroviride and the role of core clock components in secondary metabolism, development, and mycoparasitism against the phytopathogen Botrytis cinerea. eLife 2022, 11, e71358. [Google Scholar] [CrossRef]
- Cruz-Magalhães, V.; Nieto-Jacobo, M.F.; Rostás, M.; Echaide-Aquino, J.F.; Esquivel-Naranjo, E.U.; Stewart, A.; Loguercio, L.L.; Mendoza-Mendoza, A. Histidine kinase two-component response regulators Ssk1, Skn7 and Rim15 differentially control growth, developmental and volatile organic compounds emissions as stress responses in Trichoderma atroviride. Curr. Res. Microb. Sci. 2022, 3, 100139. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Fuchs, A.; Missbach, K.; Schuhmacher, R.; Zeilinger, S. Influence of Different Light Regimes on the Mycoparasitic Activity and 6-Pentyl-α-pyrone Biosynthesis in Two Strains of Trichoderma atroviride. Pathogens 2020, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Missbach, K.; Flatschacher, D.; Bueschl, C.; Samson, J.M.; Leibetseder, S.; Marchetti-Deschmann, M.; Zeilinger, S.; Schuhmacher, R. Light-Induced Changes in Secondary Metabolite Production of Trichoderma atroviride. J. Fungi 2023, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, M.; Johnston, P.R.; Ball, S.L.; Nourozi, F.; Hay, A.J.; Shoukouhi, P.; Chomic, A.; Lange, C.; Ohkura, M.; Nieto-Jacobo, M.F.; et al. Trichoderma down under: Species diversity and occurrence of Trichoderma in New Zealand. Australas. Plant Pathol. 2017, 46, 11–30. [Google Scholar] [CrossRef]
- Schmey, T.; Tominello-Ramirez, C.S.; Brune, C.; Stam, R. Alternaria diseases on potato and tomato. Mol. Plant Pathol. 2024, 25, e13435. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Ward, E. Gaeumannomyces graminis, the take-all fungus and its relatives. Mol. Plant Pathol. 2004, 5, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the Pathogenomic Features of a Global Pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Sukno, S.A.; García, V.M.; Shaw, B.D.; Thon, M.R. Root infection and systemic colonization of maize by Colletotrichum graminicola. Appl. Environ. Microbiol. 2008, 74, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Classification of Solvents. In Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 65–106. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing R: Vienna, Austria, 2024. [Google Scholar]
- Esquivel-Naranjo, E.U.; García-Esquivel, M.; Medina-Castellanos, E.; Correa-Pérez, V.A.; Parra-Arriaga, J.L.; Landeros-Jaime, F.; Cervantes-Chávez, J.A.; Herrera-Estrella, A. A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol. Microbiol. 2016, 100, 860–876. [Google Scholar] [CrossRef]
- Pezet, R.; Pont, V.; Tabacchi, R. Simple analysis of 6-pentyl-α-pyrone, a major antifungal metabolite of Trichoderma spp., useful for testing the antagonistic activity of these fungi. Phytochem. Anal. 1999, 10, 285–288. [Google Scholar] [CrossRef]
- Simon, A.; Dunlop, R.W.; Ghisalberti, E.L.; Sivasithamparam, K. Trichoderma koningii produces a pyrone compound with antibiotic properties. Soil Biol. Biochem. 1988, 20, 263–264. [Google Scholar] [CrossRef]
- Evidente, A.; Cabras, A.; Maddau, L.; Serra, S.; Andolfi, A.; Motta, A. Viridepyronone, a New Antifungal 6-Substituted 2H-Pyran-2-one Produced by Trichoderma viride. J. Agric. Food Chem. 2003, 51, 6957–6960. [Google Scholar] [CrossRef] [PubMed]
- Calcáneo-Hernández, G.; Landeros-Jaime, F.; Cervantes-Chávez, J.A.; Mendoza-Mendoza, A.; Esquivel-Naranjo, E.U. Osmotic Stress Responses, Cell Wall Integrity, and Conidiation Are Regulated by a Histidine Kinase Sensor in Trichoderma atroviride. J. Fungi 2023, 9, 939. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquivel-Naranjo, E.U.; Mancilla-Diaz, H.; Marquez-Mazlin, R.; Alizadeh, H.; Kandula, D.; Hampton, J.; Mendoza-Mendoza, A. Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma. J. Fungi 2025, 11, 9. https://doi.org/10.3390/jof11010009
Esquivel-Naranjo EU, Mancilla-Diaz H, Marquez-Mazlin R, Alizadeh H, Kandula D, Hampton J, Mendoza-Mendoza A. Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma. Journal of Fungi. 2025; 11(1):9. https://doi.org/10.3390/jof11010009
Chicago/Turabian StyleEsquivel-Naranjo, Edgardo Ulises, Hector Mancilla-Diaz, Rudi Marquez-Mazlin, Hossein Alizadeh, Diwakar Kandula, John Hampton, and Artemio Mendoza-Mendoza. 2025. "Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma" Journal of Fungi 11, no. 1: 9. https://doi.org/10.3390/jof11010009
APA StyleEsquivel-Naranjo, E. U., Mancilla-Diaz, H., Marquez-Mazlin, R., Alizadeh, H., Kandula, D., Hampton, J., & Mendoza-Mendoza, A. (2025). Light Regulates Secreted Metabolite Production and Antagonistic Activity in Trichoderma. Journal of Fungi, 11(1), 9. https://doi.org/10.3390/jof11010009








