Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
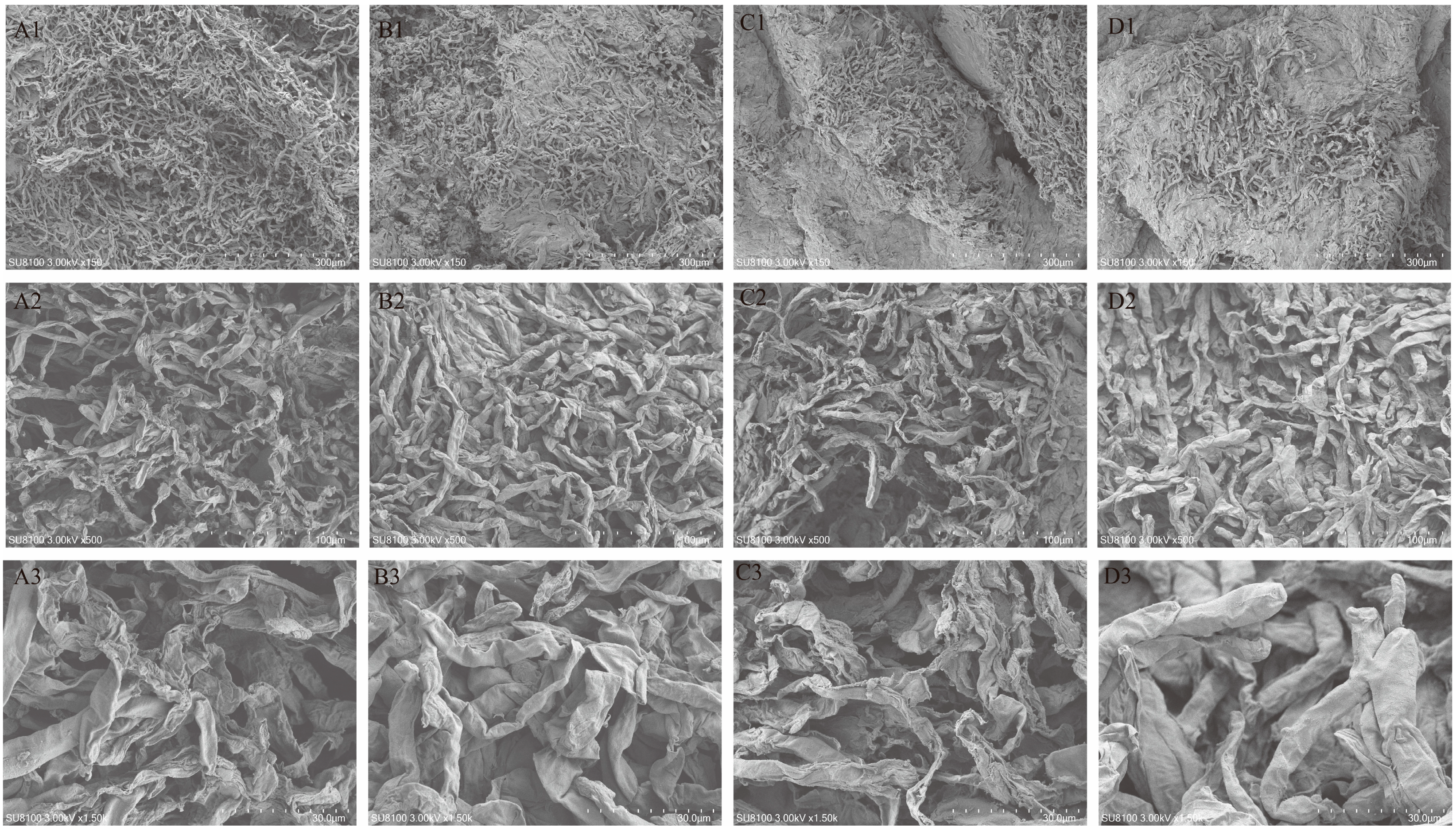
2.2. Morphological Characterization and Microstructural Analysis
2.3. Determination of Antioxidant Activity
2.4. Untargeted Metabolomics Analysis by LC-MS/MS
2.4.1. Sample Preparation and Metabolites Extraction
2.4.2. LC-MS/MS and Mass Spectrum Condition
2.4.3. Data Processing and Statistical Analysis
3. Results
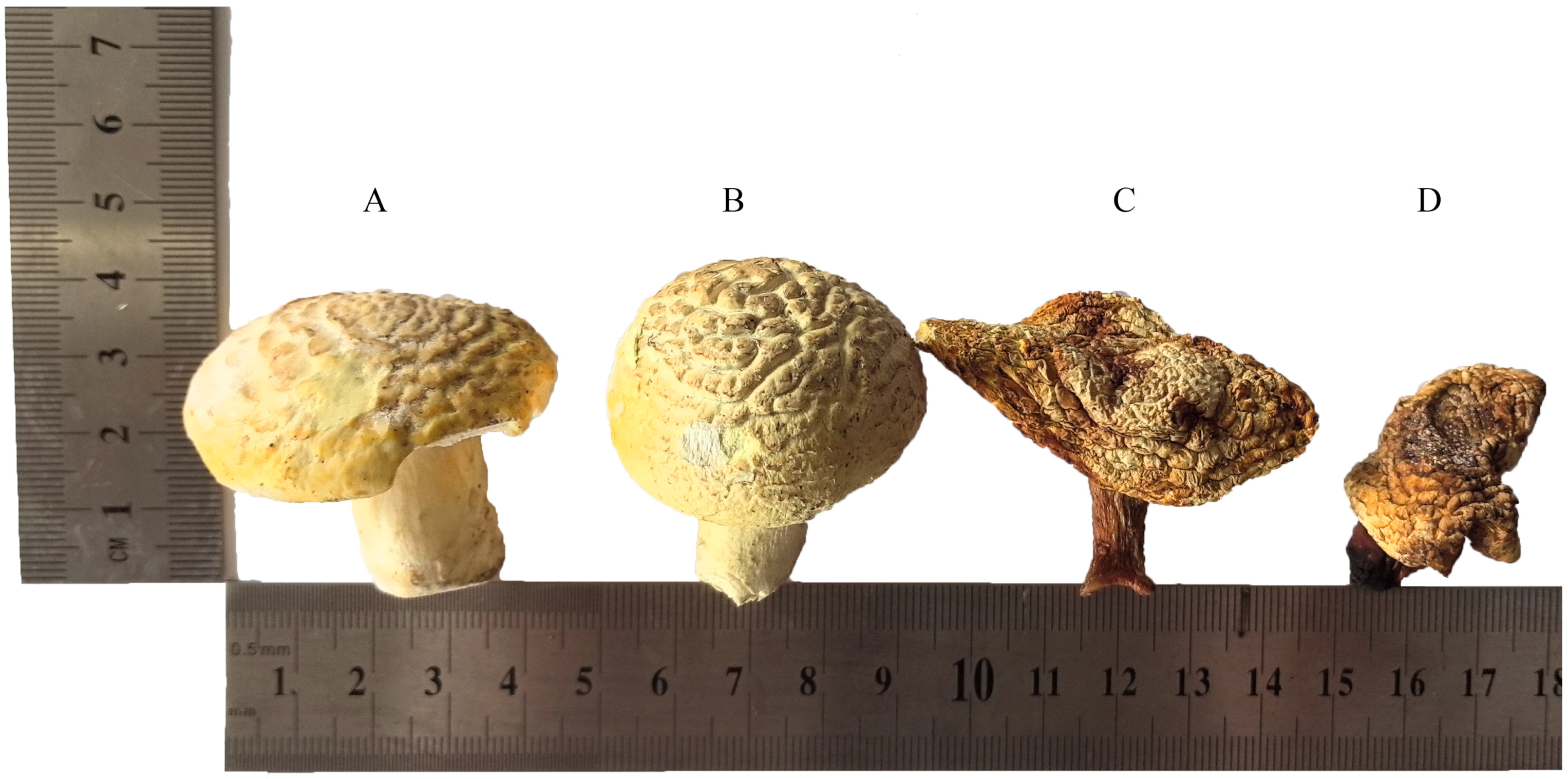
3.1. Effect of Different Drying Methods on the Morphological Characteristics of F. luteovirens
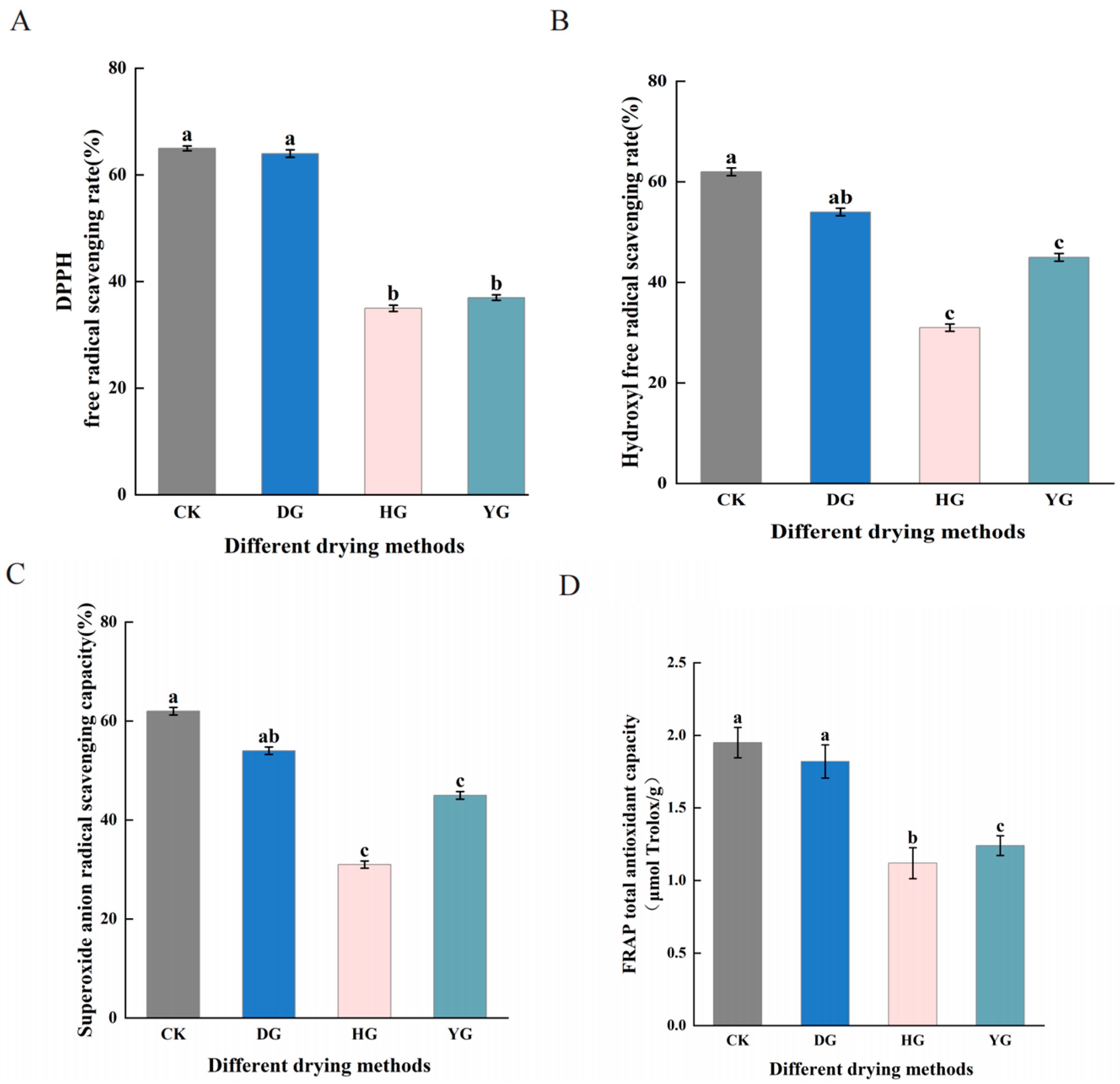
3.2. Antioxidant Activity Assay
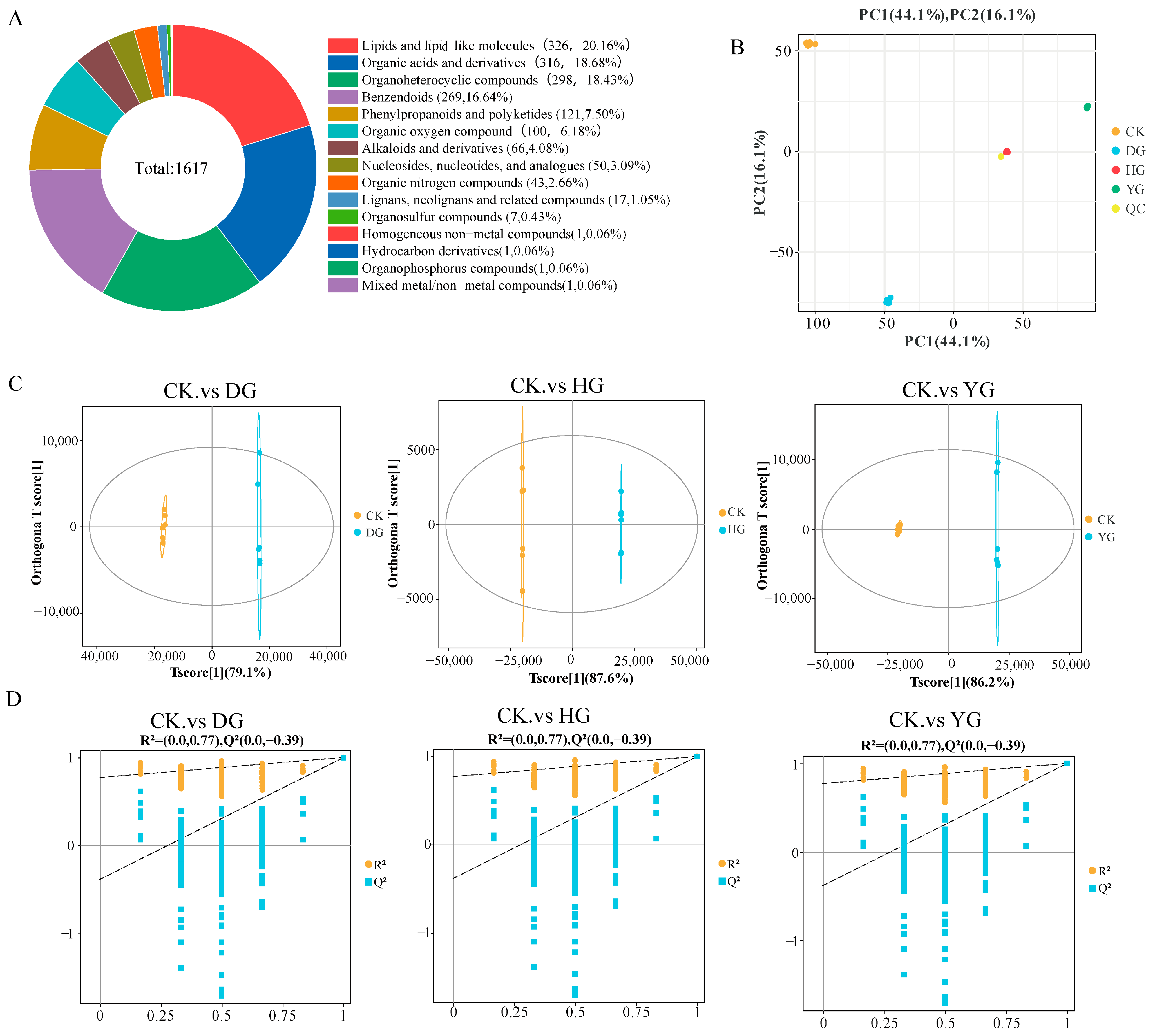
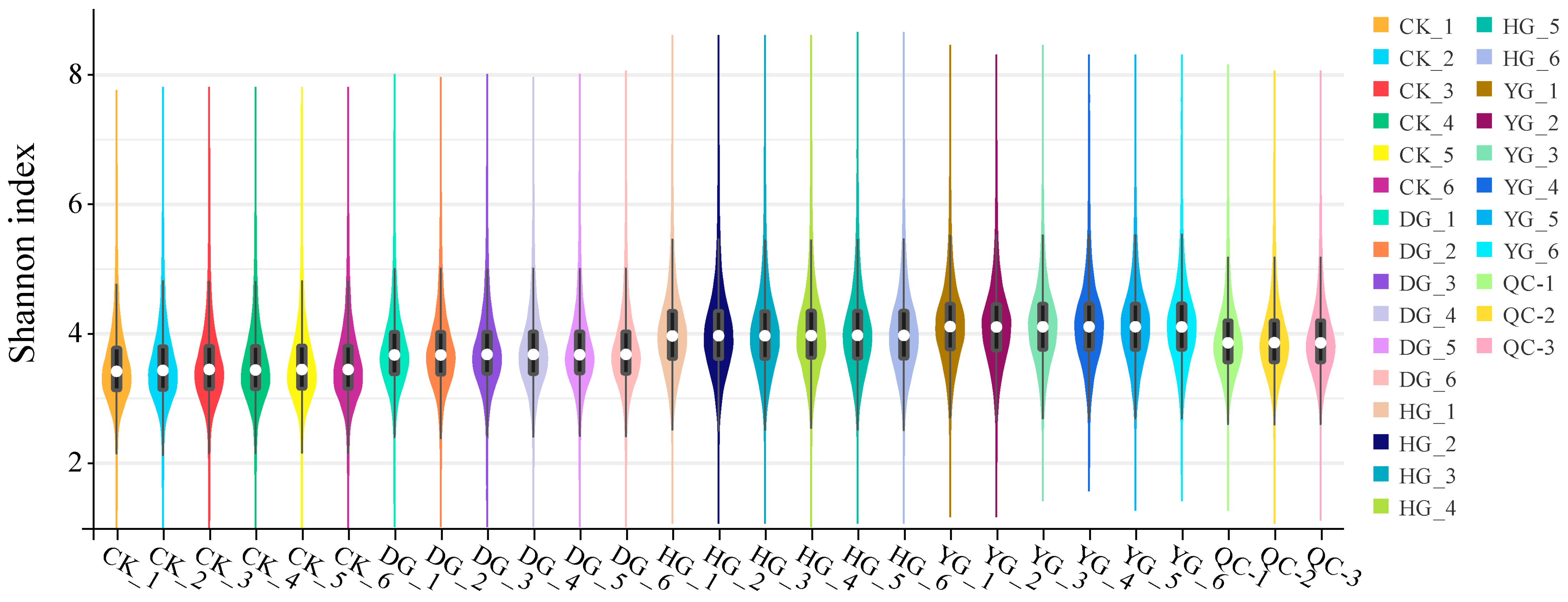
3.3. Metabolite Profiles Analysis of F. luteovirens
3.4. Correlation Between Antioxidant Capacity and Metabolites
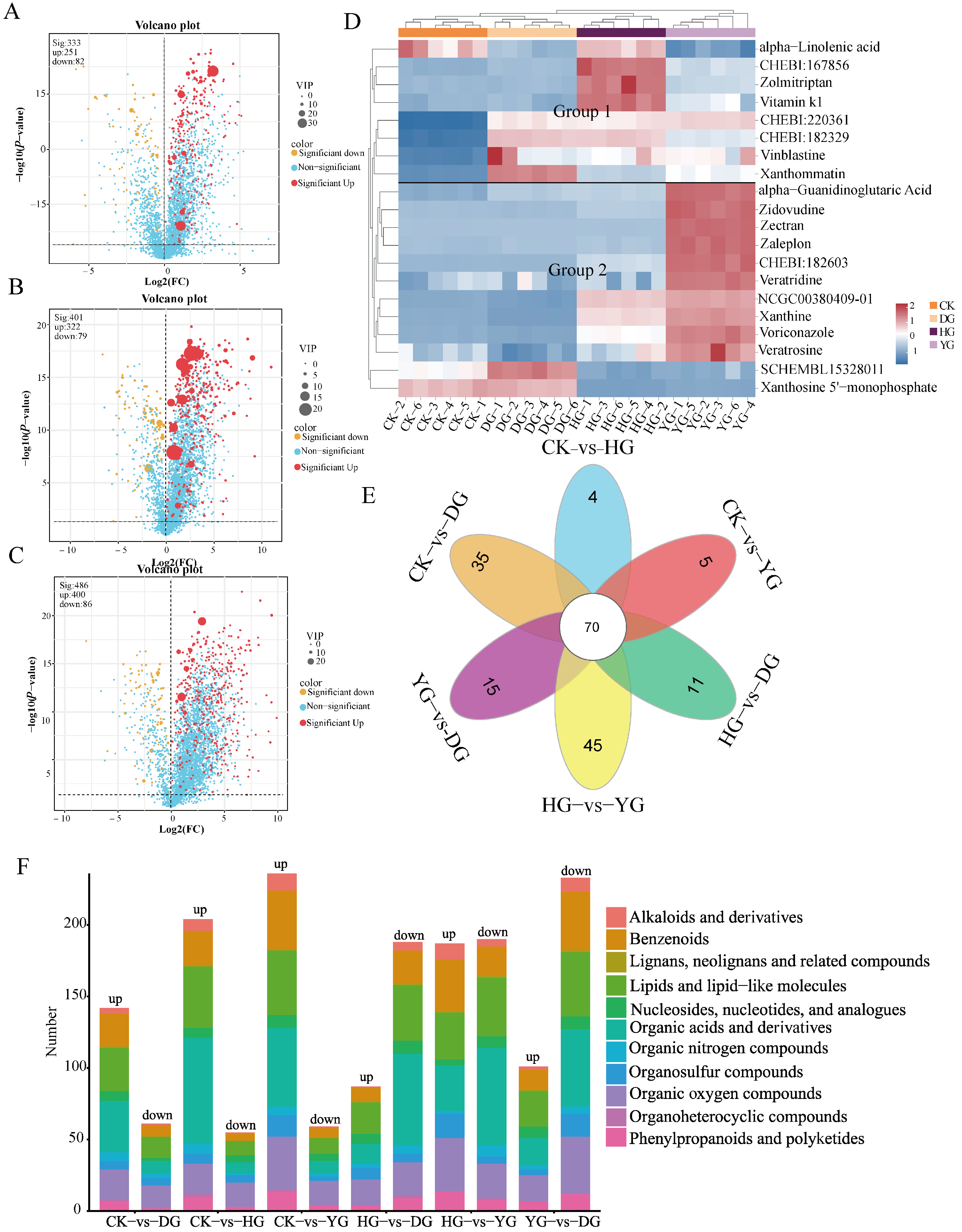
3.5. Screening of Differentially Accumulated Metabolites (DAMs)
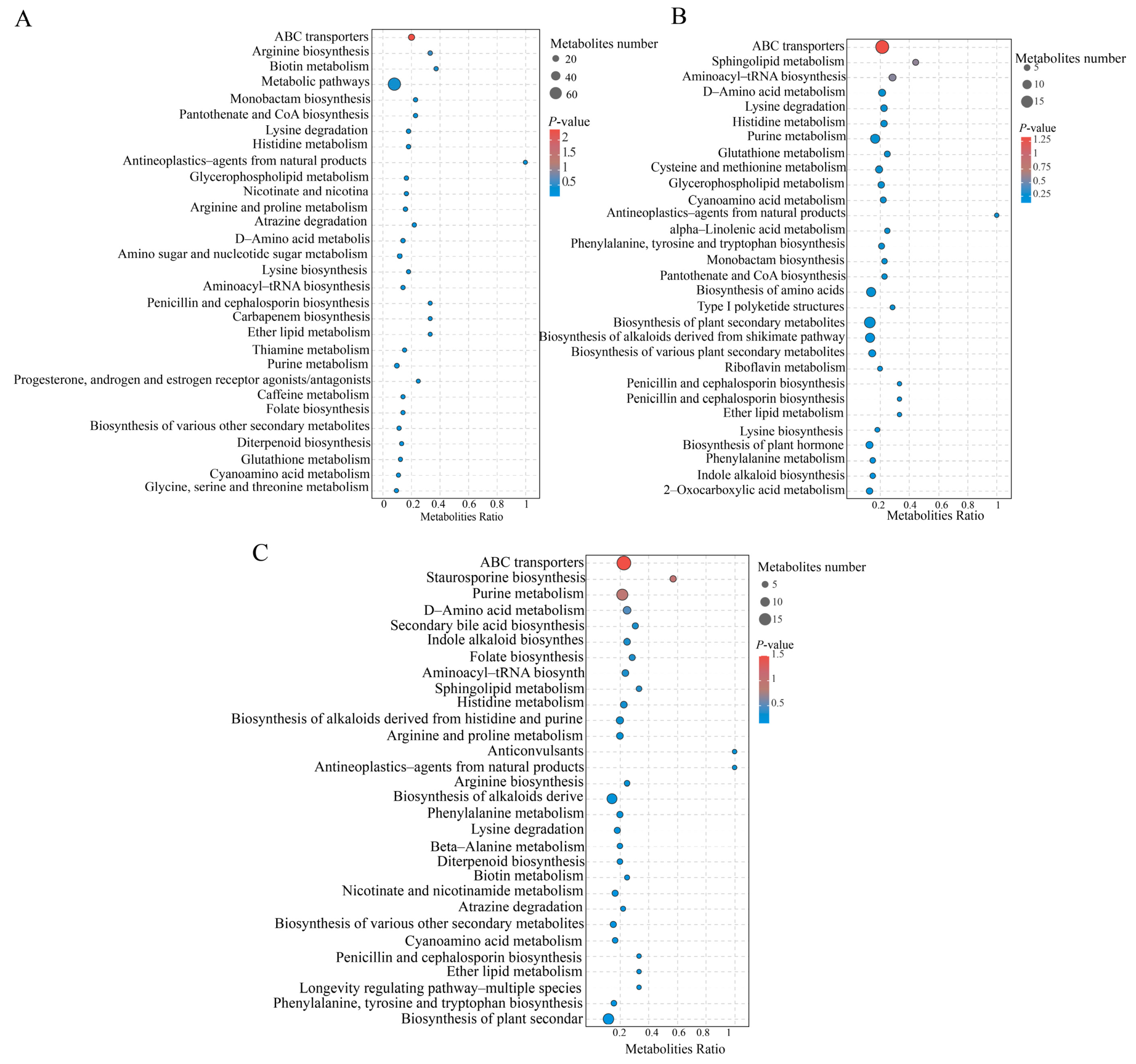
3.6. KEGG Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, X.L.; Wang, Z.X.; Qi, J. The First Whole Genome Sequencing of Agaricus bitorquis and Its Metabolite Profiling. J. Fungi 2023, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lei, J.-Y.; Li, S.-L.; Guo, L.-Q.; Lin, J.-F.; Wu, G.-H.; Lu, J.; Ye, Z.-W. Progress in biological activities and biosynthesis of edible fungi terpenoids. Crit. Rev. Food Sci. Nutr. 2023, 63, 7288–7310. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai-Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation. J. Fungi 2021, 7, 887. [Google Scholar] [CrossRef]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef]
- Fathi, F.N.; Ebrahimi, S.; Matos, L.C.; Oliveira, M.B.; Alves, R.C. Emerging drying techniques for food safety and quality: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1125–1160. [Google Scholar] [CrossRef]
- Leiva-Portilla, D.J.; Rodríguez-Núñez, K.E.; Rodríguez-Ramos, F.J.; Delgadillo Acevedo, Á.; Uribe, E. Impact on Physicochemical Composition and Antioxidant Activity of the Wild Edible Mushroom Cyttaria espinosae Subjected to Drying. Chem. Biodivers. 2020, 17, e2000642. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Xu, J. Comparison of Different Drying Methods for Recovery of Mushroom DNA. Sci. Rep. 2017, 7, 3008. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Hao, J.; Yu, W.; Kou, X. Effects of processing and storage preservation technologies on nutritional quality and biological activities of edible fungi: A review. J. Food Process Eng. 2017, 40, e12437. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Mujumdar, A.S. Comparison of three new drying methods for drying characteristics and quality of shiitake mushroom (Lentinus edodes). Dry. Technol. 2014, 32, 1791–1802. [Google Scholar] [CrossRef]
- Guo, X.; Hao, Q.; Qiao, X.; Li, M.; Qiu, Z.; Zheng, Z.; Zhang, B. An evaluation of different pretreatment methods of hot-air drying of garlic: Drying characteristics, energy consumption and quality properties. Lwt 2023, 180, 114685. [Google Scholar] [CrossRef]
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A.M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; et al. Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem. 2014, 165, 547–554. [Google Scholar] [CrossRef]
- Xue, Z.; Hao, J.; Yu, W.; Kou, X. Effects of vacuum and microwave freeze drying on microstructure and quality of potato slices. J. Food Eng. 2010, 101, 131–139. [Google Scholar]
- Guo, Y.; Chen, X.; Gong, P.; Deng, Z.; Qi, Z.; Wang, R.; Long, H.; Wang, J.; Yao, W.; Yang, W.; et al. Recent advances in quality preservation of postharvest golden needle mushroom (Flammulina velutiper). J. Sci. Food Agric. 2023, 103, 5647–5658. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Sci. Food Agric. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Yang, W.; Du, H.; Mariga, A.M.; Pei, F.; Ma, N.; Hu, Q. Hot air drying process promotes lignification of Lentinus edodes. Lwt 2017, 84, 726–732. [Google Scholar] [CrossRef]
- Bai, J.W.; Wang, Y.C.; Cai, J.R.; Zhang, L.; Dai, Y.; Tian, X.Y.; Xiao, H. W Three-dimensional appearance and physicochemical properties of Pleurotus eryngii under different drying methods. Foods 2023, 12, 1999. [Google Scholar] [CrossRef]
- Yuen, S.K.; Kalianon, K.; Atong, M. Effect of different drying temperatures on the nutritional quality of edible wild mushroom, Volvariella volvacea obtained nearby forest areas. Int. J. Adv. Res. 2014, 2, 859–864. [Google Scholar]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Wang, S.; Li, C.; Chen, C.; Wan, X.; Li, D.; Li, Y. Polysaccharides of Floccularia luteovirens Alleviate Oxidative Damage and Inflammatory Parameters of Diabetic Nephropathy in db/db Mice. Front. Biosci. 2023, 28, 82. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi. 2020, 6, 269. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Feng, X.L.; Wang, Z.X.; Xie, T.C.; Duan, Y.; Liu, C.; Gao, J.M.; Qi, J. Genomic and Metabolomic Analyses of the Medicinal Fungus Inonotus hispidus for Its Metabolite’s Biosynthesis and Medicinal Application. J. Fungi. 2022, 8, 1245. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics reviewed: A new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef]
- Norekian, T.P.; Moroz, L.L. Scanning Electron Microscopy of Ctenophores: Illustrative Atlas. Methods Mol. Biol. 2024, 2757, 163–184. [Google Scholar]
- Kong, B.-H.; Yap, C.-S.A.; Razif, M.F.M.; Ng, S.-T.; Tan, C.-S.; Fung, S.-Y. Antioxidant and Cytotoxic Effects and Identification of Ophiocordyceps sinensis Bioactive Proteins Using Shotgun Proteomic Analysis. Food Technol. Biotechnol. 2021, 59, 201–208. [Google Scholar] [CrossRef]
- Barreto, S.M.A.G.; Cadavid, C.O.M.; Moura, R.A.d.O.; Silva, G.M.M.; Araújo, S.V.F.d.; Silva Filho, J.A.A.d.; Rocha, H.A.O.; Oliveira, R.d.P.; Giordani, R.B.; Ferrari, M. In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue. Biomolecules 2020, 10, 1435. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, X.; Huang, J.; Zhao, D.; Tan, Y.; Zhang, Z.; Zhang, Z.; Zhu, L.; Wu, B.; et al. Anti-Alzheimers molecular mechanism of icariin: Insights from gut microbiota, metabolomics, and network pharmacology. J. Transl. Med. 2023, 21, 277. [Google Scholar] [CrossRef]
- Alsafra, Z.; Kuuliala, L.; Scholl, G.; Saegerman, C.; Eppe, G.; De Meulenaer, B. Characterizing the formation of process contaminants during coffee roasting by multivariate statistical analysis. Food Chem. 2023, 427, 136655. [Google Scholar] [CrossRef]
- Bello, M.A.; Ruiz-León, Y.; Sandoval-Sierra, J.V.; Rezinciuc, S.; Diéguez-Uribeondo, J. Scanning Electron Microscopy (SEM) Protocols for Problematic Plant, Oomycete, and Fungal Samples. J. Vis. Exp. 2017, 120, 55031. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Murphy, S.E.; Sweedler, J.V. Metabolomics-based mass spectrometry methods to analyze the chemical content of 3D organoid models. Analyst 2022, 147, 2918–2929. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Shi, S.; Yu, Q.; Liu, M.; Zhang, Z. Identification of cerebrospinal fluid metabolites as biomarkers for neurobrucellosis by liquid chromatography-mass spectrometry approach. Bioengineered 2022, 13, 6996–7010. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Li, S.; Wu, D.; Zhong, Y.; Li, W.; Xu, H.; Huang, L. Differentiation of Mountain- and Garden-Cultivated Ginseng with Different Growth Years Using HS-SPME-GC-MS Coupled with Chemometrics. Molecules 2023, 28, 2016. [Google Scholar] [CrossRef]
- Ali, A.M.; Monaghan, C.; Muggeridge, D.J.; Easton, C.; Watson, D.G. LC/MS-based discrimination between plasma and urine metabolomic changes following exposure to ultraviolet radiation by using data modelling. Metabolomics 2023, 19, 13. [Google Scholar] [CrossRef]
- Cocco, E.; Murgia, F.; Lorefice, L.; Barberini, L.; Poddighe, S.; Frau, J.; Marrosu, M. G 1H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neuroimmunol. Neuroinflammation 2016, 3, e185. [Google Scholar]
- Ni, Y.; Liao, Q.; Gou, S.; Shi, T.; Li, W.; Feng, R.; Zhao, X. Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens. J. Fungi 2024, 10, 618. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, N.; Li, W.; Zhang, B.; Shi, T.; Xie, M.; Yu, M. Effect of Ultrasonic Induction on the Main Physiological and Biochemical Indicators and γ-Aminobutyric Acid Content of Maize during Germination. Foods 2022, 11, 1358. [Google Scholar] [CrossRef]
- Xi, J.; Chen, X.; Du, J.; Zhong, L.; Hu, Q.; Zhao, L. Biosynthesis, behavior and fate of volatile organic sulfide in Lentinus edodes (Berk.) upon hot-air drying treatment. Food Chem. 2023, 412, 135528. [Google Scholar] [CrossRef]
- Chen, D.; Sheng, M.; Wang, S.; Chen, X.; Leng, A.; Lin, S. Dynamic changes and formation of key contributing odorants with amino acids and reducing sugars as precursors in shiitake mushrooms during hot air drying. Food Chem. 2023, 424, 136409. [Google Scholar] [CrossRef]
- Huang, Y.T.; Wu, W.; Zou, W.X.; Wu, H.P.; Cao, D.D. Drying temperature affects rice seed vigor via gibberellin, abscisic acid, and antioxidant enzyme metabolism. J. Zhejiang Univ. Sci. B 2020, 21, 796–810. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, Y.S.; Wang, Y.B.; Xiao, H.W.; Liu, W.; Ma, Y.; Zhao, X.Y. Changes in the Physical Properties and Volatile Odor Characteristics of Shiitake Mushrooms (Lentinula edodes) in Far Infrared Radiation Drying. Foods 2023, 12, 3213. [Google Scholar] [CrossRef]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef]
- He, X.; Lin, R.; Cheng, S.; Wang, S.; Yuan, L.; Wang, H.; Wang, H.; Tan, M. Effects of microwave vacuum drying on the moisture migration, microstructure, and rehydration of sea cucumber. J. Food Sci. 2021, 86, 2499–2512. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.J.; Shi, Y.; Sun, Y.; Mariga, A.M.; Zhao, L.Y.; Hu, Q.H. Comparison of freeze-drying with three different combinations of drying methods and their influence on colour, texture, microstructure and nutrient retention of button mushroom (Agaricus bisporus) slices. Food Bioprocess Technol. 2014, 7, 702–710. [Google Scholar] [CrossRef]
- Zheng, N.; Ma, Y.M.; Lei, H.Y.; Zhen, X.Y.; Wang, Y.; Zhang, Y.; Liu, T. Progress of post-harvest preservation technology of edible mushroom. Food Med. Homol. 2025, 2, 9420028. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, X.; Cai, M.; Gu, H.; E, H.; Li, X.; Zhang, Y.; Lu, H.; Zhou, C. Metabolomics Analysis of Morchella sp. From Different Geographical Origins of China Using UPLC-Q-TOF-MS. Front. Nutr. 2022, 9, 865531. [Google Scholar]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Wang, L.; Wen, H.; Yang, N.; Li, H. Effect of vacuum freeze drying and hot air drying on dried mulberry fruit quality. PLoS ONE 2023, 18, e0283303. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Ding, C.; Lu, J.; Bai, W.; Liu, J.; Lian, J.; Jia, Y. Influence of electrohydrodynamics on the drying characteristics, microstructure and volatile composition of apricot abalone mushroom (Pleurotus eryngii). Curr. Res. Food Sci. 2024, 9, 100856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; Liu, Y.; Zhang, H.L.; Wan, Q.Z.; Wang, Y.Q. Effect of different drying and grinding techniques on the physicochemical properties and biological activities of fungal polysaccharides. Food Med. Homol. 2025, 2, 9420045. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Wang, T.; Tang, C.; He, M.; Pu, X.; Zhao, T.; Li, Y. Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis. J. Fungi 2025, 11, 78. https://doi.org/10.3390/jof11010078
Xiao M, Wang T, Tang C, He M, Pu X, Zhao T, Li Y. Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis. Journal of Fungi. 2025; 11(1):78. https://doi.org/10.3390/jof11010078
Chicago/Turabian StyleXiao, Mengjun, Tao Wang, Chuyu Tang, Min He, Xiaojian Pu, Tingjing Zhao, and Yuling Li. 2025. "Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis" Journal of Fungi 11, no. 1: 78. https://doi.org/10.3390/jof11010078
APA StyleXiao, M., Wang, T., Tang, C., He, M., Pu, X., Zhao, T., & Li, Y. (2025). Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis. Journal of Fungi, 11(1), 78. https://doi.org/10.3390/jof11010078





