Dermoscopy and Ultraviolet-Enhanced Fluorescence Dermoscopy (UEFD) Increase the Accuracy of Diagnosis and Are Useful in Assessing the Effectiveness of Kerion celsi Treatment
Abstract
1. Introduction
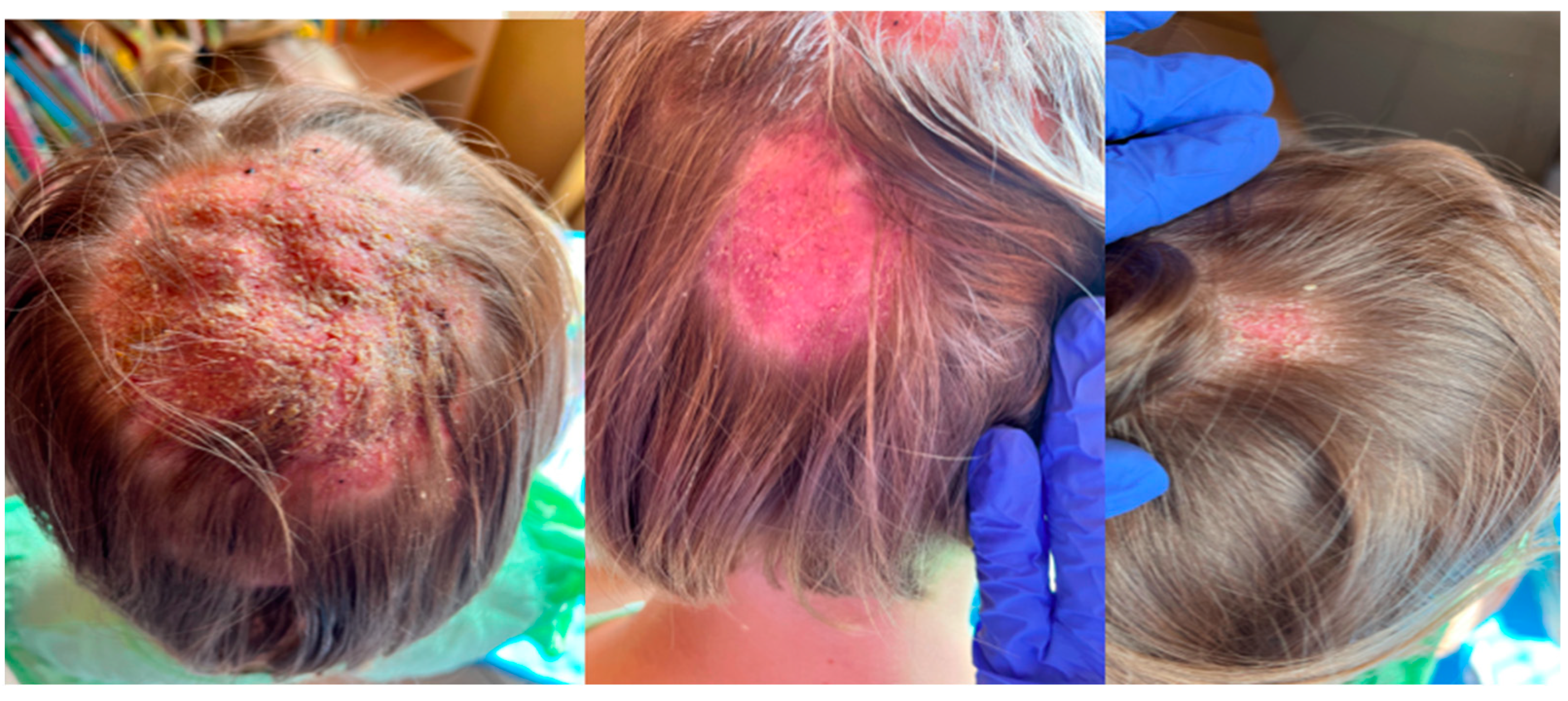
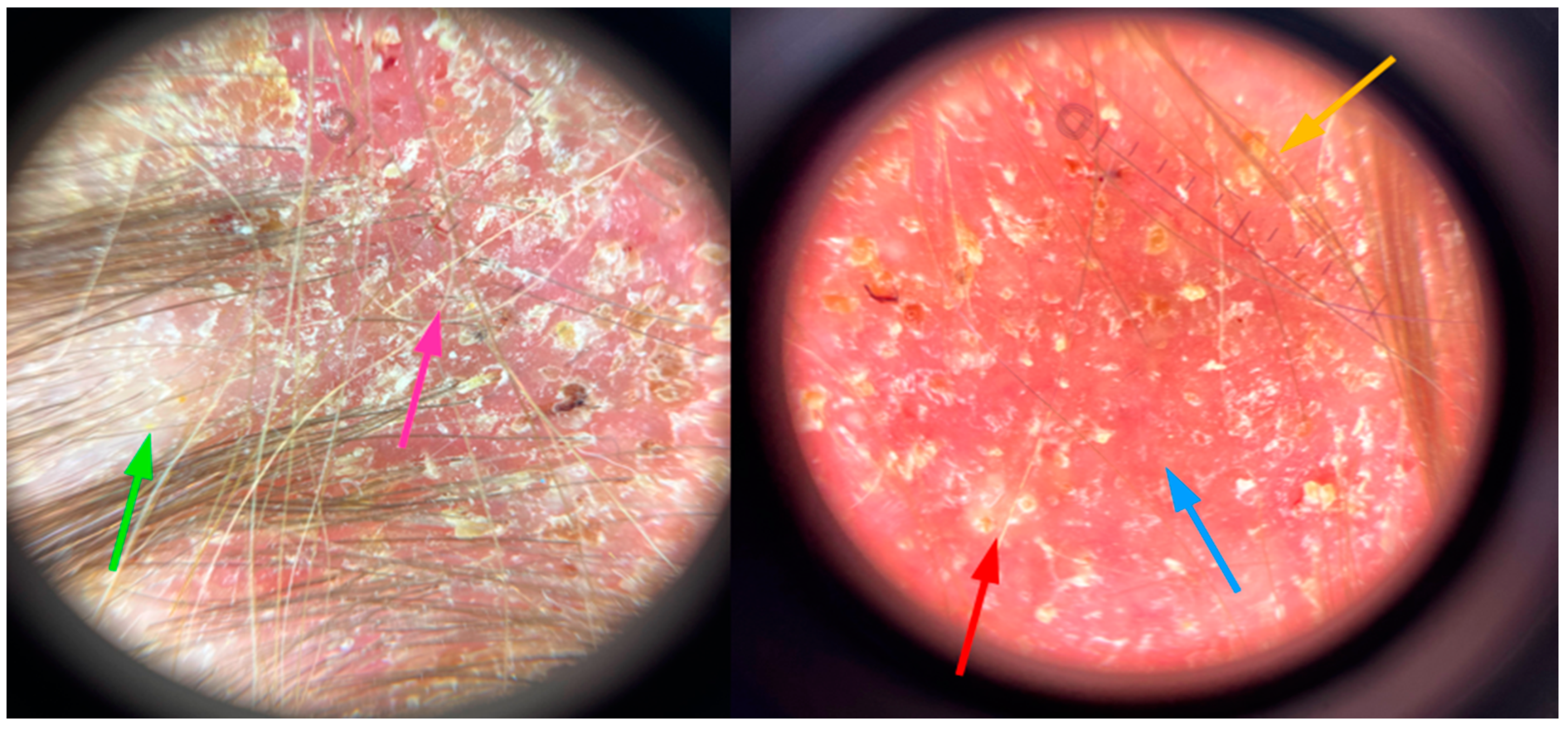
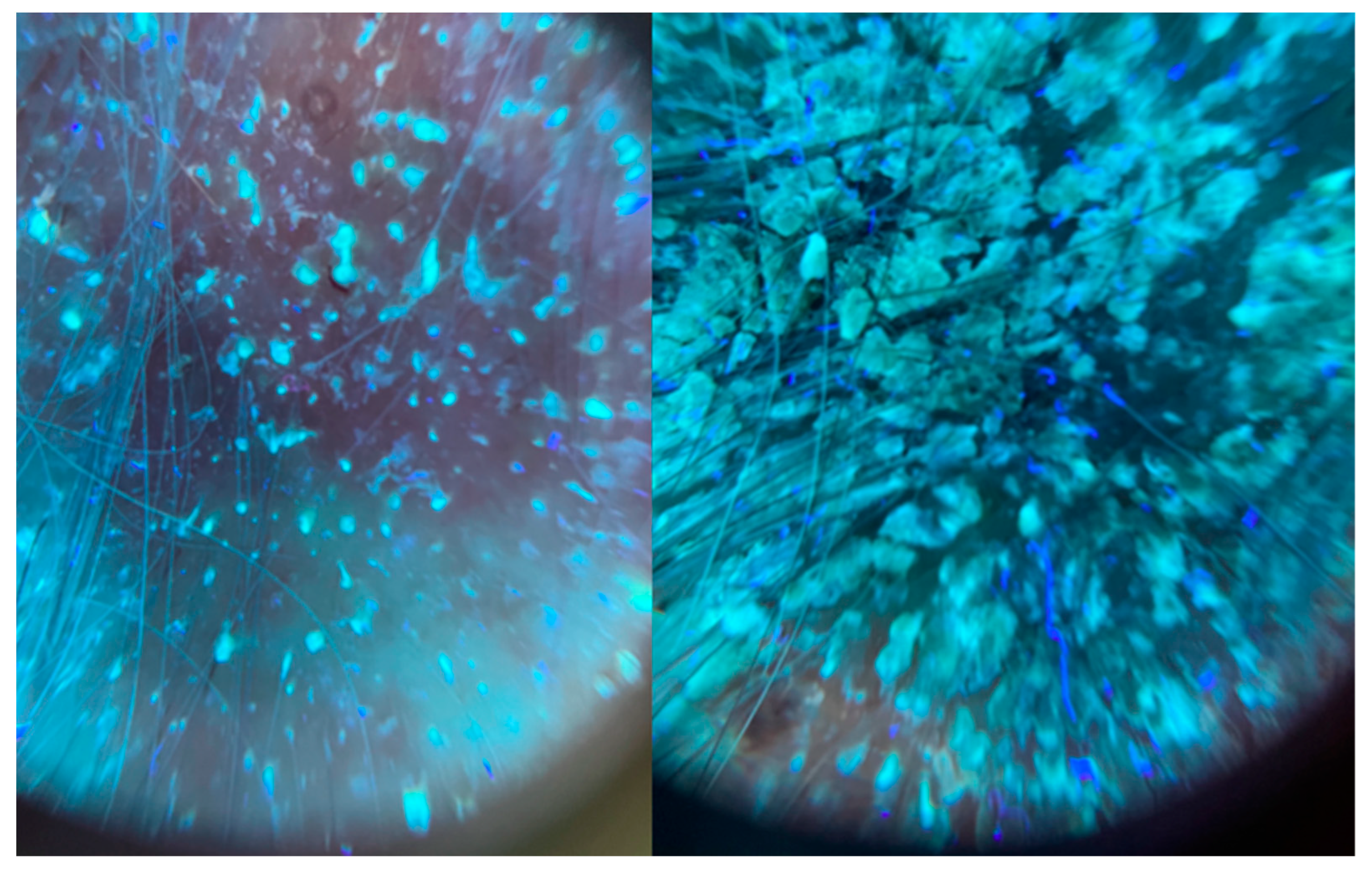
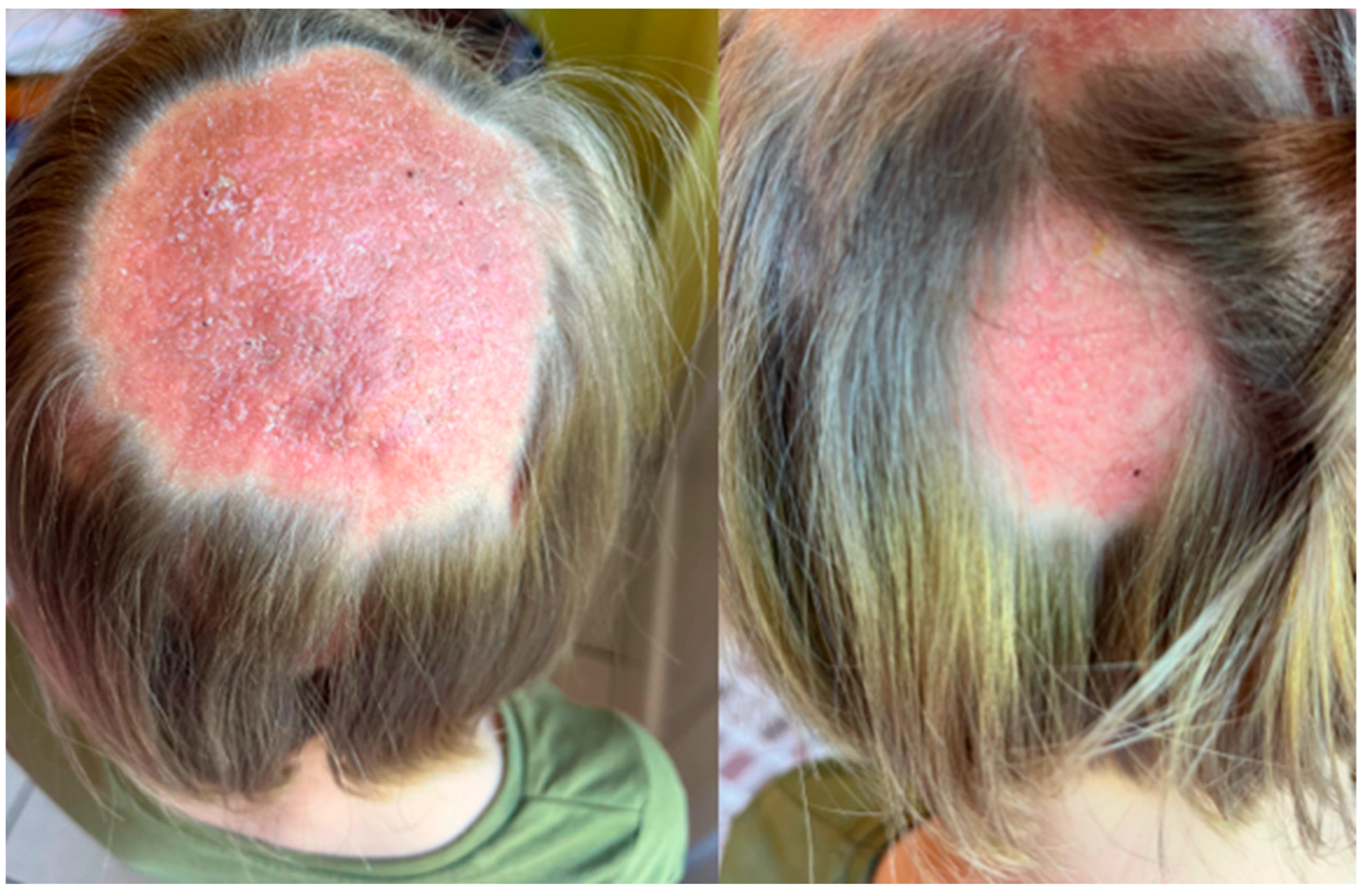
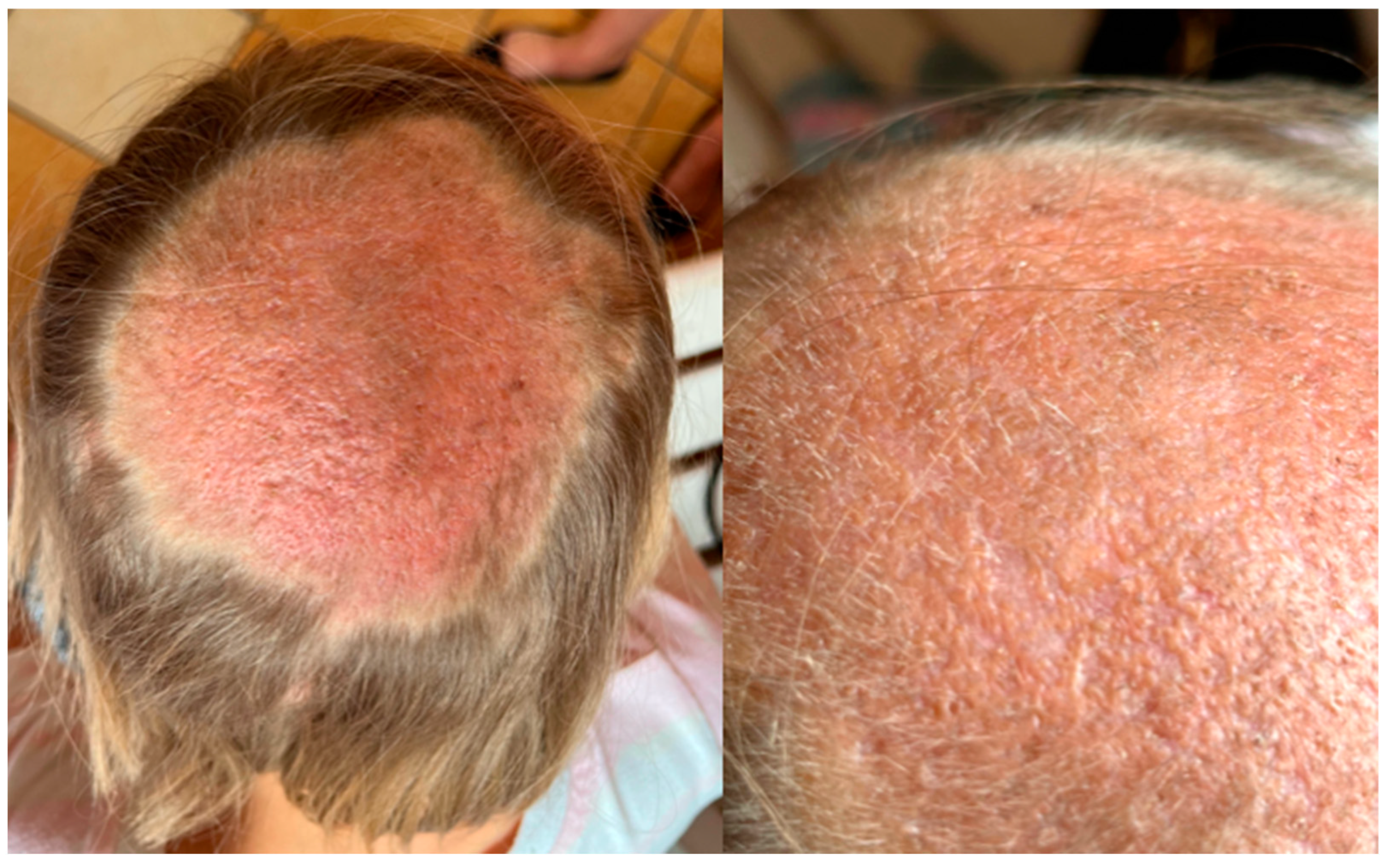
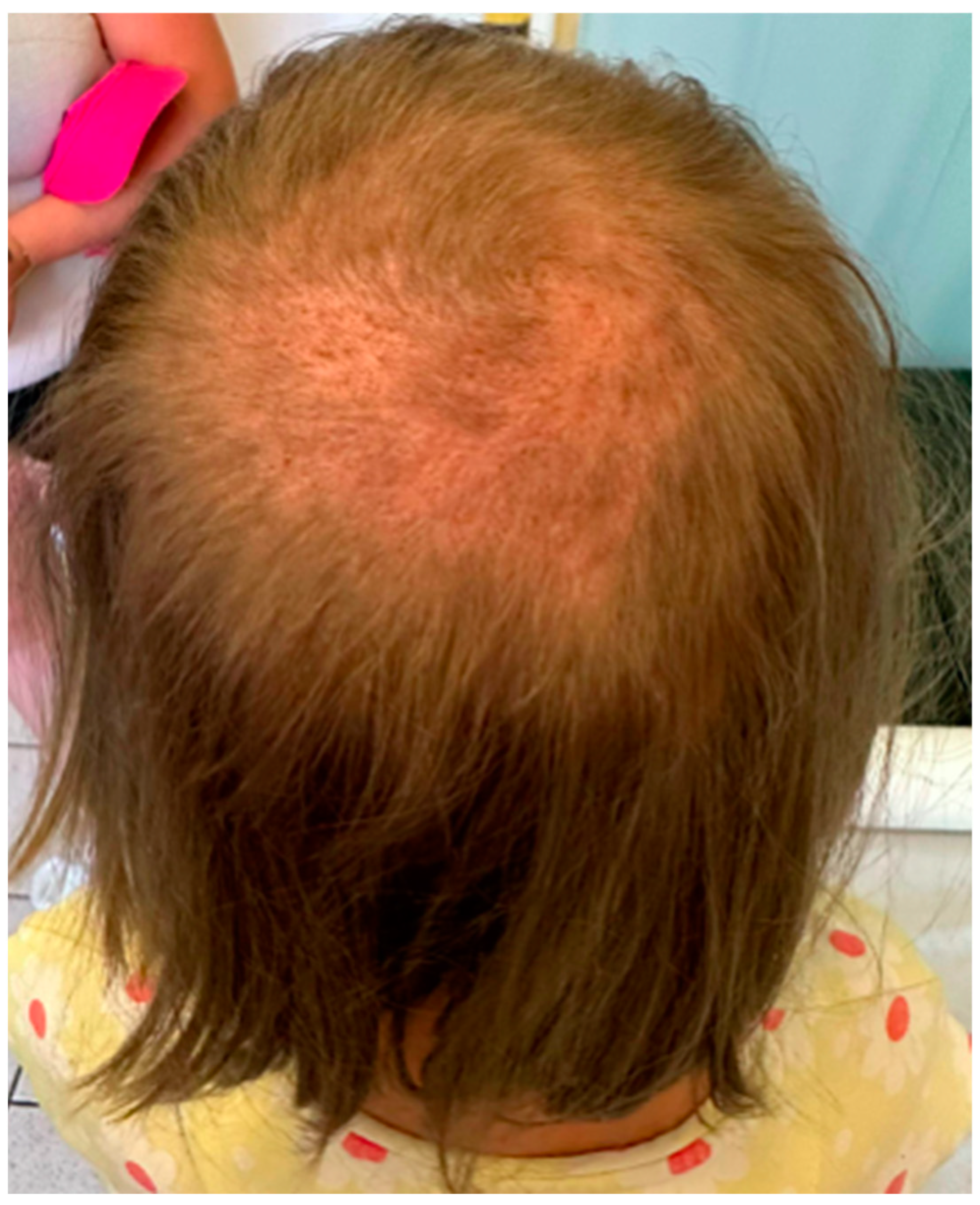
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Mays, R.; Versteeg, S.; Piraccini, B.; Shear, N.; Piguet, V.; Tosti, A.; Friedlander, S. Tinea capitis in children: A systematic review of management. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Michaels, B.D.; Del Rosso, J.Q. Tinea capitis in infants: Recognition, evaluation, and management suggestions. J. Clin. Aesthet. Dermatol. 2012, 5, 49–59. [Google Scholar]
- Adesiji, Y.O.; Omolade, B.F.; Aderibigbe, I.A.; Ogungbe, O.V.; Adefioye, O.A.; Adedokun, S.A.; Adekanle, M.A.; Ojedele, R.O. Prevalence of Tinea Capitis among Children in Osogbo, Nigeria, and the Associated Risk Factors. Diseases 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Zhu, J.; Dai, Y.; Shen, H. Successful treatment of severe kerion Celsi in an immunocompromised girl with evacuation of pus, terbinafine and short course glucocorticosteroids. J. Mycol. Med. 2016, 26, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Deh, A.; Diongue, K.; Diadie, S.; Diatta, B.A.; Diop, K.; Ndour, N.; Ndiaye, M.; Diallo, M.; Niang, S.O. Kerion celsi due to Microsporum audouinii: A severe form in an immunocompetent girl. Ther. Adv. Infect. Dis. 2021, 8, 20499361211020879. [Google Scholar] [CrossRef]
- Mayser, P.; Nenoff, P.; Reinel, D.; Abeck, D.; Brasch, J.; Daeschlein, G.; Effendy, I.; Ginter-Hanselmayer, G.; Gräser, Y.; Hipler, U.C.; et al. S1 guidelines: Tinea capitis. J. Dtsch. Dermatol. Ges. 2020, 18, 161–179. [Google Scholar] [CrossRef]
- Pietkiewicz, P.; Navarrete-Dechent, C.; Togawa, Y.; Szlązak, P.; Salwowska, N.; Marghoob, A.A.; Leszczyńska-Pietkiewicz, A.; Errichetti, E. Applications of Ultraviolet and Sub-ultraviolet Dermatoscopy in Neoplastic and Non-neoplastic Dermatoses: A Systematic Review. Dermatol. Ther. 2024, 14, 361–390. [Google Scholar] [CrossRef]
- Errichetti, E.; Pietkiewicz, P.; Bhat, Y.J.; Salwowska, N.; Szlązak, P.; Stinco, G. Diagnostic accuracy of ultraviolet-induced fluorescence dermoscopy in non-neoplastic dermatoses (general dermatology): A multicentric retrospective comparative study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 97–108. [Google Scholar] [CrossRef]
- Aguirre Sotelo, J.P.; Tarango Martinez, V.M.; Vera Cabrera, L. Kerion celsi caused by Trichophyton tonsurans in an adult. An. Bras. Dermatol. 2022, 97, 637–640. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Tinea Incognito-A Great Physician Pitfall. J. Fungi 2022, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, A.; Mueller, R.; Werckenthin, C.; Straubinger, R.; Hein, J. Dermatophytes in pet Guinea pigs and rabbits. Vet. Microbiol. 2012, 157, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Makimura, K.; Ishihara, K.; Goto, H.; Tajiri, Y.; Okuma, M.; Fujisaki, R.; Uchida, K.; Abe, S.; Iijima, M. Comparative study of direct polymerase chain reaction, microscopic examination and culture-based morphological methods for detection and identification of dermatophytes in nail and skin samples. J. Dermatol. 2009, 36, 202–208. [Google Scholar] [CrossRef]
- Ndiaye, M.; Sacheli, R.; Diongue, K.; Adjetey, C.; Darfouf, R.; Seck, M.C.; Badiane, A.S.; Diallo, M.A.; Dieng, T.; Hayette, M.-P.; et al. Evaluation of the Multiplex Real-Time PCR DermaGenius((R)) Assay for the Detection of Dermatophytes in Hair Samples from Senegal. J. Fungi 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Wittig, F.; Koch, D.; Krüger, C.; Harder, M.; Gaajetaan, G.; Dingemans, G.; Nenoff, P. Do the new molecular assays-microarray and realtime polymerase chain reaction-for dermatophyte detection keep what they promise? Hautarzt 2019, 70, 618–626. [Google Scholar] [CrossRef]
- Elmas, Ö.F.; Durdu, M. Histopathology in the Diagnosis of Tinea Capitis: When to Do, How to Interpret? Mycopathologia 2023, 188, 545–552. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Rakowska, A.; Sikora, M.; Ciechanowicz, P.; Olszewska, M.; Rudnicka, L. Trichoscopy of Tinea Capitis: A Systematic Review. Dermatol. Ther. 2020, 10, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moyano, E.; Fernández-Sánchez, A.M.; Crespo-Erchiga, V.; Martínez-Pilar, L. Kerion Celsi caused by Trichophyton tonsurans with dermatophytid reaction. Rev. Iberoam. Micol. 2021, 38, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.-R.; Luo, Y.-W.; Zhou, X.; Li, Q.-Q.; Liu, Y.-M. Adult Kerion Celsi Caused by Trichophyton tonsurans Secondary to Black Dot Tinea Capitis. Mycopathologia 2023, 188, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Pietkiewicz, P.; Salwowska, N.; Szlązak, P.; Żychowska, M.; Bhat, Y.J. Dermoscopy in Tinea Capitis/Barbae and Tinea of Glabrous Skin: A Comparative Analysis Between Polarized and Ultraviolet-Induced Fluorescence Examination to Differentiate Microsporum From Trichophyton Infections. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12999. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.; Birsan, C.; Mares, M.; Wollina, U. Kerion Celsi due to Microsporum canis infection. Hautarzt 2021, 72, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Aste, N.; Pinna, A.L.; Pau, M.; Biggio, P. Kerion Celsi in a newborn due to Microsporum canis. Mycoses 2004, 47, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Bonven, T.F.; Iversen, E.; Kragballe, K. Permanent hair loss after Kerion Celsi. Ugeskr. Laeger 1991, 153, 3151–3152. [Google Scholar]
- Foged, E.K.; Voss Jepsen, L. Hair loss following kerion Celsi—A follow-up examination. Mykosen 1984, 27, 411–414. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putek, J.; Nowicka, D.; Jankowska-Konsur, A. Dermoscopy and Ultraviolet-Enhanced Fluorescence Dermoscopy (UEFD) Increase the Accuracy of Diagnosis and Are Useful in Assessing the Effectiveness of Kerion celsi Treatment. J. Fungi 2025, 11, 52. https://doi.org/10.3390/jof11010052
Putek J, Nowicka D, Jankowska-Konsur A. Dermoscopy and Ultraviolet-Enhanced Fluorescence Dermoscopy (UEFD) Increase the Accuracy of Diagnosis and Are Useful in Assessing the Effectiveness of Kerion celsi Treatment. Journal of Fungi. 2025; 11(1):52. https://doi.org/10.3390/jof11010052
Chicago/Turabian StylePutek, Justyna, Danuta Nowicka, and Alina Jankowska-Konsur. 2025. "Dermoscopy and Ultraviolet-Enhanced Fluorescence Dermoscopy (UEFD) Increase the Accuracy of Diagnosis and Are Useful in Assessing the Effectiveness of Kerion celsi Treatment" Journal of Fungi 11, no. 1: 52. https://doi.org/10.3390/jof11010052
APA StylePutek, J., Nowicka, D., & Jankowska-Konsur, A. (2025). Dermoscopy and Ultraviolet-Enhanced Fluorescence Dermoscopy (UEFD) Increase the Accuracy of Diagnosis and Are Useful in Assessing the Effectiveness of Kerion celsi Treatment. Journal of Fungi, 11(1), 52. https://doi.org/10.3390/jof11010052





