Abstract
Apples and strawberries hold significant commercial and nutritional value but face pre- and post-harvest spoilage due to infections by Botrytis cinerea. While spoilage is conventionally managed using synthetic chemicals, there is a growing interest in utilising yeasts as biological control agents. This study aimed to assess the antifungal potential of non-Saccharomyces yeasts Suhomyces pyralidae, Meyerozyma guilliermondii, Pichia kluyveri, Zygoascus hellenicus, and Aureobasidium melanogenum against three B. cinerea strains (B05.10, IWBT-FF1, and PPRI 30807) on agar plates and in post-harvest trials on apples and strawberries. Aureobasidium melanogenum exhibited a broad range of extracellular enzyme production and inhibition rates of 55%, 52%, and 40% against the strains. In volatile organic compound (VOC) assays, P. kluyveri and S. pyralidae achieved 79% and 56% inhibition, respectively, with VOCs like isobutanol, isoamyl alcohol, 2-phenylethanol, isoamyl acetate, and 2-phenethyl acetate identified. In post-harvest trials, S. pyralidae was most effective on apples, with inhibition rates up to of 64%. The commercial fungicide Captan and S. pyralidae and P. kluyveri achieved 100% inhibition against the B. cinerea strains B05.10 and IWBT-FF1 on strawberries. These findings highlight the potential of the selected yeast species as biological control agents against B. cinerea, warranting further research into their application in commercial fruit protection.
1. Introduction
Apples (Malus domestica (Suckow) Borkh.) and strawberries (Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier) are valuable for human health, serving as primary sources of essential nutrients, including vitamins and minerals, that support a healthy lifestyle [1,2,3,4]. Their appealing sensory and nutritional characteristics make them widely consumed and processed into various products, such as cooked slices, juices, and jellies, contributing significantly to the global fresh produce market [3,5,6]. Despite their importance as major fruit crops worldwide, producers continue to encounter numerous challenges in production, storage, and market distribution [7,8].
Commercially grown fruits are destined for both local and export markets after harvest. However, pre- and post-harvest mould decay leads to significant economic losses [3,8]. Mould infections during these stages are often attributed to elevated moisture levels, excessive nutrients, low pH, and reduced fruit decay resistance as maturity progresses [9]. Among the most severe diseases affecting strawberries and apples is grey mould, caused by Botrytis cinerea, which significantly impacts yield and quality by depleting nutrients, shortening shelf life, and causing substantial financial losses [3,4,10,11]. Grey mould infection typically initiates during flowering, remaining latent until fruit maturation, at which point the pathogen proliferates extensively [12,13].
The control of B. cinerea Pers. (1794) presents a significant challenge due to the pathogen’s high genetic plasticity, with chemical control using synthetic fungicides being the most widely used strategy [6,14,15]. While synthetic fungicides effectively reduce the pre- and post-harvest fruit losses, their use has led to increased fungicide residues on produce, the emergence of fungicide-resistant mould strains, and has raised concerns regarding human health and the environmental impact [3,4,5]. Consequently, it is crucial to develop safe and effective alternative strategies for managing grey mould diseases in fruit crops [3,16].
Antagonistic fungi have proven effective against B. cinerea, which is susceptible to suppression by microorganisms such as non-Saccharomyces yeasts that produce antifungal compounds [3,6,17]. Compared to chemical control, the use of antagonist microorganisms offers several benefits, including the absence of toxic residues, environmental safety, ease of application, and cost-effectiveness [15,18]. Yeasts exhibit valuable antifungal properties, including the secretion of killer toxins such as mycocins, the production of cell wall-degrading enzymes (chitinase, β-1,3-glucanase, protease, laminarinases, and peroxidases), the synthesis of volatile organic compounds (VOCs), rapid colony formation, growth within surface wounds, competition for nutrients and space, and the induction of host resistance [3,13,14]. Killer yeasts, including the Meyerozyma guilliermondii, Suhomyces pyralidae (formerly, Candida pyralidae), Pichia kluyveri, and Hanseniaspora species, have shown antimicrobial activity against a range of fruit-spoilage fungi [17,19,20,21]. Previous research by Gomomo et al. [17] evaluated non-Saccharomyces yeasts for their ability to inhibit mycelial growth of a strain of B. cinerea in vitro and on apples, with results indicating species- and strain-dependent inhibitory effects. Building on this foundation, the present study sought to screen non-Saccharomyces yeasts for extracellular enzyme activity and to assess the inhibition of the mycelial growth and spore germination of selected yeasts against three distinct B. cinerea strains in vitro and in vivo on apples and strawberries. The yeasts that showed the most potential as biocontrol agents according to Gomomo et al. [17] were used in this study to confirm their biocontrol activity against B. cinerea.
2. Materials and Methods
2.1. Culturing Conditions and Inoculum Preparation
Twenty-three yeast isolates (Table 1) were sourced from the biobank of ARC Infruitec-Nietvoorbij (Fruit, Vine and Wine Institute of the Agricultural Research Council, Stellenbosch, South Africa). The selected yeast strains performed the best as potential biocontrol agents according to previous research findings from Gomomo et al. [17] and were selected for enzyme activity screening. The yeasts were initially cultured on yeast malt agar (YMA) media composed of 1% glucose, 0.3% malt extract, 0.3% yeast extract, 0.5% peptone, and 2% bacteriological agar and incubated at 28 °C for 48 h. For inoculum preparation, a loopful of each pure yeast colony was transferred into test tubes containing 5 mL of sterilised yeast malt broth (YMB) (Sigma-Aldrich, Johannesburg, South Africa) and incubated at 28 °C for another 48 h. Yeast cell counts were then performed using a haemocytometer under a microscope at 400× magnification to standardise the yeast inoculum concentration to 1 × 108 cells/mL.

Table 1.
Yeasts screened for the production of lytic enzymes.
Botrytis cinerea strains B05.10 and IWBT-FF1 were sourced from the South African Grape and Wine Research Institute (Stellenbosch University, South Africa), and isolate PPRI 30807 was acquired from the ARC Plant Health and Protection biobank (ARC Plant Health and Protection Institute, Pretoria, South Africa). The mould cultures were grown on potato dextrose agar (PDA, Merck, Johannesburg, South Africa) at 25 °C for 7 to 14 days. To prepare inoculum, a 5 mm mycelial disc was excised from a 5-day-old culture plate for each strain. Spores were harvested by gently scraping the plate surface with a sterile loop and rinsing with sterile distilled water to obtain a 50 mL spore suspension, which was collected in a sterile 250 mL Schott bottle. The spore concentration was adjusted to 1 × 105 spores/mL using a haemocytometer and microscope at 400× magnification.
2.2. Extracellular Lytic Enzyme Activity
The yeast isolates were evaluated for their ability to produce lytic enzymes, including proteases, chitinases, glucanases, cellulase, starch-degrading amylases, pectinase, and lipases. A 10 µL suspension of each yeast culture (±1 × 108 cells/mL) was spotted onto agar plates containing specific substrates for each enzyme assay (Figure 1). The plates were incubated at 28 °C for 4–7 days, after which enzymatic activity was assessed. Each treatment was conducted in triplicate. Enzyme activity was indicated by clear halos surrounding the yeast colonies (Figure 1), and was recorded as either (−) for no activity or (+) for activity.
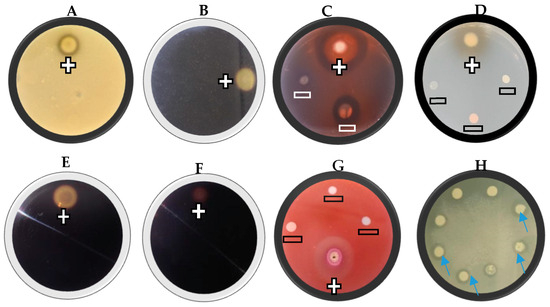
Figure 1.
A representative example of the extracellular lytic enzyme activity of selected yeast isolates, proteases (A), chitinase (B), β-1,3-Glucanase (C), β-glucosidase (D), cellulase (E), starch (F), pectinase (G), and lipase (H). The positive sign (+) represents enzyme activity and the negative sign (−) represents no enzyme activity. For lipase activity, the arrows show clear halos around the colonies. This is a representative example of three replicates.
2.2.1. Protease Activity
Protease activity was assessed using a modified protocol based on Liu et al. [22]. Assays were conducted on skim milk agar plates containing 10% skim milk powder and 2% bacteriological agar. Enzymatic activity was indicated by a clear halo around the inoculated area.
2.2.2. Chitinase Activity
Chitinase activity was determined following an adapted method from Verma and Garg [23]. Chitin agar plates, prepared with 0.1% finely ground chitin derived from shrimp as the sole carbon source and 2% bacteriological agar, were used for the assay. After incubation, Gram’s iodine was applied to the plates for 30 min. Chitinase activity was identified by the appearance of clear halos around the colonies.
2.2.3. Glucanase Activity
β-1,3-Glucanase activity was evaluated using a laminarin medium consisting of 0.5% laminarin, 0.67% yeast nitrogen base, and 2% bacteriological agar) (Sigma-Aldrich, Johannesburg, South Africa) as following the method described by Strauss et al. [24]. After incubation, the plates were stained with 0.06% Congo red for 60 min at room temperature, and excess stain was decanted. The plates were subsequently treated with 1 mol/L NaCl for 15 min. The enzymatic hydrolysis of glucan was indicated by a yellow-orange halo surrounding the colonies.
2.2.4. Glucosidase Activity
β-Glucosidase activity was determined on a selective medium containing 0.67% yeast nitrogen base (YNB, Difco), 0.5% arbutin, and 2% bacteriological agar, as outlined by Strauss et al. [24]. The pH of the medium was adjusted to 5 before autoclaving. Additionally, 10 mL of a 1% ammonium ferric citrate solution (filter-sterilised) was added to the medium before plating. Colonies exhibiting β-glucosidase activity were distinguished by a brown discolouration of the medium.
2.2.5. Cellulase Activity
Cellulase activity was evaluated on a medium containing 0.2% carboxymethyl cellulose (CMC), 1% yeast nitrogen base (1%), and 2% bacteriological agar. Following incubation, the plates were flooded with Gram’s iodine for 30 min. Cellulase activity was indicated by clear halos around the colonies.
2.2.6. Starch Degrading Activity
Yeasts were screened for starch-degrading activity on a medium comprising 0.67% YNB, 0.2% soluble starch, and 0.2% bacteriological agar at pH 6 following the protocol by Buzzini and Martini [25]. After incubation, the plates were treated with an iodine solution, and starch hydrolysis was indicated by a pale-yellow zone surrounding the colonies.
2.2.7. Pectinase Activity
Pectinase activity was assessed following an adapted method from McKay [26] using a pectinase agar medium containing 1.25% pectin (Sigma), 0.68% potassium phosphate (pH 3.5), 0.67% YNB, 1% glucose, and 2% bacteriological agar. Plates were stained with 0.1% ruthenium red, and colonies producing a purple halo were identified as positive for pectinase activity.
2.2.8. Lipase Activity
Lipase activity was tested on a tributyrin agar medium containing 0.5% peptone, 0.3% yeast extract, 1% tributyrin, and 2% bacteriological agar adjusted to pH 6, following the method of Buzzini and Martini [25]. A clear halo surrounding the colony in the opaque medium signified lipase activity.
2.3. Dual-Culture Assay
Dual-culture assays were used to assess the inhibitory effects of yeasts on mycelial growth, following the protocol by Chen et al. [27]. Four yeast strains, which displayed multiple lytic enzyme activities in initial screenings and showed the best biocontrol activity according to Gomomo et al. [17], were selected for further evaluation (Table 2). Additionally, yeast strain P. kluyveri (Y64), previously studied by Mewa-Ngongang et al. [20,28] and Gomomo et al. [17], was included as the reference strain. A 5 mm mycelial disc was positioned at the edge of the YMA plate, and 20 μL of the yeast suspension (1 × 108 cells/mL) was spotted 40 mm away from the mycelial disc (Figure 2). Incubation was conducted at 25 °C for 5–9 days.

Table 2.
Yeasts selected for the dual assays, mould spore germination, and mouth-to-mouth assays on yeast malt agar.
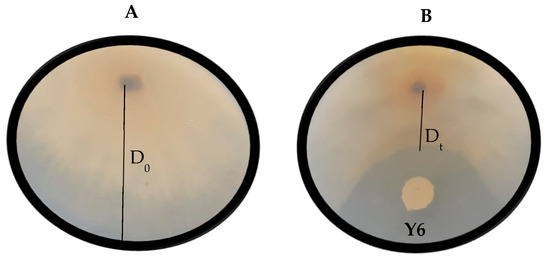
Figure 2.
Visual representation of the growth of Botrytis cinerea (A) and the antagonistic effect of yeast isolate Aureobasidium melanogenum (Y6) against B. cinerea (B) on yeast malt agar. D0 represents the horizontal growth of the mould colony on the negative control plates and Dt represents the horizontal growth of the mould colony on the yeast-treated plates. Each plate is a representative example of three replicates.
Negative control plates contained only the 5 mm diameter mycelial disc of the target mould, while positive control plates included 0.5 g/L of the commercial fungicide Captan (N-trichloromethylthio-4-cyclohexene-1,2-dicarboximide). All treatments were performed in triplicate. The percentage inhibition of mycelial growth (MGI) was calculated using the following formula:
with D0 representing the average horizontal growth of the mould colony in the negative control and Dt representing the average horizontal growth of the fungal colony on the yeast-treated plates (Figure 2), as described by Núñez et al. [29].
MGI = [(D0 − Dt)/D0] × 100
2.4. Mould Spore Germination Assay
A radial inhibition assay was conducted using the agar plate method as described by Núñez et al. [29]. Yeast cell suspensions (1 × 108 cells/mL) were prepared from yeast culture broths, and 100 µL of each suspension was spread evenly on YMA plates with a Drigalski spatula and allowed to dry. Subsequently, 15 µL of a B. cinerea spore suspension (1 × 105 spores/mL) was spotted at the centre of each plate (Figure 3), with each treatment conducted in triplicate. Negative control plates contained only the 15 µL spore suspension at the centre of the YMA. The plates were incubated at 25 °C for 5–9 days. The mould radial inhibition (MRI) was calculated as follows:
with D0 representing the average diameter of the mould growth on the negative control plates and Dt representing the diameter of the mould growth on the yeast-treated plates [29].
MRI = [(D0 − Dt)/D0] × 100
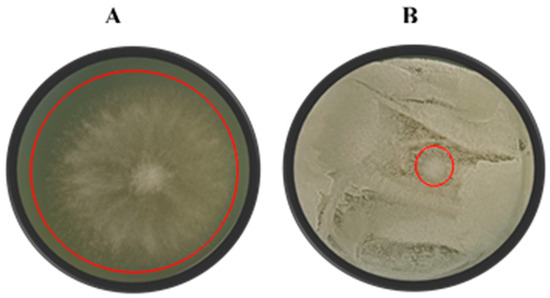
Figure 3.
A visual representation of the growth of Botrytis cinerea (A) and the antagonistic effect of yeast isolate Suhomyces pyralidae (Y63) against B. cinerea (B) on yeast malt agar. D0 represents the diameter of the mould colony on the negative control plates and Dt represents the diameter of the mould colony on the yeast-treated plates. Each plate is a representative example of three replicates. Red circles show the B. cinerea growth for the different treatments.
2.5. Volatile Organic Compound (VOC) Production Assay
The VOC production by selected yeasts was assessed using the mouth-to-mouth assay described by Medina-Córdova et al. [30]. In this assay, two YMA plates were sealed face to face using laboratory film. The bottom plate was spread with 100 µL of the yeast suspension (1 × 108 cell/mL), while a 5 mm mould mycelial disc was placed at the centre of the top plate. For the negative control, only the mycelial disc was placed in the centre of the top plate, with no yeast applied to the bottom plate. For the positive control, 0.5 g/L of the commercial fungicide Captan was spread on one YMA plate, with the mycelial disc placed on the other. The plates were incubated at 25 °C for 7 days, and each treatment was conducted in triplicate. VOC inhibition activity (VOCIA) was calculated using the following mathematical expression [29]:
with D0 representing the average diameter of the mould colony on the negative control plates and Dt representing the diameter of the mould colony on the treated plates, as shown in Figure 4.
VOCIA = [(D0 − Dt)/D0] × 100
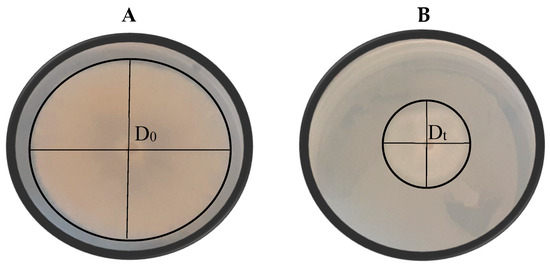
Figure 4.
Visual representation of the growth of Botrytis cinerea (A) and the antagonistic effect of selected yeast isolate Pichia kluyveri (Y64) against B. cinerea (B) on yeast malt agar. D0 represents the average diameter of the mould colony on the negative control plates and Dt represents the diameter of the mould colony on the treated plates. Each plate is a representative example of three replicates.
2.6. Extraction of Volatile Organic Compounds and Gas Chromatographic Analyses
2.6.1. Sample Preparation and Analyses
The volatile organic compounds (VOCs) produced by P. kluyveri and S. pyralidae were analysed using headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME–GC–MS), following a modified method based on Maluleke et al. [31]. Two sterile YMA layers were prepared by pouring 2 mL of agar on opposite sides of each vial. A spore suspension of B. cinerea PPRI 30807 (1 × 105 spores/mL) was prepared and 10 µL was spread on one side of the vial using an inoculation loop (LP ITAKIAN SPA, Milan, Italy). On the opposite side, 10 μL of the yeast suspension (1 × 108 cells/mL) was spread. Vials were incubated at 25 °C for 5 days, with separate control vials inoculated solely with B. cinerea spore suspension or the yeast cell suspension. Each treatment, including controls, was performed in triplicate.
For GC–MS analysis, 50 μL of a 10 ppm Anisole d8 solution was added to the centre of each vial as an internal standard. The vials were then incubated in an autosampler at 70 °C for 10 min, after which a 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fibre (Supelco, Bellafonte, PA, USA) was exposed to the headspace of each vial for 30 min at the same temperature. Following equilibration, the fibre was inserted into the GC injector at 250 °C, where compounds were desorbed over 10 min.
2.6.2. Chromatographic Conditions
Analyses were conducted using an Agilent Gas Chromatography, model 6890 N (Agilent, Palo Alto, CA, USA), coupled to an Agilent mass spectrometer detector, model 5975B Inert XL EI/CI (Agilent, Palo Alto, CA, USA), equipped with a CTC Analytics PAL autosampler. Chromatographic separation was achieved on a polar ZBWax capillary column (30 m length, 0.25 mm internal diameter, 0.25 μm film thickness). The oven temperature programme began at 40 °C, held for 17 min, followed by an increase to 240 °C at a rate of 8 °C/min, with a final hold of 5 min. Helium served as the carrier gas at a constant flow rate of 1.0 mL/min. The injector operated in a splitless mode at 250 °C throughout the analysis, with a purge flow of 50 mL/min activated after 2 min and a gas saver flow at 50 mL/min for an additional 5 min. The MS detector operated in a full scan mode, with the ion source and quadrupole temperatures set at 230 °C and 150 °C, respectively, and the transfer line at 280 °C. Compounds were identified by their retention times and mass spectra, referencing the NIST05 spectral library.
2.7. Post-Harvest Fruit Bioassays
Post-harvest biocontrol efficacy assays were conducted on “Golden Delicious” apples and “Earliglow” strawberries across 16 treatments (Table 3). Each treatment included five replicates, with each replicate comprising a rectangular fruit-packaging box containing five apples or a punnet with five strawberries. Fruit surfaces were sprayed with 70% ethanol to eliminate surface microorganisms and allowed to dry completely before wound infliction. A sterile cork borer was used to uniformly wound the fruits (approximately 5 mm diameter and 3 mm deep).

Table 3.
Treatments applied on apples and strawberries during post-harvest biocontrol trials.
After 15 min, 15 μL of sterile purified water was applied to the wound in the control treatment, while the other treatments received 15 μL of the B. cinerea spore suspension (1 × 105 spores/mL), followed by a 30 min drying period. Subsequently, 15 μL of a yeast inoculum (1 × 108 cells/mL) or 15 μL of the commercial fungicide Captan (0.5 g/L) was introduced into the wound. The negative control was treated with the three B. cinerea spore suspension only without additional yeast or fungicide. The treated fruits were incubated at ±20 °C for 4–6 days at a relative humidity of 80%. The inhibition of mould growth was characterised by an absence of visible mould development. Lesion diameters were measured, and percentage growth inhibition was calculated using previously described formulas.
2.8. Statistical Analyses
Percentage inhibition data from each assay were analysed using a one-way analysis of variance (ANOVA) using the GLM procedure of SAS software (version 9.4, SAS Institute Inc, Cary, NC, USA). The normality of standardised residuals was confirmed with the Shapiro–Wilk test. Fisher’s least significant difference (LSD) values were calculated at a 5% significance level (p = 0.05) to allow for the comparison of treatment means. A probability level of 5% was deemed significant for all statistical tests.
3. Results and Discussion
3.1. Extracellular Lytic Enzymes Activity
Among the 23 yeast strains examined, all displayed lipase activity, with additional enzyme activities varying across strains (Table 1). Notably, A. melanogenum, produced all the enzymes tested, while S. pyralidae, Z. hellenicus, and M. guilliermondii Y88 demonstrated starch-degrading enzymes, protease, glucanase, and cellulase activities. Rhodotorula dairenensis and M. guilliermondii Y65 also produced proteases and glucanases alongside lipases, whereas the remaining yeast strains exhibited activity for one additional enzyme or none.
Previous studies have documented the enzyme-producing capabilities of Aureobasidium species. Zajc et al. [10], Parafati et al. [32], and Di Francesco et al. [33] reported glucanase, pectinase, and protease activities for A. melanogenum, A. pullulans, and A. subglaciale, supporting the role of Aureobasidium spp. in producing lytic enzymes. Zajc et al. [10] and Moura et al. [34] further observed chitinase and glucanase activity in A. melanogenum, aligning with the current findings. The protease activity in S. pyralidae corroborates findings by Kantarcioǧlu and Yücel [12], Oksuz et al. [35], and Mehlomakulu et al. [36].
De Souza Ramos et al. [37] and Yang et al. [38] also reported protease and glucanase activities in Suhomyces spp., supporting this study’s observations. Additionally, Ruas et al. [39], Agirman and Erten [40], and Lorrine et al. [41] found M. guilliermondii capable of extracellular protease production, although this was strain dependent, consistent with the results here. Maluleke et al. [31] reported that chitinase and glucanase activities were common in yeasts with antagonistic activity against B. cinerea, further supporting the findings.
3.2. Dual-Culture Assay
Yeasts are known to inhibit various moulds through the production of diffusible metabolites. The five yeasts tested displayed varying levels of growth inhibition against the three B. cinerea strains, indicating that inhibition effectiveness varies depending on both yeast species and fungal strain (Figure 5).
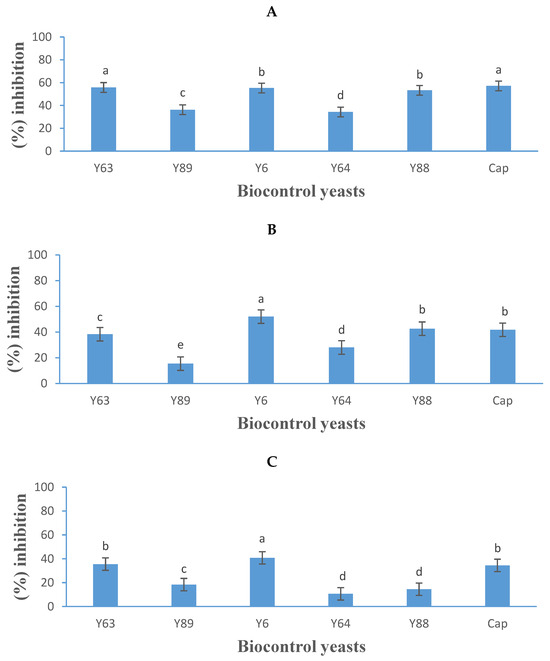
Figure 5.
Growth inhibition activity (expressed as a percentage) of five yeasts (details listed in Table 2) and Captan (Cap), a commercial fungicide against Botrytis cinerea B05.10 (A), IWBT-FF1 (B), and PPRI 30807 (C) using the dual-culture assay. Values are means of three replicates and the standard deviations are also shown. Different letters indicate significant differences (p ≤ 0.05) between treatments. The plates of negative control treatments only contained the respective moulds and were the references for determining growth inhibition.
Aureobasidium melanogenum (Y6) was the most effective, demonstrating 55%, 52%, and 40% inhibition against B. cinerea B05.10, IWBT-FF1, and PPRI 30807, respectively, whereas the commercial fungicide Captan achieved 57%, 41%, and 34% inhibition (Figure 5A–C). Suhomyces pyralidae (Y63) ranked second, inhibiting B. cinerea B05.10, IWBT-FF1, and PPRI 30807 by 56%, 38%, and 35%, respectively. Meyerozyma guilliermondii (Y88) inhibited B. cinerea strains B05.10, IWBT-FF1, and PPRI 30807 by 53%, 43%, and 15%, respectively. The reference strain P. kluyveri (Y64) showed limited inhibition with an average activity of 24% against the three B. cinerea strains.
The results of this study align with those of Di Francesco et al. [33], who also reported A. melanogenum inhibiting B. cinerea, although the yeast strain exhibited lower efficacy in their study compared to this one. Similarly, Gomomo et al. [17] reported that A. melanogenum inhibited a different strain of B. cinerea by 55% in vitro, supporting the findings of this study. Previous work by Mewa-Ngongang et al. [20] reported 100% inhibition of S. pyralidae on B. cinerea spore germination, while Gomomo et al. [17] found 62% inhibition under in vitro conditions, suggesting that S. pyralidae is more effective at preventing spore germination than controlling established mould growth. Wang et al. [42] and Cheng et al. [43] also reported antifungal activity of M. guilliermondii against B. cinerea. The reference strain P. kluyveri (Y64) showed low inhibition activity, which agrees with observations by Gomomo et al. [17] who noted its relatively weak antagonistic effect. The inhibitory effect of the highest performing yeasts, A. melanogenum, S. pyralidae, and M. guilliermondii, may be attributed to their production of cell wall-degrading enzymes (Table 1), and their ability to compete with B. cinerea for nutrients and space.
3.3. Mould Spore Germination Assay
A radial inhibition assay was used to assess the effect of S. pyralidae, P. kluyveri, A. melanogenum, M. guilliermondii, and Z. hellenicus on the spore germination of three B. cinerea strains (Figure 6). Notably, the inhibitory levels observed were higher than those in the dual-culture assay (Figure 5). Previous research by Mewa-Ngongang et al. [20] has shown that these non-Saccharomyces yeasts can inhibit mould growth through various mechanisms, such as rapid colonisation of surfaces and outcompeting spoilage moulds, thereby limiting mould proliferation. Both M. guilliermondii (Y88) and P. kluyveri (Y64) were highly effective, achieving 100% inhibition against all three B. cinerea strains (Figure 6A–C). The increased inhibition by P. kluyveri, compared to its mycelial growth inhibition in the dual-culture assay (Figure 5), underscores the stronger antagonistic effect of these yeasts on spore germination and highlights their potential as preventative treatments against moulds.
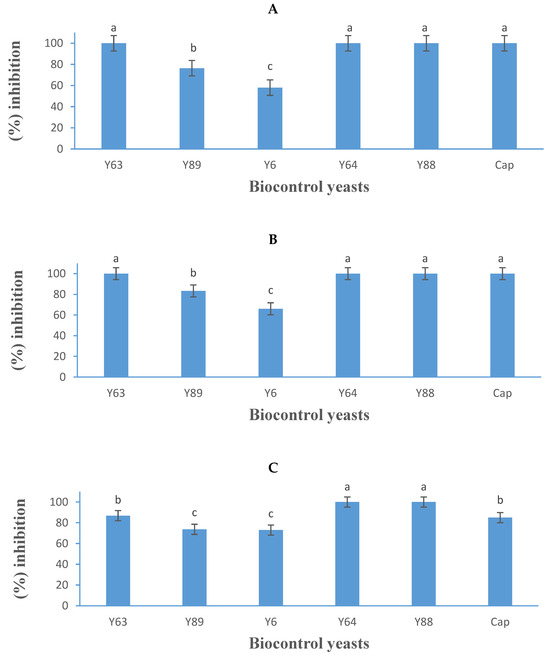
Figure 6.
Growth inhibition activity (expressed as a percentage) of five yeasts (details listed in Table 2) and Captan (Cap), a commercial fungicide against Botrytis cinerea B05.10 (A), IWBT-FF1 (B), and PPRI 30807 (C), using the mould spore germination assay. Values are means of three replicates and the standard deviations are also shown. Different letters indicate significant differences (p ≤ 0.05) between treatments. The plates of negative control treatments only contained the respective moulds and were the references for determining growth inhibition.
The complete inhibitory effect observed for M. guilliermondii in this study aligns with previous reports by Wang et al. [42] and Sepúlveda et al. [44], who noted similar efficacy against two B. cinerea strains in vitro. Similarly, the effectiveness of P. kluyveri corroborates the findings of Mewa Ngongang et al. [20,45], who also reported its strong inhibitory activity against B. cinerea. Additionally, S. pyralidae (Y63) demonstrated 100% inhibition against B. cinerea B05.10 and IWBT-FF1, and 87% inhibition against B. cinerea PPRI 30807. These results are consistent with previous studies by Carbó et al. [46], Ngongang et al. [20], and Gao et al. [47], which showed that various Candida spp. exhibit differing degrees of inhibitory activity against B. cinerea spoilage.
3.4. VOC Production Assay
The mode of action of VOCs in inhibiting B. cinerea was explored using the mouth-to-mouth assay. The results indicate that the VOCs produced by the yeasts inhibited the growth of B. cinerea, with inhibition levels varying among yeast strains (Figure 7). Notably, the B. cinerea strain PPRI 30807 exhibited higher susceptibility (mean inhibition of 73%) to yeast VOCs than strain B05.10 (mean inhibition of 38%). This pattern differed from the dual-culture assay, where the inhibition of PPRI 30807 was less pronounced, suggesting that VOCs could be a primary mode of action against this particular strain.
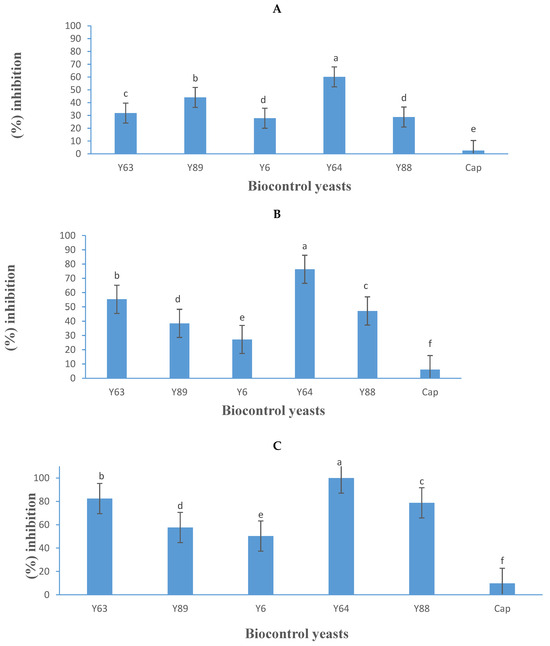
Figure 7.
Growth inhibition activity (expressed as a percentage) of five yeasts (details listed in Table 2) and Captan (Cap), a commercial fungicide against Botrytis cinerea B05.10 (A), IWBT-FF1 (B), and PPRI 30807 (C) based on volatile organic compound production. Values are means of three replicates and the standard deviations are also shown. Different letters indicate significant differences (p ≤ 0.05). The negative control treatments only contained the respective moulds and were the references for determining growth inhibition.
In the VOC assay trial, P. kluyveri (Y64) demonstrated the highest inhibition, achieving 60%, 76%, and 100% inhibition against B. cinerea B05.10, IWBT-FF1, and PPRI 30807, respectively (Figure 7). Unlike in the diffusible metabolite assay (Figure 5) where P. kluyveri (Y64) exhibited lower inhibition, its stronger performance in the VOC assay points toward VOC production as its primary antagonistic mechanism. This observation aligns with findings by Nägeli et al. [48] who reported P. kluyveri as an effective inhibitor of B. cinerea growth under in vitro conditions, emphasising its reliance on VOCs for mould suppression.
Other yeasts also demonstrated notable VOC-based inhibition. Suhomyces pyralidae (Y63) achieved inhibition rates of 32%, 55%, and 82% against strains B05.10, IWBT-FF1, and PPRI 30807, respectively, while M. guilliermondii Y88 exhibited 29%, 47%, and 79% inhibition (Figure 7). Previous studies by Mewa-Ngongang et al. [20] and Choińska et al. [49] also reported the effective VOC-mediated inhibition of B. cinerea by S. pyralidae and M. guilliermondii, supporting the current findings. Zygoascus hellenicus (Y89) showed moderate inhibition, with rates of 44%, 38%, and 58% against the three B. cinerea strains, respectively (Figure 7), further illustrating the potential of VOC production as a biological control strategy against mould proliferation.
3.5. VOC Extraction and Gas Chromatographic Analyses
The VOCs produced by P. kluyveri and S. pyralidae were shown to play a significant role in inhibiting B. cinerea growth during in vitro trials (Figure 7). The VOCs produced by P. kluyveri and S. pyralidae were analysed using solid-phase microextraction coupled with gas chromatography–mass spectrometry (SPME-GC–MS), identifying a total of 29 compounds, of which seven were consistently present across all replicates. The key compounds included alcohols (isobutanol, isoamyl alcohol, 2-phenylethanol), esters (isoamyl acetate, 2-phenethyl acetate), gamma butyrolactone (γ-decanolactone), and a fatty acid methyl ester (methyl palmitate) (Table 4). These VOCs were produced by the yeast isolates alone or in conjunction with B. cinerea. Notably, isoamyl alcohol, 2-phenylethanol, 2-phenethyl acetate, and methyl palmitate were also detected when B. cinerea was cultured independently.

Table 4.
Major volatile compounds (VOCs) produced by Pichia kluyveri, Suhomyces pyralidae, and Botrytis cinerea PPRI 30807, identified through gas chromatography–mass spectrometry (GC-MS).
Suhomyces pyralidae produced all seven VOCs in monoculture, with isobutanol, isoamyl acetate and 2-phenethyl acetate concentrations slightly elevated in the presence of B. cinerea. These VOCs, particularly isobutanol, isoamyl acetate, and 2-phenethyl acetate, are likely contributors to the inhibition of B. cinerea, aligning with findings of Li et al. [50] who reported isoamyl acetate’s antagonistic effects against grey mould on blueberries. Further, Zou et al. [51] demonstrated the antifungal efficacy of isoamyl acetate against B. cinerea mycelial growth, and Hanseniaspora uvarum effectively controlled B. cinerea in strawberries and cherries, with 2-phenylethyl acetate identified as the predominant VOC [52]. Phenylethyl acetate has also shown strong inhibitory activity against Aspergillus ochraceus and Mucor spp. growth [49,53].
When co-cultured with B. cinerea, S. pyralidae produced slightly lower levels of isoamyl alcohol and 2-phenylethanol, yet these VOCs continued to contribute to its antagonistic effect. Calvo et al. [54] reported complete inhibition of B. cinerea growth in vivo by isoamyl alcohol, with additional studies by Maluleke et al. [31] and Zou et al. [51], linking isoamyl alcohol and 2-phenylethanol to B. cinerea inhibition. The observed inhibition of B. cinerea may be due to the combined or synergistic effects of VOCs produced by S. pyralidae.
Pichia kluyveri, when cultured alone, produced high concentrations of isoamyl acetate and 2-phenethyl acetate; however, these levels significantly decreased when co-cultured with B. cinerea. Lower concentrations of isoamyl alcohol and 2-phenylethanol were also observed in co-culture, yet these VOCs may still be central to B. cinerea suppression.
Previous research on biocontrol yeasts, such as P. kudriavzevii, P. occidentalis, W. anomalus, H. uvarum, and C. intermedia, demonstrates that VOCs effectively inhibited B. cinerea spore germination and mycelial growth [14,31,49,55]. Ethanol and 2-phenylethanol are specifically highlighted as potent antifungal agents against B. cinerea and Alternaria alternata [56,57]. Additionally, transcinnamaldehyde has shown to inhibitory effects on B. cinerea mycelial growth and conidia germination, significantly reducing infections in cherry tomatoes [58]. VOCs such as 3-methyl-1-butanol, 2-phenylethanol, 2-ethyl-1-hexanol, 4-methyl-ethyl ester, and ethyl acetate have also been identified as effective in inhibiting B. cinerea spore germination and mycelial growth [59,60,61,62].
The observed decrease in VOC concentrations in the presence of B. cinerea may result from interspecies competition for oxygen and/or carbon dioxide within the test environment, with the biocontrol efficacy of the yeast potentially deriving from the synergistic effects of VOCs and elevated carbon dioxide levels [14,63]. Additionally, B. cinerea may produce defensive compounds that alter the metabolic pathways of biocontrol yeasts, resulting in shifts in VOC production profiles [64].
3.6. Post-Harvest Fruit Bioassays
The application of yeast-based biocontrol agents demonstrated significant efficacy in reducing the spoilage of B. cinerea in apples and strawberries, resulting in marked reductions in fruit decay (Figure 8 and Figure 9). In apple trials, S. pyralidae (Y63) effectively inhibited the growth of B. cinerea strains B05.10, IWBT-FF1, and PPRI 30807 by 64%, 40%, and 25%, respectively (Figure 8). Aureobasidium melanogenum displayed inhibitory activity against these strains by 21%, 24%, and 26%, respectively, while P. kluyveri achieved inhibition rates of 11%, 17%, and 16%, respectively. In comparison, the commercial fungicide Captan exhibited stronger inhibitory effects, reducing the growth of B. cinerea B05.10, IWBT-FF1, and PPRI 30807 by 92%, 59%, and 17%, respectively. These findings align with prior studies. Guerrero Prieto et al. [65] and Carbó et al. [46] reported the antagonistic properties of Candida spp., such as C. oleophila and C. sake, against B. cinerea in various fruit contexts, consistent with the inhibitory effects observed for S. pyralidae in this study. The activity of A. melanogenum corroborates the findings of Di Francesco et al. [33], who demonstrated similar antagonistic effects of Aureobasidium species in vivo. Furthermore, P. kluyveri’s inhibitory effects and the minimum concentration of 1 × 102 cells/mL required for inhibition by P. kudriavzevii against B. cinerea are consistent with Maluleke et al. [31], who highlighted the antimicrobial properties of Pichia spp. targeting B. cinerea.
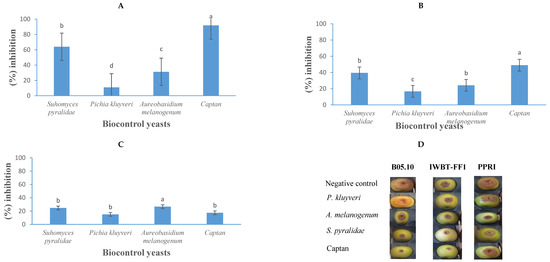
Figure 8.
The growth inhibition activity (%) of Suhomyces pyralidae (Y63), Aureobasidium melanogenum (Y6), and Pichia kluyveri (Y64) against Botrytis cinerea B05.10 (A), IWBT-FF1 (B), and PPRI 30807 (C) during post-harvest trials on apples. Values are means of five replicates and the standard deviations are also shown. The different letters indicate significant differences (p ≤ 0.05) between treatments. (D) Photographs of apples showing lesion diameters. Each set is a representative example of 25 apples. For the negative control treatments, the apples were only infected with the respective moulds, therefore no growth inhibition was observed.
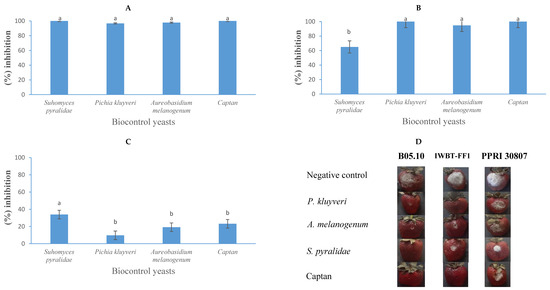
Figure 9.
The growth inhibition activity (expressed as a percentage) of Suhomyces pyralidae (Y63), Aureobasidium melanogenum (Y6), and Pichia kluyveri (Y64) against Botrytis cinerea B05.10 (A), IWBT-FF1 (B), and PPRI 30807 (C) during post-harvest trials on strawberries. Values are means of five replicates and the standard deviations are also shown. The different letters indicate significant differences (p < 0.05) between treatments. (D) Photographs of strawberries showing lesion diameters. Each set is a representative example of 25 strawberries. For the negative control treatments, the strawberries were only infected with the respective moulds, therefore no growth inhibition was observed.
In strawberry trials, yeast strains and Captan demonstrated greater inhibition, particularly against B. cinerea strains B05.10 and IWBT-FF1, compared to their performance in apples. The results indicate that the extent of inhibition is not solely dependent on the specific yeast or mould species but is also influenced by the type of fruit substrate. Suhomyces pyralidae (Y63) achieved 100%, 65%, and 34% inhibition against B. cinerea strains B05.10, IWBT-FF1, and PPRI 30807, respectively. Similarly, A. melanogenum (Y6) exhibited 98%, 95%, and 19% antagonistic activity against the same strains. Pichia kluyveri demonstrated 98%, 100%, and 10% inhibition of B. cinerea strains B05.10, IWBT-FF1, and PPRI 30807, respectively. Captan, the commercial fungicide, exhibited 100%, 100%, and 23% inhibition against these strains. These findings align with previous studies. The strong inhibitory effects of Aureobasidium spp., observed in this study, are consistent with the work of Zajc et al. [66,67], who highlighted the antagonistic capabilities of Aureobasidium species under in vivo conditions. The efficacy of P. kluyveri supports the findings of Nägeli et al. [48] and Maluleke et al. [31], who demonstrated the potential of Pichia spp. in suppressing B. cinerea. Furthermore, the high efficacy of Captan observed in this study is consistent with reports by Guerrero Prieto et al. [65], who documented its effectiveness against B. cinerea in apples.
Overall, the study indicates that the degree of inhibition is influenced by the specific yeast or mould strain as well as the fruit type. The susceptibility of B. cinerea strains varied based on the host fruit, highlighting the importance of fruit type in modulating the antagonistic effects of yeast biocontrol agents. The potential application of these yeast species, either individually or in combination, offers promising alternatives to traditional chemical fungicides for managing B. cinerea spoilage, presenting a sustainable solution for the agricultural industry.
4. Conclusions
The study confirmed that A. melanogenum synthesises enzymes capable of hydrolysing cell walls. In the direct-contact inhibition assays, cell suspensions of S. pyralidae and A. melanogenum exhibited the strongest antagonistic effects against B. cinerea, with the yeasts significantly impeding spore germination. In fruit trials, S. pyralidae, A. melanogenum, and P. kluyveri each demonstrated distinct inhibitory capacities against B. cinerea, varying by fruit type, with results comparable to those of commercial fungicides. This suggests that these yeasts hold promise as biocontrol agents for reducing post-harvest spoilage in place of chemical fungicides. The antagonistic mechanism of P. kluyveri was linked to the production of VOCs, with isobutanol, isoamyl acetate, and 2-phenethyl acetate identified as key antifungal agents. The primary VOCs contributing to this inhibition were identified as belonging to the alcohol and ester groups. Future investigations should focus on assessing the individual and synergistic antimicrobial effects of these VOCs against B. cinerea. Additionally, research should explore the use of biocontrol yeasts in pre-harvest treatments to inhibit mould growth, exploring various yeast combinations and integrating yeast applications with commercial fungicides to mitigate resistance development.
Author Contributions
Z.G.: investigation, data curation, writing—original draft, methodology, formal analysis; M.F.: supervision, conceptualization, writing—review and editing; M.M.-N.: conceptualization, methodology, writing—review and editing; B.S.C.: conceptualization, resources, writing—review and editing; J.W.H.: resources, methodology, writing—review and editing; M.v.d.R.: formal analysis, writing—review and editing; L.M.: methodology, formal analysis, writing—review and editing; M.E.S.: resources, writing—review and editing; H.W.d.P.: conceptualization, supervision, methodology, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Research Council (ARC) and the National Research Foundation (NRF) of South Africa (Grant Number 138631).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank the Agricultural Research Council (ARC), Agricultural Sector Education Training Authority (AgriSETA), and the National Research Foundation (NRF) of South Africa for funding and/or infrastructure. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors alone, and the NRF accepts no liability whatsoever in this regard. The authors thank the students, interns, researchers, technicians, and research assistants for their contributions.
Conflicts of Interest
The authors hereby declare that they have no conflicts of interest.
References
- Al-Hindi, R.R.; Al-Najada, A.R.; Mohamed, S.A. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. Afr. J. Microbiol. Res. 2011, 5, 443–448. [Google Scholar]
- Abo-Elyousr, K.A.; Al-Qurashi, A.D.; Almasoudi, N.M. Evaluation of the synergy between Schwanniomyces vanrijiae and propolis in the control of Penicillium digitatum on lemons. Egyp. J. Biol. Pest Control 2021, 31, 66. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against post-harvest grey mould in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in post-harvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Lutz, M.C.; Lopes, C.A.; Sosa, M.C.; Sangorrin, M.P. Semi-commercial testing of regional yeasts selected from North Patagonia Argentina for the biocontrol of pear post-harvest decays. Biol. Control 2020, 150, 104246. [Google Scholar] [CrossRef]
- Guigón-López, C.; Holguín-Ibarra, P.D.; Torres-Zapien, J.H.; García-Cruz, I.; Villapando, I.; Salas-Salazar, N.A. Metarhizium anisopliae reduces conidial germination and mycelium growth of the apple grey mould Botrytis cinerea. Biol. Control 2021, 160, 104660. [Google Scholar] [CrossRef]
- Rico, D.; Barcenilla, B.; Meabe, A.; González, C.; Martín-Diana, A.B. Mechanical properties and quality parameters of chitosan-edible algae (Palmaria palmata) on ready-to-eat strawberries. J. Sci. Food Agric. 2019, 99, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Nybom, H.; Ahmadi-Afzadi, M.; Rumpunen, K.; Tahir, I. Review of the impact of apple fruit ripening, texture and chemical contents on genetically determined susceptibility to storage rots. Plants 2020, 9, 831. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialisation of post-harvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Zajc, J.; Gostinčar, C.; Černoša, A.; Gunde-Cimerman, N. Stress-tolerant yeasts: Opportunistic pathogenicity versus biocontrol potential. Genes 2019, 10, 42. [Google Scholar] [CrossRef]
- Iqbal, M.; Jützeler, M.; França, S.C.; Wäckers, F.; Andreasson, E.; Stenberg, J.A. Bee-vectored Aureobasidium pullulans for biological control of grey mould in strawberry. Phytopathology 2022, 112, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kantarcioǧlu, A.S.; Yücel, A. Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains: Phospholipase-and Protease-Aktivitat bei klinischen Candida-Isolaten mit Bezug zur Herkunft der Stämme. Mycoses 2002, 45, 160–165. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for post-harvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, L.; Lin, M.; Yu, T. Post-harvest Biology and Technology Recombinant expression of antimicrobial peptides in Pichia pastoris: A strategy to inhibit the Penicillium expansum in pears. Postharvest Biol. Technol. 2021, 171, 111298. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Dai, K.; Xu, F.; Wang, H.; Shao, X. Yeasts from intertidal zone marine sediment demonstrate antagonistic activities against Botrytis cinerea in vitro and in strawberry fruit. Biol. Control 2021, 158, 104612. [Google Scholar] [CrossRef]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Hoff, J.; Van der Rijst, M.; Okudoh, V.; Kriel, J.; du Plessis, H.W. Control of mould spoilage on apples using yeasts as biological control agents. Pol. J. Food Nutr. Sci. 2022, 72, 119–128. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Mangieri, N.; Maghradze, D.; Foschino, R.; Valdetara, F.; Cantoral, J.M.; Vigentini, I. Wild grape-associated yeasts as promising biocontrol agents against Vitis vinifera fungal pathogens. Front. Microbiol. 2017, 8, 2025. [Google Scholar] [CrossRef] [PubMed]
- Mewa-Ngongang, M.; du Plessis, H.W.; Ntwampe, S.K.O.; Chidi, B.S.; Hutchinson, U.F.; Mekuto, L.; Jolly, N.P. The use of Candida pyralidae and Pichia kluyveri to control spoilage microorganisms of raw fruits used for beverage production. Foods 2019, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawali, S.S.; Al-Sadi, A.M.; Ali Khalifa Alsheriqi, S.; Nasser Al-Sabahi, J.; Velazhahan, R. The potential of antagonistic yeasts and bacteria from tomato phyllosphere and fructoplane in the control of Alternaria fruit rot of tomato. All Life 2021, 14, 34–48. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, S.; Deng, L.; Min, J.; Zeng, K. Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 2019, 152, 100–110. [Google Scholar] [CrossRef]
- Verma, K.; Garg, N. Detection of Chitinase on Chitin Agar Plates. Int. J. Sci. Res. 2019, 8, 1186–1189. [Google Scholar]
- Strauss, M.L.A.; Jolly, N.P.; Lambrecht, M.G.; Van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Martini, A. Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J. Appl. Microbiol. 2002, 93, 1020–1025. [Google Scholar] [CrossRef]
- McKay, A.M. A plate assay method for the detection of fungal polygalacturonase secretion. FEMS Microbiol. Lett. 1988, 56, 355–358. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, R.Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef]
- Mewa-Ngongang, M.; Du Plessis, H.W.; Hlangwani, E.; Ntwampe, S.K.; Chidi, B.S.; Hutchinson, U.F.; Jolly, P. Activity interactions of crude biopreservatives against spoilage yeast consortia. Ferment 2019, 5, 53. [Google Scholar] [CrossRef]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Maluleke, E.; Jolly, N.P.; Patterton, H.G.; Setati, M.E. Antifungal activity of non-conventional yeasts against Botrytis cinerea and non-Botrytis grape bunch rot fungi. Front. Microbiol. 2022, 13, 986229. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Foggia, M.; Zajc, J.; Gunde-Cimerman, N.; Baraldi, E. Study of the efficacy of Aureobasidium strains belonging to three different species: A. pullulans, A. subglaciale and A. melanogenum against Botrytis cinerea of tomato. Ann. Appl. Biol. 2020, 177, 266–275. [Google Scholar] [CrossRef]
- Moura, V.S.; Pollettini, F.L.; Ferraz, L.P.; Mazzi, M.V.; Kupper, K.C. Purification of a killer toxin from Aureobasidium pullulans for the biocontrol of phytopathogens. J. Basic Microbiol. 2021, 61, 77–87. [Google Scholar] [CrossRef]
- Oksuz, S.; Sahin, I.; Yildirim, M.; Gulcan, A.; Yavuz, T.; Kaya, D.; Koc, A.N. Phospholipase and proteinase activities in different Candida species isolated from anatomically distinct sites of healthy adults. Jpn. J. Infect. Dis. 2007, 60, 280. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- de Souza Ramos, L.; Barbedo, L.S.; Braga-Silva, L.A.; dos Santos, A.L.S.; Pinto, M.R.; da Graça Sgarbi, D.B. Protease and phospholipase activities of Candida spp. isolated from cutaneous candidiasis. Rev. Iberoam. Micol. 2015, 32, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Solis, N.V.; Marshall, M.; Garleb, R.; Zhou, T.; Wang, D.; Swidergall, M.; Pearlman, E.; Filler, S.G.; Liu, H. Control of β-glucan exposure by the endo-1, 3-glucanase Eng1 in Candida albicans modulates virulence. PLoS Pathog. 2022, 18, 1010192. [Google Scholar] [CrossRef]
- Ruas, F.A.D.; Barboza, N.R.; Castro-Borges, W.; Guerra-Sá, R. Manganese alters expression of proteins involved in the oxidative stress of Meyerozyma guilliermondii. J. Proteom. 2019, 196, 173–188. [Google Scholar] [CrossRef]
- Agirman, B.; Erten, H. Biocontrol ability and action mechanisms of Aureobasidium pullulans GE17 and Meyerozyma guilliermondii KL3 against Penicillium digitatum DSM2750 and Penicillium expansum DSM62841 causing post-harvest diseases. Yeast 2020, 37, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Lorrine, O.E.; Rahman, R.N.Z.R.A.; Tan, J.S.; Khairuddin, R.F.R.; Salleh, A.B.; Oslan, S.N. Determination of Putative Vacuolar Proteases, PEP4 and PRB1 in a Novel Yeast Expression Host Meyerozyma guilliermondii strain SO using bioinformatics tools. Pertanika J. Sci. Technol. 2022, 30, 777–797. [Google Scholar] [CrossRef]
- Wang, X.; Glawe, D.A.; Kramer, E.; Weller, D.; Okubara, P.A. Biological control of Botrytis cinerea: Interactions with native vineyard yeasts from Washington State. Phytopathology 2018, 108, 691–701. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, L.; Li, D.; Gao, Z.; Teng, J.; Nie, X.; Guo, F.; Wang, C.; Wang, X.; Li, S.; et al. Combining the biocontrol agent Meyerozyma guilliermondii with UV-C treatment to manage postharvest grey mould on kiwifruit. Biol. Control 2023, 180, 105198. [Google Scholar] [CrossRef]
- Sepúlveda, X.; Vargas, M.; Vero, S.; Zapata, N. Indigenous yeasts for the biocontrol of Botrytis cinerea on table grapes in Chile. J. Fungi 2023, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Mewa Ngongang, M.; Du Plessis, H.W.; Boredi, C.S.; Hutchinson, U.F.; Ntwampe, K.S.; Okudoh, V.I.; Jolly, N.P. Physiological and antagonistic properties of Pichia kluyveri for curative and preventive treatments against post-harvest fruit fungi. Pol. J. Food Nutr. Sci. 2021, 71, 245–253. [Google Scholar] [CrossRef]
- Carbó, A.; Teixidó, N.; Usall, J.; Torres, R. Verifying the biocontrol activity of novel film-forming formulations of Candida sake CPA-1: Resilience in relation to environmental factors, rainfall episodes, and control of Botrytis cinerea on different hosts. J. Sci. Food Agric. 2019, 99, 4969–4976. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, R.; Xiong, B. Management of postharvest diseases of kiwifruit with a combination of the biocontrol yeast Candida oleophila and an oligogalacturonide. Biol. Control 2021, 156, 104549. [Google Scholar] [CrossRef]
- Nägeli, L.; Schuler, M.; Segessemann, T.; Frei, D.; Frey, J.E.; Wolfe, K.H.; Ahrens, C.H.; Freimoser, F.M. Genome sequence data of the strongly antagonistic yeast Pichia kluyveri isolate APC 11.10 B as a foundation for analysing biocontrol mechanisms. Data Brief 2023, 49, 109394. [Google Scholar] [CrossRef] [PubMed]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Anton Leeuw. 2020, 113, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Wu, C.; Yuan, Y.; Ni, X.; Wu, T.; Chang, R.; Wang, Y. Volatile organic compounds produced by Metschnikowia pulcherrima yeast T-2 inhibited the growth of Botrytis cinerea in post-harvest blueberry fruits. Hort Plant. J. 2024. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Zhu, J.; Sun, J.; Shao, X. Volatile organic compounds of Scheffersomyces spartinae W9 have antifungal effect against Botrytis cinerea on strawberry fruit. Foods 2023, 12, 3619. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbial. 2020, 92, 103556. [Google Scholar] [CrossRef] [PubMed]
- Masoud, W.; Poll, L.; Jakobsen, M. Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 2005, 22, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against post-harvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qin, X.; Wu, Y.; Yu, W.; Liu, J.; Xi, Y.; Dou, G.; Wang, L.; Xiao, H. Biocontrol of grey mould of cherry tomatoes with the volatile organic monomer from Hanseniaspora uvarum, trans-cinnamaldehyde. Food Bioproc. Tech. 2019, 12, 1809–1820. [Google Scholar] [CrossRef]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of post-harvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Che, H.J.; Zhang, J.; Yang, L.; Jiang, D.H.; Li, G.Q. Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biol. Control 2012, 62, 53–63. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Zajc, J.; Gunde-Cimerman, N.; Aprea, E.; Gasperi, F.; Placì, N.; Caruso, F.; Baraldi, E. Bioactivity of volatile organic compounds by Aureobasidium species against grey mould of tomato and table grape World. J. Microbiol. Biotechnol. 2020, 36, 171. [Google Scholar]
- Altieri, M.A. Linking ecologists and traditional farmers in the search for sustainable agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile metabolism of wine grape Trincadeira: Impact of infection with Botrytis cinerea. Plants 2022, 11, 141. [Google Scholar] [CrossRef]
- Guerrero Prieto, V.M.; Jacobo Cuéllar, J.L.; Parra Quezada, R.Á.; Linares Marrufo, M.I.; Ojeda Barrios, D.L.; Hernández Rodríguez, O.A.; Robles Hernández, L.; Berlanga Reyes, D.I.; Cabanillas Mata, I.J. Botrytis cinerea Pers. in post-harvest apple fruit, control with Candida oleophila Montrocher strains and/or synthetic fungicides. Nov. Sci. 2019, 11, 69–84. [Google Scholar]
- Zajc, J.; Cernosa, A.; Di Francesco, A.; Casteria, R.; De Curtis, F.; Lima, G.; Badri, H.; Jijakli, H.; Ippolito, A.; Gostincar, C.; et al. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genom. Biol. 2020, 10, 163. [Google Scholar]
- Zajc, J.; Černoša, A.; Sun, X.; Fang, C.; Gunde-Cimerman, N.; Song, Z.; Gostinčar, C. From glaciers to refrigerators: The population genomics and biocontrol potential of the black yeast Aureobasidium subglaciale. Microbiol. Spectr. 2022, 10, 01455-22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).