Two New Species of the Genus Diderma (Physarales, Didymiaceae) in China with an Addition to the Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Studies
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Data Analysis
3. Results
3.1. Phylogenetic Analysis
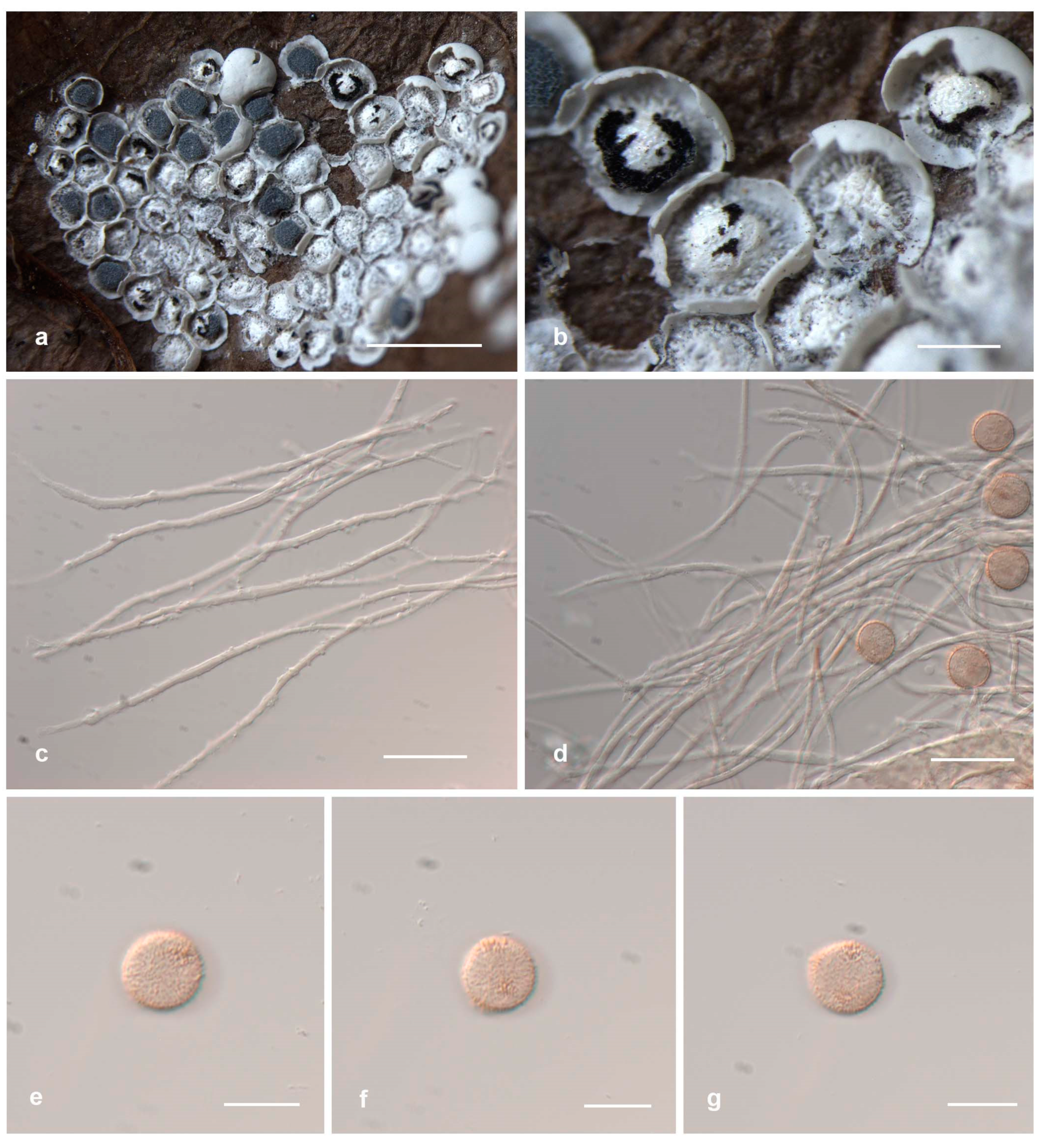
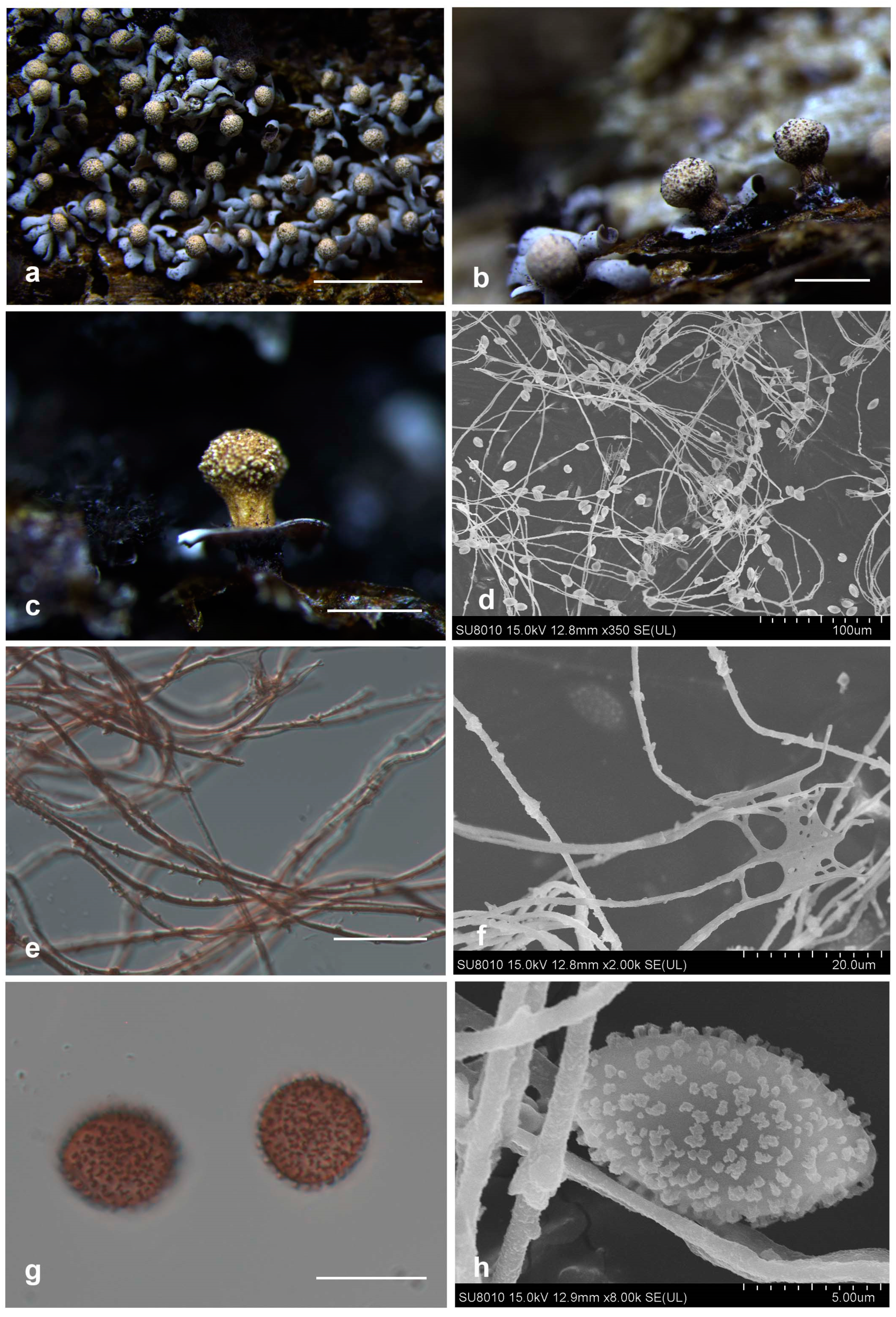
3.2. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, S.L.; Schnittler, M.; Novozhilov, Y.K. Myxomycete diversity and distribution from the fossil record to the present. Biodivers. Conserv. 2008, 17, 285–301. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.W.; Alexopoulos, C.J. The Myxomycetes. The Myxomycètes; University of Iowa Press: Iowa City, IA, USA, 1969; pp. ix + 560. [Google Scholar]
- García-Martín, J.M.; Zamora, J.C.; Lado, C. Multigene phylogeny of the order Physarales (Myxomycetes, Amoebozoa): Shedding light on the dark-spored clade. Persoonia 2023, 51, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Nmf, D. Neues Magazin für die Botanik in Ihrem Ganzen Umfange: Band 1; Saraswati Press: Kolkata, India, 1794. [Google Scholar]
- Leontyev, D.V.; Schnittler, M.; Stephenson, S.L.; Novozhilov, Y.K.; Shchepin, O.N. Towards a phylogenetic classification of the Myxomycetes. Phytotaxa 2019, 399, 209–238. [Google Scholar] [CrossRef]
- Prikhodko, I.S.; Shchepin, O.N.; Bortnikova, N.A.; Novozhilov, Y.K.; Gmoshinskiy, V.I.; Moreno, G.; López-Villalba, N.; Stephenson, S.L.; Schnittler, M. A three-gene phylogeny supports taxonomic rearrangements in the family Didymiaceae (Myxomycetes). Mycol Prog. 2023, 22, 11. [Google Scholar] [CrossRef]
- Lado, C.; Eliasson, U. Taxonomy and systematics: Current knowledge and approaches on the taxonomic treatment of Myxomycetes: Updated version. In Myxomycetes, 2nd ed.; Rojas, C., Stephenson, S.L., Eds.; Academic Press: New York, NY, USA, 2022; pp. 269–324. [Google Scholar]
- Rostafiński, J.T. Versuch Eines Systems der Mycetozoen. F. Wolff 1873, 9–13. Available online: https://BiotaNZ.landcareresearch.co.nz/references/1cb0f8b9-36b9-11d5-9548-00d0592d548c (accessed on 19 July 2024).
- Chow, Z.H. Taxonomic information of Myxomycetes. Jilin Agricultural University, Institute of Microbiology; Chinese Academy of Sciences: Beijing, China, 1978; pp. 157–174. [Google Scholar]
- Lado, C. (2005–2024) An Online Nomenclatural Information System of Eumycezotozoa. Available online: http://www.nomen.eumycetozoa.com (accessed on 1 July 2024).
- Song, W.L.; Lin, D.; Li, M.; Du, Q.; Chen, S.L. Four new records of myxomycetes from China. Phytotaxa 2022, 567, 181–188. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, S.Z.; Wang, G.W.; Chen, S.L. Two new species and two new records of myxomycetes from subtropical forests in China. Phytotaxa 2018, 350, 51–63. [Google Scholar] [CrossRef]
- Zhao, H.N.; Rao, G.; Yang, X.Y.; Li, X.; Yu, H.L.; Zhang, B.; Li, Y. Two new species of Diderma (Physarales, Didymiaceae) from northern China. Phytotaxa 2022, 572, 61–63. [Google Scholar] [CrossRef]
- Chen, S.L.; Yan, S.Z.; Li, Y. Species of the genus Diderma from China and their distributions. Mycosystema 2013, 32, 200–206. [Google Scholar]
- Liu, C.H.; Chang, J.H. Myxomycetes of Taiwan XXI. The genus Diderma. Taiwania 2011, 56, 118–124. [Google Scholar] [CrossRef]
- Royal, B.G. Edinburgh. Flora of British Fungi: Colour Identification Chart; HM Stationery Office: Edinburgh, UK, 1969. [Google Scholar]
- Wang, W.; Wang, W.; Wei, S.W.; Huang, W.; Qi, B.; Wang, Q.; Li, Y. Design of potentially universal SSU primers in myxomycetes using next-generation sequencing. J. Microbiol. Methods 2021, 184, 106203. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Okun, M.V.; Erastova, D.A.; Shchepin, O.N.; Zemlyanskaya, I.V.; García-Carvajal, E.; Schnittler, M. Description, culture and phylogenetic position of a new xerotolerant species of Physarum. Mycologia 2013, 105, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Yan, S.Z.; Chen, S.L. Further resolving the phylogeny of Myxogastria (slime molds) based on COI and SSU rRNA genes. Russ. J. Genet. 2015, 51, 46–53. [Google Scholar] [CrossRef]
- Feng, Y.; Schnittler, M. Sex or no sex? Group I introns and independent marker genes reveal the existence of three sexual but reproductively isolated biospecies in Trichia varia (Myxomycetes). Org. Divers. Evol. 2015, 15, 631–650. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Prikhodko, I.S.; Shchepin, O.N. A new species of Diderma from Bidoup Nui Ba National Park (southern Vietnam). Protistology 2019, 13, 126–132. [Google Scholar] [CrossRef]
- Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F. The Clustal–X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 63, 215–228. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Catalano, S.A.; Torres, A. Parsimony analysis of phylogenomic datasets (II): Evaluation of PAUP*, MEGA and MPBoot. Cladistics 2022, 38, 126–146. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.V.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.V.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhaxybayeva, O.; Gogarten, J.P. Bootstrap, Bayesian prob-ability and maximum likelihood mapping: Exploring newtools for comparative genome analyses. BMC Genom. 2002, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Shchepin, O.N.; López, V.Á.; Inoue, M.; Prikhodko, I.S.; Erastova, D.A.; Okun, M.V.; Woyzichovski, J.; Yajima, Y.; Gmoshinskiy, V.I.; Moreno, G.; et al. DNA barcodes reliably differentiate between nivicolous species of Diderma (Myxomycetes, Amoebozoa) and reveal regional differences within Eurasia. Protist 2024, 175, 126023. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, D.V.; Schnittler, M. The phylogeny and phylogenetically based classification of myxomycetes. In Myxomycetes: Biology, Systematics, Biogeography and Ecology; Rojas Alvarado, C., Stephenson, S.L., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2022; pp. 97–124. [Google Scholar] [CrossRef]
- Shchepin, O.; Novozhilov, Y.; Woyzichovski, J.; Bog, M.; Prikhodko, I.; Fedorova, N.; Gmoshinskiy, V.; Borg, D.M.; Dagamac, N.H.A.; Yajima, Y.; et al. Genetic structure of the protist Physarum albescens (Amoebozoa) revealed by multiple markers and genotyping by sequencing. Mol. Ecol. 2022, 31, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Dagamac, N.H.A.; Rojas, C.; Novozhilov, Y.K.; Moreno, G.H.; Schlueter, R.; Schnittler, M. Speciation in progress? A phylogeographic study among populations of Hemitrichia serpula (Myxomycetes). PLoS ONE 2017, 12, e0174825. [Google Scholar] [CrossRef]
- Leontyev, D.V.; Schnittler, M.; Stephenson, S.L. A critical revision of the Tubifera ferruginosa-complex. Mycologia 2015, 107, 959–985. [Google Scholar] [CrossRef]
- Leontyev, D.V.; Buttgereit, M.; Kochergina, A.; Shchepin, O.N.; Schnittler, M. Two independent genetic markers support separation of the myxomycete Lycogala epidendrum into numerous biological species. Mycologia 2023, 115, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, D.V.; Ishchenko, Y.; Schnittler, M. Fifteen new species from the genus Lycogala (Myxomycetes). Mycologia 2023, 115, 524–560. [Google Scholar] [CrossRef] [PubMed]
- Nandipati, S.C.; Haugli, K.; Coucheron, D.H.; Haskins, E.F.; Johansen, S.D. Polyphyletic origin of the genus Physarum (Physarales, Myxomycetes) revealed by nuclear rDNA mini-chromosome analysis and group I intron synapomorphy. BMC Evol. Biol. 2012, 12, 166. [Google Scholar] [CrossRef]
- Cainelli, R.; Haan, M.; Meyer, M.; Bonkowski, M.; Fiore-Donno, A.M. Phylogeny of Physarida (Amoebozoa, Myxogastria) based on the small-subunit ribosomal RNA gene, redefinition of Physarum pusillum s. str. and reinstatement of P. gravidum Morgan. J. Eukaryot Microbiol. 2020, 67, 327–336. [Google Scholar] [CrossRef]
- Ronikier, A.; Janik, P.; Haan, M.; Kuhnt, A.; Zankowicz, M. Importance of type specimen study for understanding genus boundaries-taxonomic clarifications in Lepidoderma based on integrative taxonomy approach leading to resurrection of the old genus Polyschismium. Mycologia 2022, 114, 1008–1031. [Google Scholar] [CrossRef]
- García-Martín, J.M.; Mosquera, J.; Lado, C. Morphological and molecular characterization of a new succulenticolous Physarum (Myxomycetes, Amoebozoa) with unique polygonal spores linked in chains. Eur. J. Protistol. 2018, 63, 13–25. [Google Scholar] [CrossRef]
- Gmoshinskiy, V.I.; Prikhodko, I.S.; Bortnikov, F.M.; Shchepin, O.N.; Schnittler, M. Morphology and phylogeny of Diderma aurantiacum (myxomycetes)-A new species for Russia from the far east. Hobocmu Cucemamuku Hu3wux Oacmehuu 2023, 57, 27–42. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Mitchell, D.W.; Okun, M.V.; Shchepin, O.N. New species of Diderma from Vietnam. Mycosphere 2014, 5, 554–564. [Google Scholar] [CrossRef]
- Hoppe, T.; Schnittler, M. Characterization of myxomycetes in two different soils by TRFLP- analysis of partial 18S rRNA gene sequences. Mushroom Research Foundation. Mycosphere 2015, 6, 216–227. [Google Scholar] [CrossRef]
- Novozhilov, Y.K.; Shchepin, O.N.; Prikhodko, I.; Schnittler, M. A new nivicolous species of Diderma (Myxomycetes) from Kamchatka, Russia. Nova Hedwig. Z. Fur Kryptogamenkd. 2022, 114, 181–196. [Google Scholar] [CrossRef]
- Bortnikov, F.M.; Shchepin, O.N.; Gmoshinskiy, V.I.; Prikhodko, I.S.; Novozhilov, Y.K. Diderma velutinum, a new species of Diderma (Myxomycetes) with large columella and triple peridium from Russia. Bot. Pacifica 2018, 7, 47–51. [Google Scholar] [CrossRef]
- Lado, C.; Treviño, Z.I.; García-Martín, J.M.; Basanta, D.W. Diachea mitchellii: A new myxomycete species from high elevation forests in the tropical Andes of Peru. Mycologia 2022, 114, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Wikmark, O.G.; Haugen, P.; Lundblad, E.W.; Haugli, K.; Johansen, S.D. The molecular evolution and structural organization of group I introns at position 1389 in nuclear small subunit rDNA of myxomycetes. J. Eukaryot Microbiol. 2007, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Janik, P.; Lado, C.; Ronikier, A. Range-wide Phylogeography of a nivicolous protist Didymium nivicola Meyl. (Myxomycetes, Amoebozoa): Striking contrasts between the northern and the southern hemisphere. Protist 2020, 171, 125771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Y.; Liu, S.Y.; Stephenson, S.L.; Hsiang, T.; Qi, B.; Li, Z. Morphological and molecular characterization of the new aethaloid species Didymium yulii. Mycologia 2021, 113, 926–937. [Google Scholar] [CrossRef]
- Fiore-Donno, A.M.; Berney, C.; Pawlowski, J.; Baldauf, S.L. higher-order phylogeny of plasmodial slime molds (Myxogastria) based on elongation factor 1-A and small subunit rRNA gene sequences. J. Eukaryot Microbiol. 2005, 52, 201–210. [Google Scholar] [CrossRef]
- Borg, D.M.; Brejnrod, A.D.; Unterseher, M.; Hoppe, T.; Feng, Y.; Novozhilov, Y.; Sørensen, S.J.; Schnittler, M. Genetic barcoding of dark-spored myxomycetes (Amoebozoa)-Identification, evaluation and application of a sequence similarity threshold for species differentiation in NGS studies. Mol. Ecol. Resour. 2018, 18, 306–318. [Google Scholar] [CrossRef]



| Scientific Name | Voucher/Specimen Numbers | GenBank Accession Numbers | |||
|---|---|---|---|---|---|
| nSSU | EF-1α | COI | |||
| D. clavatocolumellum | HMJAU 11084-1 | PP165417 | PP178585 | PP261313 | This study |
| D. clavatocolumellum | HMJAU 11084-2 | PP165418 | PP982177 | PP261314 | This study |
| D. globosum | HMJAU M20016 | PP165444 | PP178604 | This study | |
| D. radiatum | HMJAU M20026-1 | PP165459 | PP178614 | PP261321 | This study |
| D. radiatum | HMJAU M20026-2 | PP165460 | PP178615 | PP261322 | This study |
| D.shaanxiense | HMJAU M20033 | PP165469 | PP178624 | PP261324 | This study |
| D.shaanxiense | HMJAU M20034 | PP165470 | PP982178 | PP261325 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tuo, Y.; Li, Y.; Hu, J.; Sossah, F.L.; Dai, D.; Liu, M.; Guo, Y.; Zhang, B.; Li, X.; et al. Two New Species of the Genus Diderma (Physarales, Didymiaceae) in China with an Addition to the Distribution. J. Fungi 2024, 10, 514. https://doi.org/10.3390/jof10080514
Li X, Tuo Y, Li Y, Hu J, Sossah FL, Dai D, Liu M, Guo Y, Zhang B, Li X, et al. Two New Species of the Genus Diderma (Physarales, Didymiaceae) in China with an Addition to the Distribution. Journal of Fungi. 2024; 10(8):514. https://doi.org/10.3390/jof10080514
Chicago/Turabian StyleLi, Xuefei, Yonglan Tuo, You Li, Jiajun Hu, Frederick Leo Sossah, Dan Dai, Minghao Liu, Yanfang Guo, Bo Zhang, Xiao Li, and et al. 2024. "Two New Species of the Genus Diderma (Physarales, Didymiaceae) in China with an Addition to the Distribution" Journal of Fungi 10, no. 8: 514. https://doi.org/10.3390/jof10080514
APA StyleLi, X., Tuo, Y., Li, Y., Hu, J., Sossah, F. L., Dai, D., Liu, M., Guo, Y., Zhang, B., Li, X., & Li, Y. (2024). Two New Species of the Genus Diderma (Physarales, Didymiaceae) in China with an Addition to the Distribution. Journal of Fungi, 10(8), 514. https://doi.org/10.3390/jof10080514







