3. Taxonomy
- 1.
Inosperma calamistratum (Fr.) Matheny & Esteve-Rav.;
- 2.
Inosperma gracilentum E. Larss. & Esteve-Rav., sp. nov.;
- 3.
Inosperma neohirsutum Esteve-Rav., Pancorbo & E. Larss., sp. nov.;
- 4.
Inosperma praetermissum (P. Karst.) Esteve-Rav., E. Larss. & Pancorbo comb. nov.;
- 5.
Inosperma subhirsutum (Kühner) Matheny & Esteve-Rav.
- 6.
Inosperma geraniodorum (J. Favre) Matheny & Esteve-Rav.;
- 7.
Inosperma geminum E. Larss. & Vauras, sp. nov.;
- 8.
Inosperma turietoense Pancorbo & Esteve-Rav., sp. nov.;
- 9.
Inosperma veliferum (Kühner) Matheny & Esteve-Rav.
Inosperma calamistratum (Fr.) Matheny & Esteve-Rav., Mycologia 112(1): 94, 2019.
MycoBank No. 830345
≡ Agaricus calamistratus Fr., Systema Mycologicum 1: 256, 1821, nom. sanct. MycoBank No. 239098.
≡ Inocybe calamistrata (Fr.) Gillet, Les Hyménomycètes ou Description de tous les Champignons qui Croissent en France 1: 513, 1876. MycoBank No. 232510.
= Agaricus hirsutus Lasch, Linnaea 4: 546, 1829, nom. sanct. Systema Mycologicum 3 (Index): 23, 1832. MycoBank No. 461481.
= Inocybe hirsuta (Lasch: Fr.) Quél., Mémoires de la Société d’Emulation de Montbéliard 2, 5: 178, 1872. MycoBank No. 191743.
= Inosperma hirsutum (Lasch: Fr.) Matheny & Esteve-Rav., Mycologia 112(1): 103, 2019. MycoBank No. 831768.
Neotype. Sweden, Småland, Femsjö: between moss and grass, not far from the edge of a deciduous forest, 135–170 m alt., 1 September 1979, leg. M. Moser, IBF 19790433, GenBank accession: ITS1 (PP431543), ITS2 (PP431547). Neotype designated by T.W. Kuyper ([
4]: 36). MBT 10016187.
Descriptions and selected iconography. Konrad and Maublanc ([
45], pl 89, 90), Bresadola ([
46], pl 720 Figure 1), Heim ([
47], pl 3 Figure 2), Kühner ([
48]: 200–201)
, Phillips ([
49]: 148), Moser and Jülich ([
50], pl 9 Figure 1), Kuyper ([
4]: 35–36, pro parte), Bon ([
51]: 235), Leisner and Kalamees ([
52]: 102, pl 4), Stangl ([
53]: 54–57, pl 3), Nespiak ([
54]: 20–22), Courtecuisse and Duhem ([
55], n° 1022), Bon ([
5]: 26, pro parte), Courtecuisse ([
56]: 152, 500), Breitenbach and Kränzlin ([
57]: 46–47 n° 8, pro parte), Dähncke ([
58]: 646), Ferrari ([
59]: 54–56, 328 top), Roux ([
60]: 772), Eyssartier and Roux ([
61]: 872), Outen and Cullington ([
62]: 16), Ludwig ([
63]: 203–204, pl 129.18 C-D, pro parte), Laessoe and Petersen ([
64]: 658).
Additional microscopic examination of the neotype of I. calamistrata. Basidiospores (9.0–)9.5–11.5(–11.8) × (4.5–)4.6–5.6(–5.7) µm, Spavg = 10.4 × 5.1 µm, Q = (1.8–)1.83–2.3(–2.6), Qavg = 2.0 (n = 30), smooth, subcylindrical to narrowly ellipsoid in face view, phaseoliform to subphaseoliform in profile, pale greenish glaucous. Basidia clavate, mainly four-spored, 36–40 × 7.5–9.5 µm, with evenly distributed intracellular greenish pigment. Pleurocystidia absent. Lamellar trama pale, hardly pigmented, except for cells with greenish content. Lamellar edge sterile, uniform white. Cheilocystidia tightly packed, subcylindrical to narrowly clavate, (22.1–)26.2–40.1 × 7.2–9.7(–10.2) µm, Chavg = 31.3 × 8.3 µm (n = 16), subcylindrical to subclavate or clavate, with rounded apex, usually two- to three-septate at base, hyaline, thin-walled. Clamp connections present.
Distribution. Based on the data obtained in our study,
I. calamistratum is widespread throughout Europe, as also suggested by Courtecuisse and Duhem [
55], although it is not as common as it has been considered until now. The high number of observations and records of this species may be due to its apparent ease of recognition, based on its colouration and the appearance of the surfaces of the pileus and stipe. Most records reported from the northern boreal to alpine zones do not correspond to
I. calamistratum but probably refer to
I. praetermissum,
I. gracilentum and
I. subhirsutum. Furthermore, records from similar bioclimatic areas may correspond to
I. neohirsutum, with which it is more easily confused.
There are no records in the GenBank and UNITE databases referring to other continents, such as North America and Asia (Matheny, pers. comm.). The only record matching the sequence obtained from the neotype corresponds to AM882938 (EL 19-04, GB 0240829), from Sweden. The deposited sequences matching other similar species, especially I. praetermissum and I. subhirsutum, are more frequent.
Ecology. Inosperma calamistratum occurs in Europe in humid to very moist habitats on poor to rich soils, usually acidic, in both continental and Atlantic climates, in nemoral to montane forests, often along paths. In Sweden, where the species was described, it occurs from the nemoral to boreo-nemoral zone, usually found in mixed conifer-dominated forests of Vaccinium uliginosum type, on granitic bedrock ground, and associated with Betula pendula, Picea abies and Pinus sylvestris. We are not aware of any records from the northern boreal to the alpine zone, where other species occur. In Europe, it is associated and likely forms ectomycorrhizae with both broadleaved trees (Betula, Alnus, Quercus and Fagus) and conifers (Pinus, Picea, Larix and Pseudotsuga) in natural and reforested woodlands (e.g., AH 46636 and ARAN 00235).
Etymology. Derived from the Latin calamister, meaning crisp or curled, in reference to the scaly hirsute or squarrose surface of the pileus and stipe.
Additional specimens examined. France, Corsica, Haute-Corse, Corte, Restonica Valley: 42°15′7.44″ N, 9° 3′39.09″ E, in Pinus nigra subsp. laricio forest in acidic soil, 1130 m alt., 8 November 2019, leg. P.A. Moreau, N. Subervielle & E. Larsson, EL 362-19 (GB 0237702), GenBank accession: ITS-LSU (OR803784). Portugal, Viseu, Moselos: 40°40′55.68″ N, 7°57′29.54″ W, mixed partially reforested forest with Pinus pinaster, Pseudotsuga menziesii, Acacia melanoxylon, Eucalyptus sp. and Quercus robur, in acidic soil, 540 m alt., 7 November 1996, leg. F. Esteve-Raventós, AH 46636, GenBank accession: ITS (PP431514), LSU (PP431535), RPB1 (PP478161). Guarda, Serra do Estrela, Manteigas: 40°25′15″ N, 7°35′23″ W, under Betula alba in acidic soil, 1295 m alt., 10 Nov. 2015, leg. M.A. Ribes, J.F. Mateo, M. Parreño & F. Pancorbo, AH 51034 (dupl. FP 15111006). Spain, Aragón, Valle de Hecho, Selva de Oza: 42°50′6″ N, 0°42′33″ W, under Abies alba and Fagus sylvatica in acidic soil, 1150 m alt., 17 September 2023, leg. F. Pancorbo & F. Esteve-Raventós, AH 58564. Asturias, Allande, San Emiliano: 43°15′53.13″ N, 6°49′33.76″ W, in Quercus suber forest, in acidic soil, 260–270 m alt., 22 December 2020, leg. I. Martín, AH 56397 (dupl. ERD 8644), GenBank accession: ITS (PP431517), LSU (PP431537). Castilla-La Mancha, Guadalajara, Peñalba de la Sierra, ribera del arroyo de Cañamar: 41° 8′43.02″ N, 3°23′23.73″ W, under Alnus glutinosa and Quercus pyrenaica nearby, in acidic soils, 1290 m alt., 14 July 2002, leg. J.P. Campos & J.C. Campos, AH 29995. Castilla y León, Segovia, Valsaín-Puerto de Navacerrada: 40°49′22.86″ N, 4°0′49.90″ W, in Pinus sylvestris forest in acidic soil, 1360 m alt., 22 November 2008, leg. A. Sánchez, AH 40200, GenBank accession: ITS (PP431518), LSU (PP431538), RPB1 (PP478162). Ibidem: 10 October 2015, leg. A. Sánchez, AH 46926. Castilla y León, Zamora, Galende, bank of river Tera: 42°6′55.18″ N, 6°41′10.42″ W, under Alnus glutinosa in acidic soil, 1000 m alt., 19 October 1999, leg. M. Castro-Cerceda, AH 27000, GenBank accession: ITS (PP431515). Galicia, Lugo, Cervantes, Vilarnovo: 42°53′37.11″ N, 6°58′38.87″ W, in humid Castanea sativa forest, in acidic soil, 660 m alt., 4 October 1994, leg. F. Esteve-Raventós, AH 36309, GenBank accession: ITS (PP431513), LSU (PP431534), RPB1 (PP478160), RPB2 (PP478207). Madrid (Community), Rascafría: 40°51′32.67″ N, 3°54′39.53″ W, in boggy soil in Pinus sylvestris forest with Betula alba and Salix atrocinerea, in acidic soil, 1278 m alt., 22 August 2013, leg. F. Pancorbo, AH 44420 (dupl. FP 13082210). Madrid (Community), Cercedilla: under Pinus sylvestris in acidic soil, 22 November 2021, leg. P. Miranda, AH 49307. Navarra (Nafarroa), Areso-Labaki: 43°5′29.42″ N, 1°57′3.52″ W, in a Larix decidua reforested forest, 530 m alt., 30 August 2014, leg. P. Arrillaga, ARAN 00235, GenBank accession: ITS (PP431516), LSU (PP431536). País Vasco (Euskadi), Guipúzcoa (Gipuzkoa), Irún, Peñas de Aia (Aiako Harria): in reforested Larix decidua forest, 350–400 m alt., 18 November 1991, leg. J.M. Lekuona, AH 22169. Sweden, Bohuslän, Resteröd, Ulvesund: along path in mixed coniferous forest under Pinus sylvestris and Betula pendula on acidic soil, 25 July 2004, leg. E. Larsson, EL 19-04 (GB 0240829), GenBank accession: ITS-LSU (AM882938)—as Inocybe calamistrata. Västergötland, Vänersborg, Toltorp: in mixed coniferous forest on acid soil, 16 September 2016, leg. J. Olsson, JO 120916 (GB 0181745), GenBank accession: ITS-LSU (OR803781), RPB2 (PP092166). Västergötland, Trollhättan, Jonstorp: in pasture with conifer and deciduous trees, 14 October 2017, leg. J. Olsson, EL 446-17 (GB 0237701), GenBank accession: ITS-LSU (OR803783), RPB1 (PP092178), RPB2 (PP092167). Västergötland, Sandhult, Sandhults hembyggdgård: in a pasture close to Pinus sylvestris, Betula pendula and Quercus robur on acidic soil, 17 September 2013, leg. E. Larsson, EL 404-13 (GB 0237700), GenBank accession: ITS-LSU (OR803782).
Notes.
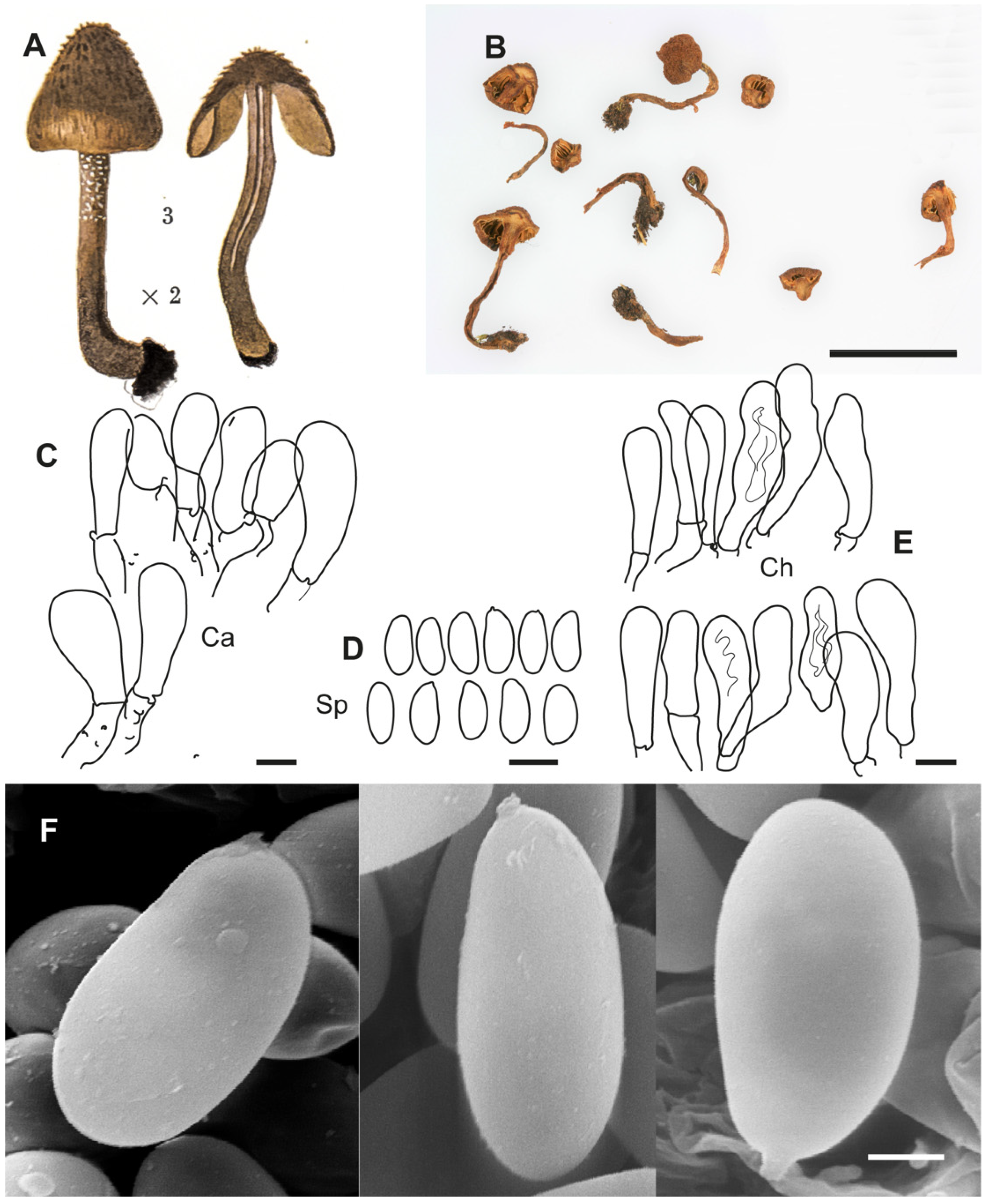
Inosperma calamistratum (≡
Agaricus calamistratus Fr.) is the type of the genus
Inosperma (Kühner) Matheny & Esteve-Rav. [
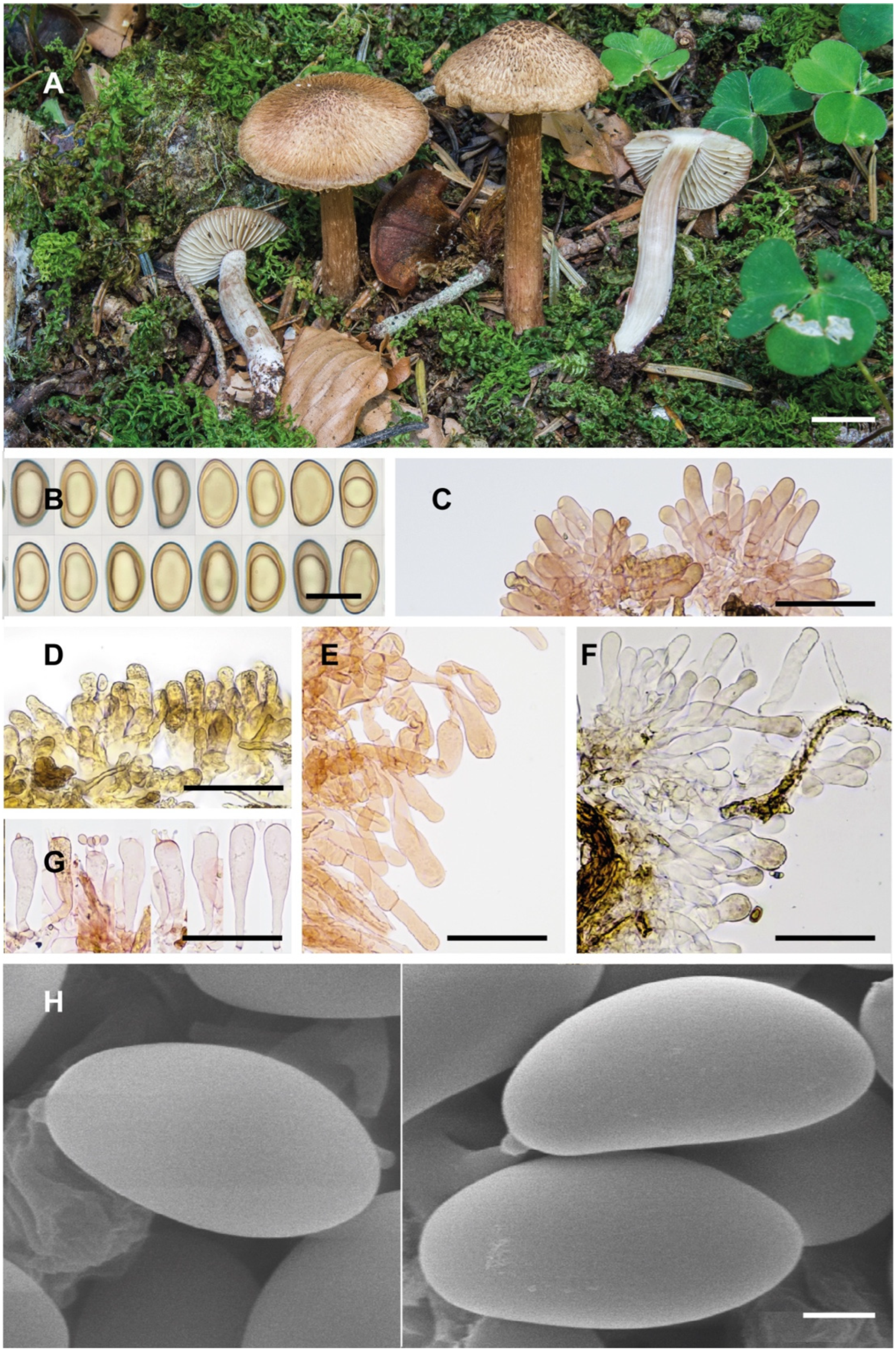
2]. Because of its morphological and ecological peculiarities, it is easy to recognise and has been frequently recorded in Europe. The surface of the pileus and stipe is characteristically scaly–hirsute, even squarrose (
Figure 9A–C). The blue-green colour at the base of the stipe is also very distinctive, although this character can vary with environmental conditions, age and degree of imbibition of the basidiomata. Microscopically, the narrow spores show a marked tendency to be phaseoliform in profile (
Figure 2A,D), and the lamellar edge consists of numerous claviform to subcylindrical, two- to three-septate cheilocystidia, barely longer than 40 µm (
Figure 2C), mixed with some basidia, often greenish pigmented. Its odour is very peculiar and variable, and it has been defined in different ways in the literature, such as rancid, resinous, sour, reminiscent of fish or with a sweet fruity component. In any case, it is not like that of pelargonium, which is also present in other related species, especially those defined here as the Geraniodorum group. Based on the results of our study, we are convinced that
I. calamistratum has often been confused with other species, and its presence in northern boreal to alpine zones seems most likely to be excluded in view of the results obtained. Records from high altitudes and boreo-alpine latitudes correspond to other morphologically very close species that have been identified in the past as ecological forms or variants of
I. calamistratum ([
65]: 77).
On the European continent,
I. calamistratum shares similar or common habitats with
I. neohirsutum, and both occur in moist temperate forests of
Fagaceae (
Fagus,
Quercus and
Castanea), coniferous or mixed. Morphologically they are also similar in appearance, although
I. calamistratum often produces larger basidiomata, with a longer, slender and elastic stipe (30–80 × 2–8 mm), the scales of the pileus and stipe are thinner and often recurved, giving it a hirsute to squarrose appearance, and are usually distributed over the entire surface as they develop. In
I. neohirsutum, the scales are conspicuously aggregated on the central part of the pileus during development, then appear thicker as they tend to fuse and take on a pyramidal appearance. There are also differences in spore Q, and although both have a clear tendency to be phaseoliform in profile,
I. neohirsutum has slightly wider spores with a lower Q (Q
avg 1.7 vs. 2.0). Other species of similar appearance, such as
I. gracilentum,
I. praetermissum and
I.
subhirsutum, show differences in spore shape and size, inhabit different ecosystems and are well-separated phylogenetically (
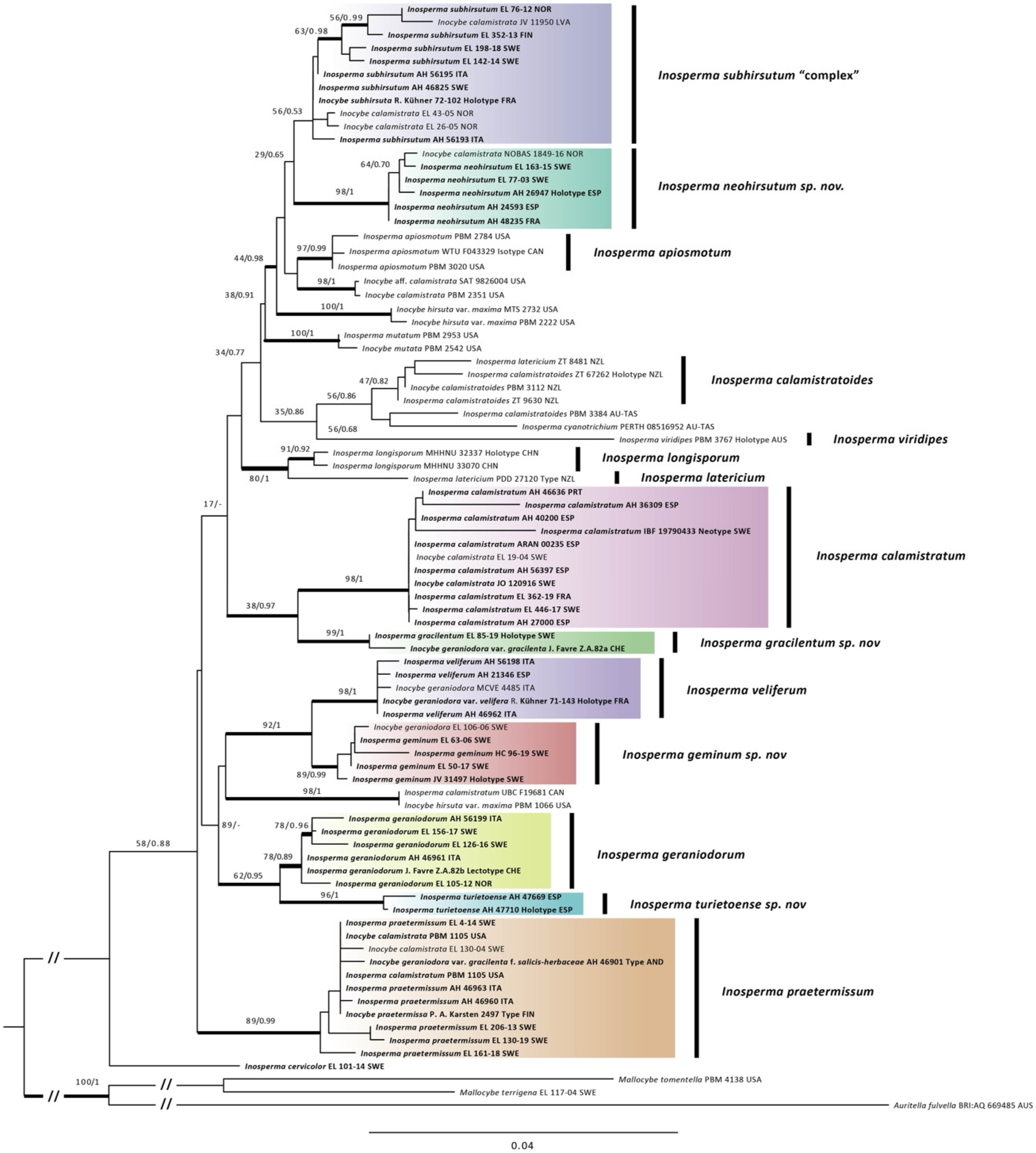
Figure 1).
There are several morphologically similar species in North America, but they are all distinct phylogenetically [
12,
66].
Inosperma mucidiolens (Grund & D.E. Stuntz) Matheny & Esteve-Rav. (=
Inocybe calamistrata var.
mucidiolens Grund & D.E. Stuntz) produces a characteristic odour of green corn, while the basidiomata of
Inosperma apiosmotum (Grund & D.E. Stuntz) Matheny & Esteve-Rav. smell particularly of ripe pears. Other species, such as
Inosperma maximum (A.H. Sm.) Matheny & Esteve-Rav., are characterised by their large, robust size and long stipe (55–120 × 2.5–6 mm).
Also similar in appearance to
I. calamistratum are the species
Inosperma longisporum,
I. squamulosobrunneum and
I. squamulosohinnuleum. These have been recently described from China, from montane coniferous forests in subtropical environments. All three species show macroscopic and microscopic differences from
I. calamistratum and phylogenetic characters closer to North American species than to European species [
21]. Other similar species, especially in pileus and stipe cover, have been recorded from the Australian continent and Southeast Asia, e.g.,
Inosperma calamistratoides (E. Horak) Matheny & Esteve-Rav. and
Inosperma latericium (E. Horak) Matheny & Esteve-Rav., but with different micromorphological characters and rather distant phylogenetically [
7,
67,
68].
Figure 2.
Inosperma calamistratum Neotype IBF 19790433. (A) Basidiospores. (B) Basidia. (C) Cheilocystidia. (D) Spore SEMs. Scale bars: 10 µm (A–C); 2 µm (D).
Figure 2.
Inosperma calamistratum Neotype IBF 19790433. (A) Basidiospores. (B) Basidia. (C) Cheilocystidia. (D) Spore SEMs. Scale bars: 10 µm (A–C); 2 µm (D).
Inosperma gracilentum E. Larss. & Esteve-Rav., sp. nov.
MycoBank No. 850652
= Inocybe geraniodora var. gracilenta J. Favre, Ergebnisse der Wissenschaftlichen Untersuchungen des Schweizerischen Nationalparks 5: 84, 1955. MycoBank No. 346937 (nom. inval., Art. 39.1).
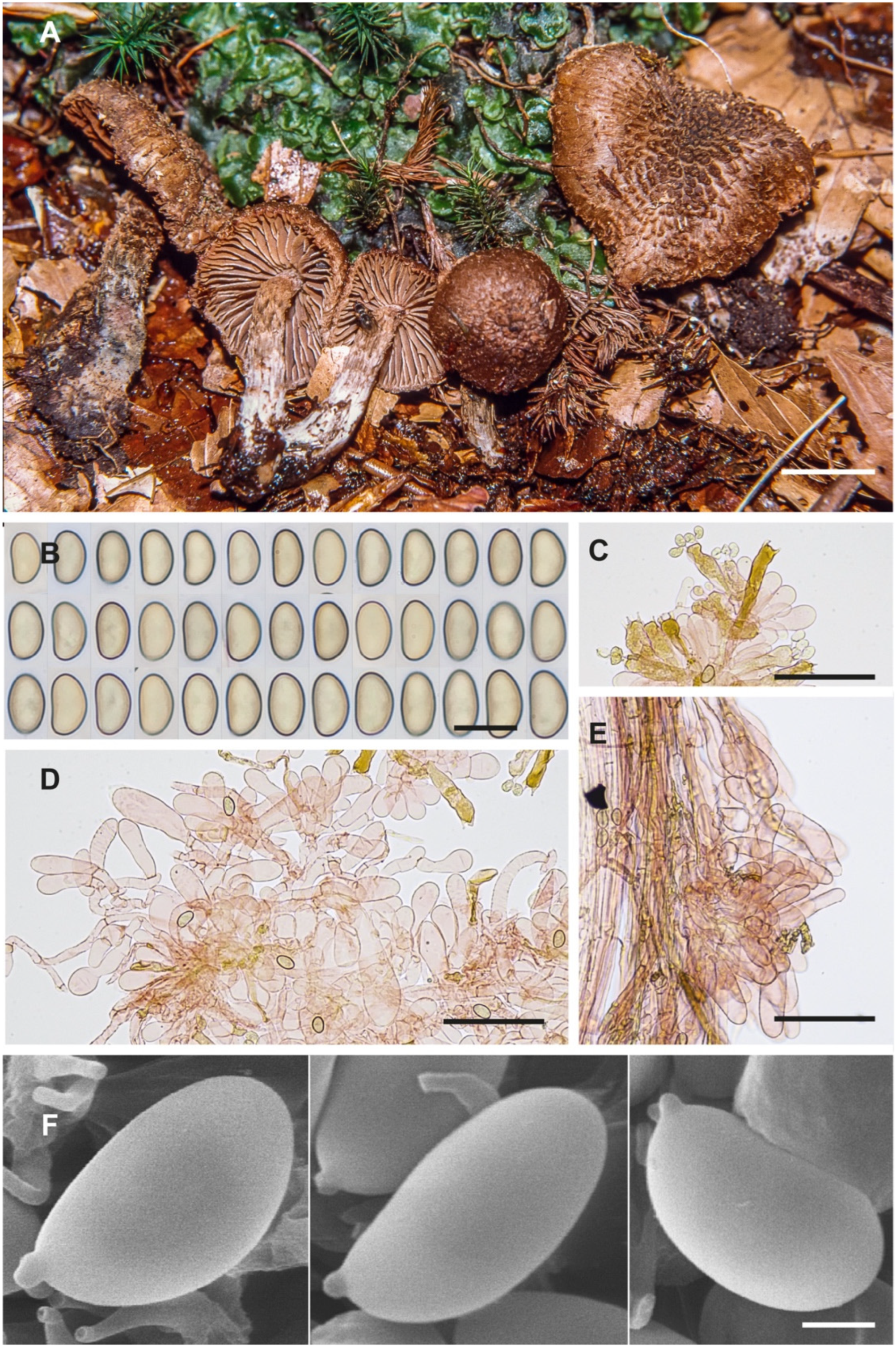
Diagnosis. Inosperma gracilentum differs from other morphologically similar species such as I. praetermissum and I. calamistratum by the long ellipsoid spores, which are slightly concave and sometimes slightly phaseoliform in lateral view, and by the shorter cheilocystidia. It is phylogenetically distinct from all other species of the Calamistratum group.
Holotype. Sweden, Åsele lappmark, Vilhelmina, Klimpfjäll, Frimstjakke: 65°14′42.8″ N, 14°24′06.6″ E, alpine heath with Dryas octopetala, Salix reticulata and Bistorta vivipara, on calcareous ground, 1016 m alt., 22 August 2019, leg. E. Larsson, EL 85-19 (GB 0207620, isotype in AH 56238), GenBank accession: ITS-LSU (OR817726).
Description. Pileus 5–25 mm; when young, hemispherical, conical to obtuse conical with recurved margin; later, conico-convex to plano-convex; surface dry, margin fibrillose to finely scaly, centre of disc scaly to recurved scaly, ochraceous brown to reddish brown, velipellis ochraceous, sometimes not observed or only present in centre of pileus, fugacious.
Lamellae rather sparse, broadly adnate to emarginate (L = 26–40), interspersed with lamellulae, initially pale beige, turning ochraceous brown with age, edge pale fimbriate.
Stipe 15–30 × 1–3 mm, dry, equal to slightly bulbous, at the base bluish green, more ochraceous brown at apex, fibrillose, squamulose, flocculose at apex.
Context ochraceous brown and bluish green at the base of stipe and in the middle of the pileus, more or less reddish on the upper part of the stipe when cut.
Odour distinctly of pelargonium or fishy.
Basidiospores variable, (10.2–)10.8–13.7(–14.4) × 6.0–7.4(–7.7) µm, Sp
avg = 12.1 × 6.5 µm, Q = (1.63–)1.70–2.04(–2.08), Q
avg = 1.8 (n = 67/1), smooth, ochraceous brown, long ellipsoid, some adaxially plane to slightly concave, typically depressed in the supra-apicular region (
Figure 3B,F), with obtuse apex, apiculus small and not distinct.
Basidia (31.2–)34.3–50.6(–52.5) × (9.0–)9.6–12.0(–12.1) µm, Ba
avg = 42.4 × 11.0 µm (n = 15/1), narrowly clavate, four-spored, hyaline.
Pleurocystidia absent.
Cheilocystidia (21.3–)22.7–41.7(–44.6) × (9–)9.6–14.1(–15.9) µm, Ch
avg = 30.4 × 11.8 µm, (n = 25/1), mostly pyriform to clavate, less often subcylindrical, hyaline or full or brownish-green pigment, thin-walled.
Caulocystidia present near apex, like cheilocystidia but generally shorter, 20–42 × 9–16 µm (n = 35/1).
Clamp connections present.
Distribution. Known only from the alpine areas of Europe in Sweden and Switzerland, where its presence is confirmed by molecular data. Its distribution range may be wider; however, the species seems to be rare, and few confirmed collections are known. There are no ITS sequences in GenBank nor in the UNITE database that match or are close to the samples studied.
Ecology. Found growing in the alpine zone on calcareous soils among Salix reticulata, S. retusa, Dryas octopetala and Bistorta vivipara.
Etymology. Refers to the Latin word gracilentus, which means slender, thin.
Additional specimens examined. Switzerland, Grisons, National Park, between Sur il Foss and Alp Minger: 46°42′31″ N, 10°15′31″ E, calcareous soil in a Salix retusa carpet, 2250 m alt., 17 August 1951, Herb. J. Favre Z.A.82a (G 00551725), GenBank accession: ITS1 (PP431544), ITS2 (PP431548).
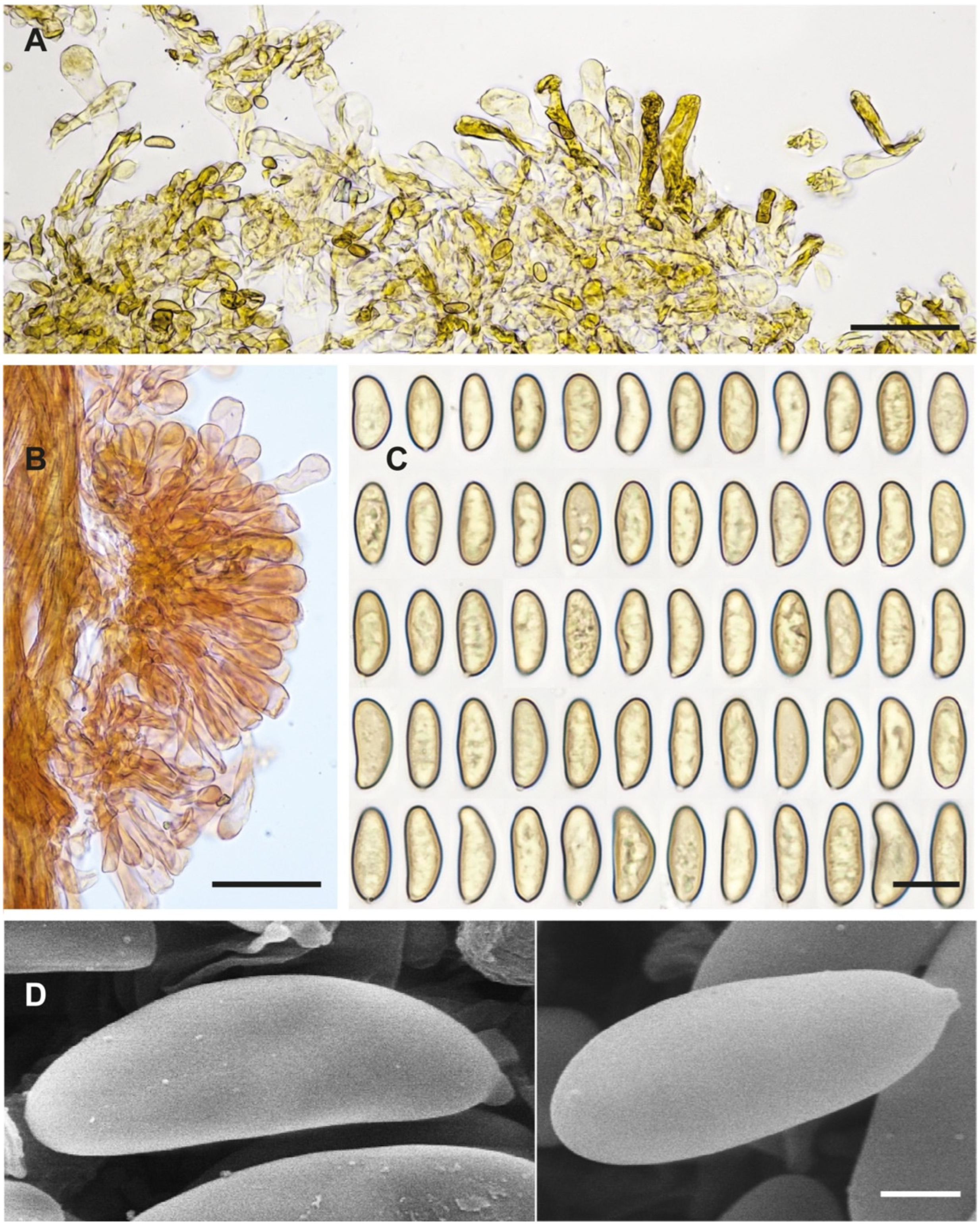
Notes. Favre ([
69]: 84) very briefly described a “varieté gracilenta” for
Inocybe geraniodora, and the voucher collection (
Figure 4) was examined by us (G!). This variety was not validly published by Favre because no Latin diagnosis was given (nom. inval., Art 39.1). Favre’s collection, based on the molecular data obtained, represents the same taxon as the Swedish holotype proposed here. It is a distinct species from
Inosperma geraniodorum.
The holotype includes many specimens that have been examined in detail for their macro- and micromorphological characters, and ITS-LSU sequences were successfully obtained.
Inosperma gracilentum is similar to
I. praetermissum in morphological characters and habitat. It differs in the spores, which are broader with an ellipsoid and more regular outline, often adaxially slightly concave to hardly subphaseoliform, often depressed in the supra-apicular region (
Figure 3B,F). The cheilocystidia are also much shorter in
I. gracilentum, barely exceeding 35(–40) µm in length (
Figure 3D). From the available data, it seems to be a rare species, and to us it is only known from two alpine localities in Europe.
Inosperma praetermissum is more widespread in the Northern Hemisphere in the boreal zone and is also known from North America and Asia.
Inosperma gracilentum can also resemble
I. subhirsutum and the two can easily be confused, but they are genetically distinct (
Figure 1). Both show a different appearance of the pileus and stipe surface, which is often more fibrillose and lanose (“mallocyboid”) in
I. subhirsutum and more hirsute–strigose in
I. gracilentum. Both can be separated in terms of micromorphology by the spore measurements, since
I. gracilentum has narrower spores (Q
avg = 1.8) than
I. subhirsutum (Q
avg = 1.6).
Inosperma subhirsutum has more regular ellipsoid to ovo-ellipsoid spores, often flattened in profile (more reminiscent of the spores of
I. geraniodorum), it has longer cheilocystidia and is collected more frequently and regularly in the alpine zone of the Alps and Fennoscandia.
Figure 3.
Inosperma gracilentum Holotype EL 85-19 (GB 0207620). (A) Basidiomata. (B) Basidiospores. (C) Caulocystidia. (D) Cheilocystidia. (E) Basidia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F).
Figure 3.
Inosperma gracilentum Holotype EL 85-19 (GB 0207620). (A) Basidiomata. (B) Basidiospores. (C) Caulocystidia. (D) Cheilocystidia. (E) Basidia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F).
Figure 4.
Inocybe geraniodora var.
gracilenta Herb. J. Favre Z.A.82a (G 00551725). (
A) Favre ([
69]: pl. VI Figure 3). (
B) Voucher material. (
C) Caulocystidia. (
D) Spores. (
E) Cheilocystidia. (
F) Spore SEMs. Scale bars: 10 mm (
B); 10 µm (
C–
E); 2 µm (
F).
Figure 4.
Inocybe geraniodora var.
gracilenta Herb. J. Favre Z.A.82a (G 00551725). (
A) Favre ([
69]: pl. VI Figure 3). (
B) Voucher material. (
C) Caulocystidia. (
D) Spores. (
E) Cheilocystidia. (
F) Spore SEMs. Scale bars: 10 mm (
B); 10 µm (
C–
E); 2 µm (
F).
Inosperma neohirsutum Esteve-Rav., Pancorbo & E. Larss., sp. nov.
MycoBank No. 850656
Diagnosis. Inosperma neohirsutum is similar to I. calamistratum, but differs from it due to its smaller size, less coriaceous flesh and a strongly hirsute scaly pileus on the central disc, which, as it develops, forms a characteristic squarrose patch of thick recurved, agglutinated and welded scales, which contrasts sharply with the fibrillose margin. The spores are also broader (Qavg = 1.8). The closest ITS sequences in BLAST correspond to the American species Inosperma apiosmotum (Grund & D.E. Stuntz) Matheny & Esteve-Rav. with 94% similarity.
Holotype. Spain, Castilla y León, Segovia, Riofrío de Riaza, Puerto de La Quesera, Hayedo de La Pedrosa: 41°12′59.59″ N, 3°24′26.70″ W, in very humid soil among mosses in Fagus sylvatica forest, on acid granitic soil, 1700 m alt., 1 September 2001, leg. F. Esteve-Raventós & M. Villarreal, AH 26947 (isotype in GB: 0266843), GenBank accession: ITS (PP431510), LSU (PP431532).
Description. Pileus 15–25(–30) mm; when young, hemispherical convex to campanulate, sometimes obtusely conical, with deflexed, wavy margin; later, conico-convex to plano-convex, sometimes with a slightly depressed centre; dry, not hygrophanous, margin crenate; surface fibrillose–lacerate to finely scaly towards the edge, strongly scaly and hirsute–squarrose on the disc, formed by clusters of recurved concolourous scales, these often aggregated and coarse in the centre, sometimes appearing as pyramidal aggregates with age. Colour uniformly brown to chocolate brown on a lighter background as the surface breaks into scales; velipellis not observed. Lamellae moderately dense (L = 30–40), narrowly adnate to emarginate, subventricose, interspersed with numerous lamellulae l = 1–2(–3), initially pale beige, turning ochraceous brown with age, becoming concolourous to pileus, edge pale, fimbriate. Stipe 15–35(–40) × (2.5–)3–5 mm, dry, cylindrical, tapering upwards to subclavate; with age, brown to concolourous to pileus, usually bluish green at base but sometimes absent or very pale, apex ochraceous to brown; surface regularly squamulose to squarrose, covered with concolourous recurved scales, apex only flocculose. Context pale ochraceous to buff, hardly greenish to glaucous at the base of stipe, slightly reddening on the upper part of the stipe when cut. Odour sometimes faint, aromatic, fruity–acidic, sometimes with a scent of pelargonium or fish. Basidiospores (8.2–)9.0–10.9(–12.0) × (4.5–)5–6.1(–6.3) µm, Spavg = 9.9 × 5.5 µm, Q = (1.54–)1.61–2.01(–2.23), Qavg = 1.8 (n = 307/3), smooth, ellipsoid, phaseoliform in profile, ochraceous brown. Basidia 36.1–50.0(–56.4) × 9.1–13.7(–14.1), Baavg = 42.3 × 11.0 µm, clavate, mainly four-spored, hyaline, often with intracellular brown pigment. Pleurocystidia absent. Cheilocystidia rather short, (16.5–)23.3–53.0(–68.2) × (8.3–)8.9–15.9(–17.5) µm, Chavg = 35.2 × 12.0 µm (n = 133/3), mostly broadly clavate to spheropedunculate, less often subclavate to subcylindrical, sometimes subcapitate, usually one- to two-septate at the base and then resembling the Opuntia-like arrangement of cheilocystidia in certain Mallocybe spp. Caulocystidia present near stipe apex, grouped in clusters, similar to cheilocystidia, (19.3–)19.6–42.0–65.2(–67.1) × (6.6–)7.6–11.2–15.2(–17.1) µm, Caavg = 42.0 × 11.2 µm, (n = 28/2). Clamp connections present.
Distribution. The distribution of I. neohirsutum on the European continent is still unclear. It is very likely that some European records of I. calamistratum represent I. neohirsutum. To date, its presence has been confirmed in France, Norway, Sweden and Spain. However, according to the available distribution data and habitat preferences, it is likely to be widespread throughout the continent, thriving in humid forests in mainly temperate to mild climates of continental, Atlantic and hemiboreal type. It is unlikely that it will be found in colder climates of the northern boreal and alpine zones. There is only one sequence available matching I. neohirsutum, in the UNITE database, from Arendal, Norway, collected under Quercus and Corylus (UDB07673483/NOBAS 1849-16, as Inocybe calamistrata).
Ecology. It grows in both moist broadleaved forests of Fagaceae (Fagus and Quercus) and Betulaceae (Corylus) and mixed conifer-dominated forests, either in acidic or calcareous soils.
Etymology. From Greek néos, meaning new, and Latin hirsus, meaning hirsute, hairy, shaggy. Refers to a new or different interpretation of Inocybe hirsuta.
Additional specimens examined. France, Nouvelle-Aquitaine, Pyrénées-Atlantiques, Osse-en-Aspe, Forêt d’Issaux: 42°59′44″ N, 0°41′51″ W, in very humid soil among mosses in Fagus sylvatica forest in calcareous soil, 1044 m alt., 13 October 2018, leg. F. Pancorbo, AH 48235 (dupl. FP 18101301), GenBank accession: ITS (PP431512), LSU (PP431533). Spain, Castilla y León, Segovia, Riofrío de Riaza, Puerto de La Quesera, Hayedo de La Pedrosa: 41°12′59.59″ N, 3°24′26.70″ W, in very humid soil among mosses in Fagus sylvatica forest, on acid, granitic soil, 1700 m alt., 9 September 1986, leg. F. Esteve-Raventós, G. Moreno & C. Illana, AH 09624. Ibidem: 1 October 1989, leg. F. Esteve-Raventós & A. Altés, AH 18865. Ibidem: 16 October 1996, leg. F. Esteve-Raventós & M. Villarreal, AH 21333. Ibidem: 22 September 1993, leg. F. Esteve-Raventós & M. Heykoop, AH 22146. Ibidem: 14 September 1990, leg. F. Esteve-Raventós, G. Moreno & M. Heykoop, AH 24593, GenBank accession: ITS (PP431511). Ibidem: 27 August 1995, leg. P. Juste, AH 24959 (dupl. in Herb. Asociación Micológica de Tudela de Duero n° 1180). Sweden, Västergötland, Östad, Risveden, Långevattnet: in mixed coniferous forest close to Pinus sylvestris and Betula pendula on acidic soil, 20 September 2015, leg. E. Larsson, EL 163-15 (GB 0207661), GenBank accession: ITS-LSU (OR831119), RPB2 (PP092169). Västergötland, Ödenäs, close to the church: in mixed coniferous forest on acidic soil, 17 October 2003, leg. E. Larsson, EL 77-03 (GB 0150442), GenBank accession: ITS-LSU (AM882945)—as Inocybe cf. calamistrata.
Notes.
Agaricus hirsutus was described by Lasch [
70] with a succinct description that could apply to both
I. calamistratum and
I. neohirsutum. The habitat is noted as “in fagetis humidis”, a habitat that may support both species. Kuyper [
4] considered
Inocybe hirsuta to be a synonym of
I. calamistrata, a treatment with which we agree, since it is impossible to separate the two species with the available data from the protologues. It is very likely that among the numerous records of
I. calamistratum in Europe, some of them could correspond to
I. neohirsutum. In our study, molecular analysis has confirmed the existence of two distinct species with rather similar morphological characters.
One of the macroscopic differences between the two species mostly lies in the size of the basidiomata.
Inosperma calamistratum often produces larger basidiomes, with a normally long, fibrous and elastic stipe, whereas
I. neohirsutum is smaller, with a more fragile stipe (
Figure 9E–H). It is not improbable to assume that Lange’s [
71] interpretation of
Inocybe calamistrata f.
gracilis J.E. Lange (nom. inval., art. 36.1) may correspond, at least in part, to
I. neohirsutum. However, a studied collection (AH 58564) under
Abies and
Fagus from Aragón (Spain) showed a small size reminiscent of f.
gracilis and is in molecular agreement with
I. calamistratum. In these cases, to separate
I. calamistratum and
I. neohirsutum, it is necessary to analyse other morphological characters, such as the appearance of the pileus surface, the cheilocystidia, and the spore shape and size.
Inosperma neohirsutum is probably not an uncommon species from the humid, temperate forests of Europe, in both deciduous and coniferous forests. So far, it has been overlooked or probably misinterpreted as a smaller form of
I. calamistratum. It has a very peculiar macroscopic character, namely, the squarrose aspect (reminiscent of
Inocybe hystrix, for example) on the central disc of the pileus, which contrasts sharply with the more fibrillose to subsquamose margin, especially in adult specimens when the pileus is fully extended (
Figure 5A and
Figure 9E–H). In
I. calamistratum, the scales are very abundant, dense, sharp, thinner and mostly distributed over the whole surface of the pileus. The shape of the spores also differs between the two species, and although phaseoliform in both, this characteristic is somewhat less pronounced in
I. neohirsutum (
Figure 5B,F), where the Q
avg is smaller (1.8 vs. 2.0). The trend in the morphology of the cheilocystidia of the two species also appears to be different, being narrower and subcylindrical in
I. calamistratum. Because of its habitat,
I. neohirsutum cannot be confused with other similar northern boreal and alpine species, such as
I. gracilentum,
I. subhirsutum and
I. praetermissum.
In our phylogenetic analysis,
I. neohirsutum appears to be closely related to
I. subhirsutum, which has a lower Q
avg of the spores, showing an ovoid to broadly ellipsoid outline, and to the North American species
Inosperma apiosmotum (Grund & D.E. Stuntz) Matheny & Esteve-Rav., which is also small to medium in size and emits a typical odour of ripe pears. Grund and Stuntz [
72] already noted in their observations its resemblance to
Inocybe calamistrata and
I. hirsuta.
Inosperma apiosmotum and
I. neohirsutum show similarities in the arrangement of scales on the pileus ([
72],
Figure 2 and
Figure 6) and in microscopic characters, such as the shape and size of spores and cheilocystidia.
Figure 5.
Inosperma neohirsutum (Holotype AH 26947 for (A–E), AH 48235 for (F)). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Cheilocystidia. (E) Caulocystidia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F).
Figure 5.
Inosperma neohirsutum (Holotype AH 26947 for (A–E), AH 48235 for (F)). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Cheilocystidia. (E) Caulocystidia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F).
Inosperma praetermissum (P. Karst.) Esteve-Rav., E. Larss. & Pancorbo, comb. nov.
MycoBank Transfer No. 850526
≡ Inocybe praetermissa P. Karst., Meddelanden af Societas pro Fauna et Flora Fennica 11: 3, 1885. MycoBank No. 239808
= Inocybe geraniodora var. gracilenta f. salicis-herbaceae Bon & Ballarà, Revista Catalana de Micologia 19: 145, 1996. MycoBank No. 446932
Holotype. Finland, Tavastia Australis, Tammela, Mustiala: under Pinus on a roadside, 30 Aug, 1867, Herb. P.A. Karsten 2497 (H), GenBank accession: ITS2 (PP431551).
Description. Karsten [
73], Breitenbach and Kränzlin ([
57], photo as
Inocybe calamistrata, no. 8), Jamoni ([
74], as
Inocybe calamistrata), Bon and Ballarà ([
75], as
Inocybe geraniodora var.
gracilenta f.
salicis-herbaceae), Armada et al. ([
76], as
Inosperma cf.
calamistratum).
Additional microscopic examination of the holotype of I. praetermissum. The details given are mainly based on T.W. Kuyper’s revision and annotations in 1984 and our supplementary information. Caulocystidia were not examined due to the scarcity of material. Basidiospores (10.6–)11.2–13.1(–14.2) × 5.1–5.7(–6.0) µm, Spavg = 12.2 × 5.3 µm, Q = (1.9–)2.1–2.6), Qavg = 2.2 (n = 30), [Kuyper: (10.0–)10.5–12.0(–13.0) × 5.0–5.5(–6.0) µm, Q = (1.9–)2.0–2.4(–2.6)] smooth, thin-walled, ellipsoid, subphaseoliform in profile, yellowish greenish. Basidia clavate, mainly four-spored, hyaline. Pleurocystidia absent. Cheilocystidia (30–)35–55(–60) × 8–11 µm, cylindrical, subclavate or clavate, sometimes flexuose, with rounded apex, hyaline, thin-walled. Clamp connections present.
Additional microscopic examination of the holotype of I. geraniodora var. gracilenta f. salicis-herbaceae. Basidiospores (10.6–)11.6–14.5(–15.8) × (4.8–)5.0–6.2(–7.0) µm, Spavg = 13.2 × 5.5 µm, Q = (1.92–)2.05–2.68(–2.94), Qavg = 2.3 (n = 92), smooth, thin-walled, elongated ellipsoid to bacilliform, subphaseoliform in profile. Basidia clavate, predominantly four-spored. Pleurocystidia absent. Cheilocystidia (44.3–)45.8–63.5(–63.9) × (9.5–)10.4–16.6(–16.7) µm, Chavg = 54.8 × 13.0 µm (n = 20), cylindrical, subclavate, with rounded apex, with brown-greenish pigment, thin-walled. Caulocystidia present near apex, (51.2–)51.9–68.2 × (10.8–)11.5–16.0(–16.8) µm, Caavg = 60.2 × 14.3 µm (n = 13) clustered, cheilocystidia-like. Clamp connections present.
Distribution. The sequenced collections studied by us and those deposited in GenBank suggest a wide distribution of I. praetermissum on the European continent, always in cold bioclimates, whether boreal–alpine, subalpine, altimontane or hemiboreal. Its distribution ranges from Greenland and the Nordic countries to the Pyrenees as the southernmost limit. In addition to the sequences from Finland (where it was originally found), the Alps and the Pyrenees, there are other sequences deposited in GenBank from the Swiss Alps (MK838291 and MT095693). It also occurs in cold mountainous areas of the northeastern Czech Republic (OM793002) in the Giant Mountains, under Picea (M. Vasutova, pers. comm.). In North America, I. praetermissum also seems to occur in similar boreal-alpine ecosystems and montane coniferous forests (JQ801386 and OQ701112). Several sequences (clones) from alpine meadows in China with the presence of Polygonum and Kobresia (FJ827203, FJ378766 and OL850876) may also correspond to I. praetermissum.
Ecology. On both calcareous and acidic soils, under conifers (Pinus, Picea, Abies and Tsuga) and Betula pubescens in montane–subalpine and hemiboreal areas, and in the alpine zone with shrubs or herbaceous plants (Salix herbacea, S. retusa, S. lapponum, Dryas spp., Betula nana, Bistorta vivipara and Kobresia).
Etymology. From the Latin praetermissus, meaning overlooked, neglected.
Additional specimens examined. Andorra, Arcalís: in alpine area dominated by Salix herbacea, 2350 m alt., 26 August 1995, leg. J. Ballarà, holotype of I. geraniodora var. gracilenta f. salicis-herbaceae, JB 1620/95 (isotype in AH 46901), GenBank accession: ITS1 (PP431546), ITS2 (PP431550). Italy, Trentino-Alto Adige, Passo Venegiotta, at the foot of Cima Mulaz: 46°19′35″ N, 11°49′38″ E, in calcareous Salix retusa and S. reticulata scrub, 2314 m alt., 30 August 2005, leg. E. Bizio, EB 2005083005 (dupl. AH 56197). Trentino-Alto Adige, Parco di Paneveggio, Pale di San Martino, Malga Juribrutto: 46°19′36″ N, 11°46′51″ E, in Picea abies forest, on quartz–porphyry calcareous soil, 1800 m alt., 17 August 2019, leg. E. Bizio, EB 2019081705 (dupl. AH 46960), GenBank accession: ITS (PP431527). Trentino-Alto Adige, Parco di Paneveggio, Baita Segantini, Passo Rolle: 46°17′53″ N, 11°48′18″ E, in calcareous alpine scrubland, 2200 m alt., 30 August 2019, leg. E. Bizio, EB 2019083003 (dupl. AH 46963), GenBank accession: ITS (PP431528). Trentino-Alto Adige, Pale di Gerda: 43°32′17″ N, 11°58′48″ E, in calcareous alpine scrubland, 2250 m alt., 24 August 2019, leg. R.J. Ferrari, AH 46977 (dupl. FRJ 036-2019). Spain, Cataluña (Catalonia), Girona, Vall de Núria, ras de l’Ortigar: 42°23′46″ N, 2°08′40″ E, in calcareous alpine scrubland under Dryas octopetala and Salix retusa, 2220 m alt., 10 August 1999, leg. J. Vila, JVG 990810-3 (dupl. AH 26722). Sweden, Bohuslän, Grinneröd, Norra fjället: in mixed forest close to Picea abies and Corylus avellana on acid soil, 6 July 2014, leg. E. Larsson, EL 4-14 (GB 0243035), GenBank accession: ITS-LSU (OR831122). Bohuslän, Uddevalla, Kuröds skalgrusbankar: under Betula pendula and Corylus avellana, 2 October 2004, leg. E. Larsson, EL 130-04 (GB 0240811), GenBank accession: ITS-LSU (AM882944)—as Inocybe calamistrata. Bohuslän, Resteröd, Ulvesund, Grinddalen: in mixed coniferous forest close to Picea abies and Corylus avellana on acid soil, 2 October 2004, leg. E. Larsson, EL 139-04 (GB 0240820). Torne lappmark, Jukkasjärvi, Abisko, along Rakkasjokka: alpine heath with Salix herbacea and Bistorta vivipara, 24 August 2013, leg. J. Vauras, EL 206-13 (GB 0243036), GenBank accession: ITS-LSU (OR831121), RPB1 (PP092180), RPB2 (PP092171). Åsele lappmark, Vilhelmina, Fiehteres: alpine heath, snowbed area with Salix herbacea, 1070 m alt., 21 August 2019, leg. E. Larsson, EL 70-19 (GB 0243039), GenBank accession: ITS-LSU (OR831123). Pite lappmark, Arjeplog, northeast side of Ákharis: alpine heath with Salix spp. and Betula nana, 14 August 2018, leg. J. Vauras, EL 161-18 (GB 0243037), GenBank accession: ITS-LSU (OR831120), RPB2 (PP092170). Jämtland, Frostviken, Raavre: alpine heath, moist with Salix herbacea and Bistorta vivipara, 800 m alt., 23 August 2019, leg. J. Vauras, EL 130-19 (GB 0243038), GenBank accession: ITS-LSU (OR831124).
Notes.
Inosperma praetermissum is morphologically similar to
I. calamistratum,
I. gracilentum and
I. subhirutum. After the revision of Karsten’s type, Kuyper [
4] considered
I. praetermissum to be a synonym of
I. calamistratum. However, the molecular study has allowed for a distinction between the two species.
Inosperma praetermissum is relatively common in the hemiboreal, subalpine and alpine areas of the Northern Hemisphere and has probably been interpreted as a form or ecological variant of
I. calamistratum. The latter prefers more temperate and very humid ecosystems, either continental or Atlantic, either mesophilic or montane, but the two co-occur in the hemiboreal zone in Fennoscandia. Apart from being genetically different,
I. praetermissum can be separated, as it is smaller and more fragile in appearance than
I. calamistratum.
Inosperma praetermissum shows a clear tendency to have a pileus not as squarrose as that of
I. calamistratum, with paler, woolly–fibrillose and looser scales that are not as prominent or as sharply defined, especially in the centre of the pileus (
Figure 10A–C). Also often observed is the presence of a persistent ochraceous veil, resembling a fibrillose covering. In addition, the cheilocystidia are slightly longer than those of
I. calamistratum, with a more elongated claviform outline, usually reaching 60 µm in length (
Figure 6D). Care should be taken when observing the blue-greenish colour of the stipe and when perceiving the odour, as both can sometimes go unnoticed or blurred, especially if the specimens are old or soaked after heavy rain. Micromorphologically, the spores (
Figure 6C,E) are somewhat similar in appearance to those of
I. calamistratum, being subphaseoliform to phaseoliform in profile, although they are longer on average and often appear narrowly phaseoliform (12.2 × 5.3 µm vs. 10.4 × 5.1 µm; Q
avg = 2.2 vs. 2.0). The odour of
I. praetermissum was described by Karsten as unpleasant (“inamoenus”) and strong (“gravis”) and has been noticed in some specimens as smelling like fish brine.
Bon [
5] mentioned the possible presence (most probably in the Alps) of
Inocybe praetermissa (s.str. P. Karst.) at subalpine levels under conifers and
Vaccinium bushes, to which he attributes a pileus surface not as hirsute as in
I. calamistratum, an odour that sometimes eventually develops into that of
I. cervicolor (earthy–mouldy), and elongated, narrow spores 12–14(–15) × 5–6(–7) µm. This interpretation agrees with the data obtained in the collections studied.
Inosperma gracilentum, which also occurs in alpine and boreal areas, can be confused with I. praetermissum, but the former is distinguished by its molecular characteristics, shorter cheilocystidia barely exceeding 40 µm in length, and the more ellipsoidal and somewhat broader spores (Spavg 12.2 × 5.3 µm, Qavg = 2.2 vs. Spavg 12.1 × 6.5 µm, Qavg = 1.8).
Inosperma subhirsutum collections growing in hemiboreal mixed Alnus forests and in subalpine Betula forests often have larger basidiomata than the average for the alpine zone, and they can then be confused in their macromorphology with I. pratermissum, but the two are clearly separated by spore morphology.
DNA extraction was successful in the holotype of
Inocybe geraniodora var.
gracilentum f.
salicis-
herbaceae, despite its poor condition. Based on the characters described in the protologue and on the study of the holotype (
Figure 7), we consider it a synonym of
I. praetermissum. The holotype of
I. praetermissum shows slightly smaller and narrower spores (L/l
avg 12.2 × 5.3 vs. 13.4 × 5.7; Q
avg 2.2 vs. 2.3), although the other characters overlap or coincide in both. As in the case of
I. geraniodora var.
gracilenta f.
salicis-herbaceae, the shapes of the spores of
I. praetermissum are narrowly subphaseoliform in profile, and the cheilocystidia share similar dimensions and morphology. Finally, the phylogenetic study (ITS) indicates that both taxa are cospecific. The odour was also described as unpleasant and fishy.
Figure 6.
Inosperma praetermissum Holotype Herb. P.A. Karsten 2497 (H). (A,B) Voucher material and label. (C) Basidiospores. (D) Cheilocystidia. (E) Spore SEM. Scale bars: 10 mm (A); 10 µm (C,D); 2 µm (E).
Figure 6.
Inosperma praetermissum Holotype Herb. P.A. Karsten 2497 (H). (A,B) Voucher material and label. (C) Basidiospores. (D) Cheilocystidia. (E) Spore SEM. Scale bars: 10 mm (A); 10 µm (C,D); 2 µm (E).
Figure 7.
Inocybe geraniodora var. gracilenta f. salicis-herbaceae Holotype JB 1620/95. (A) Cheilocystidia. (B) Caulocistydia. (C) Basidiospores. (D) Spore SEMs. Scale bars: 50 µm (A,B); 10 µm (C); 2 µm (D).
Figure 7.
Inocybe geraniodora var. gracilenta f. salicis-herbaceae Holotype JB 1620/95. (A) Cheilocystidia. (B) Caulocistydia. (C) Basidiospores. (D) Spore SEMs. Scale bars: 50 µm (A,B); 10 µm (C); 2 µm (D).
Inosperma subhirsutum (Kühner) Matheny & Esteve-Rav., Mycologia 112(1): 105, 2019.
MycoBank No. 830402
≡ Inocybe subhirsuta Kühner, Documents Mycologiques 19(74): 25, 1988. MycoBank No. 135080
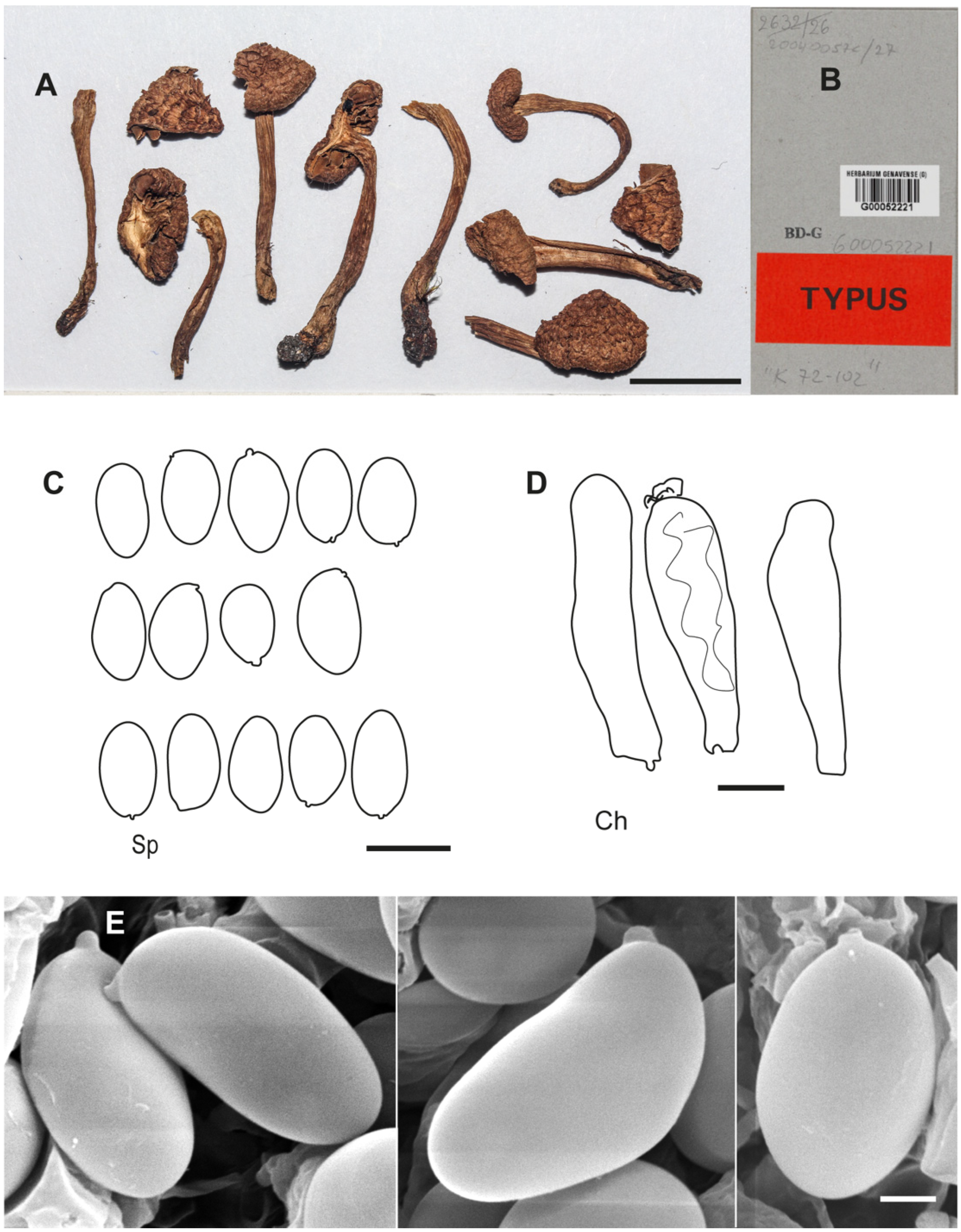
Holotype. France, Savoie, Parc National de la Vanoise, Haute Maurienne, Plan des Évettes: 45°21′48.93″ N, 7°6′43.04″ E, under Salix reticulata in calcareous soil, 2500 m alt., 12 August 1972, Herb. R. Kühner 72-102 (G 00052221), GenBank accession: ITS (PP065739).
Description. Kühner [
77], Bon [
65].
Additional microscopic examination of the holotype of I. subhirsutum. Basidiospores 10.7–13.0(–13.7) × 6.2–7.8 µm, Spavg = 11.8 × 7.0 µm, Q = (1.44–)1.46–1.85, Qavg = 1.6 (n = 21), smooth, thin-walled, variable in shape, ovo-ellipsoid to ovo-subamygdaliform, broadly ellipsoid to ellipsoid, often somewhat flattened in profile view, rarely subphaseoliform. Basidia clavate, mainly four-spored, hyaline, often with greenish-brown pigment. Hymenial trama greenish brown. Pleurocystidia absent. Gill edge heterogeneous. Cheilocystidia (35–)37.8–45.3 × 9.1–13.0 µm, Chavg = 41.1 × 11.1 µm (n = 3), cylindrical, narrowly clavate to clavate, with rounded apex, hyaline, often with olive greenish content, thin-walled. Caulocystidia present near apex, grouped in clusters, cheilocystidia-like. Clamp connections present.
Distribution. Inosperma subhirsutum was first found in France in the alpine zone. It is more than likely that the species thrives at high altitudes in the Alps, as we have found by studying several samples from Italy. It is logical to assume its presence in other European countries with alpine ranges, such as Switzerland, Austria, etc., of which we have no records to date. Its presence in the Pyrenees is not known yet. According to the data collected by Jacobsson and Larsson [
78], the species seems to be frequent and widely distributed in the boreal and alpine zones of Fennoscandia and Iceland. This is confirmed by several collections with sequences deposited both in GenBank (AM882946 and AM882947) and the UNITE database (UDB001195 and UDB07673483).
Ecology. In Europe, it grows in alpine, subalpine and boreal areas, mainly on calcareous soils, but also sometimes on neutral or slightly acidic soils. It occurs mainly in communities of various shrubs and small Salicaceae, sometimes mixed with Betula nana or Bistorta vivipara. In calcareous soils, it is most often associated with Salix reticulata, S. retusa, S. glauca and Dryas octopetala. It can also be found with Salix herbacea on soils with a more acidic component or of a sandy nature due to soil washing. It is known to occur in the Alps in subalpine areas (e.g., in Lago Dobbiaco, Italy, EB 20140807), close to Salix near watercourses in coniferous forests (Picea). In boreal areas, it can also be found in coniferous vegetation, associated with Salix, or in mixed forests with Populus tremula, Alnus incana and Betula pubescens (GB 0243043). The ectomycorrhizal relationship of I. subhirsutum with Salicaceae especially seems obvious, as well as its localisation in hemiboreal, subalpine and alpine zones.
Etymology. From the Latin sub-, meaning under, beneath, behind and near, and hirsus (variant of hirtus), meaning shaggy, rough, hairy, referring to the appearance of the pileus.
Additional specimens examined. Finland, Ostrobottnia ultima, Rovaniemi, Kylmäoja: moist area with Populus tremula, Betula pubescens and Alnus incana, 200 m alt., 3 September 2013, leg. E. Larsson, EL 352-13 (GB 0243043), GenBank accession: ITS-LSU (OR817728). Italy, Piemonte, Vercelli, Alagna Valsesia, Monte Rosa, Conca delle Pisse: 45°52′53.8″ N, 7°53′2″ E, in acidic soil under Salix herbacea shrub, 2515 m alt., 26 August 1992, leg. E. Bizio, EB 1992082601 (dupl. AH 56195), GenBank accession: ITS (PP431507), LSU (PP431530). Trentino-Alto Adige, Bolzano, Dobbiaco, Lago di Dobbiaco: 46°41′46″ N, 12°13′12″ E, in calcareous, sandy soil, among mosses on the edge of a torrent near Salix sp. and Picea abies, 1260 m alt., 7 August 2014, leg. E. Bizio, EB 2014080711 (dupl. AH 56193), GenBank accession: ITS (PP431509), LSU (PP431531). Norway, Sør-Trøndelag, Oppdal, Dovre, Kongsvoll: moist area with Betula nana and Salix reticulata on calcareous soil, 19 August 2012, leg. E. Larsson, EL 76-12 (GB 0243034), GenBank accession: ITS-LSU (OR817729), RPB2 (PP092163). Hordaland, Ulvik, Finse, Blåisen: snowbed area with and Salix herbacea, 1375 m alt., 11 August 2005, leg. E. Larsson, EL 26-05 (GB 0248023), GenBank accession: ITS2-LSU (AM882946), RPB1 (PP092179), RPB2 (PP092165)—as Inocybe calamistrata. Hordaland, Ulvik, Finse, Sandalsnut: alpine meadow on calcareous soil, with Dryas octopetala and Salix reticulata, 12 August 2005, leg. E. Larsson, EL 43-05 (GB 0248040), GenBank accession: ITS-LSU (AM882947)—as Inocybe calamistrata. Sweden, Jämtland, Åre, Mt Åreskutan BaseCamp: 63°25′42.78″ N, 13°4′45.04″ E, in Salix herbacea shrubland, 1250 m alt., 26 July 2018, leg. J.C. Zamora, AH 46825, GenBank accession: ITS (PP431508). Medelpad, Alnön, Storsjönäset: moist forest with Betula pubescens and Salix spp., 75 m alt., 12 September 2014, leg. E. Larsson, EL 142-14 (GB 0243040), GenBank accession: ITS-LSU (OR817727), RPB1 (PP092177), RPB2 (PP092162). Pite lappmark, Arjeplog, Årjep Rivatjåkkå, NE of Skärrim: moist alpine meadow on calcareous ground, under Salix reticulata, 15 August 2018, leg. E. Larsson, EL 198-18 (GB 0243041), GenBank accession: ITS-LSU (OR817730), RPB2 (PP092164). Åsele lappmark, Vilhelmina, Lasterfjället, Tjårronjunjes NV side: alpine heath on calcareous soil, with Salix reticulata, 19 August 2019, leg. E. Larsson, J.B. Jordal & J. Vauras, EL 11-19 (GB 0454414), GenBank accession: ITS-LSU (PP512979).
Notes.
Inosperma subhirsutum is another hemiboreal to alpine species in the Calamistratum group. From its morphological and organoleptic characteristics, it seems to show some similarities with the species of the Geraniodorum group in terms of the appearance and shape of the pileus, its surfaces, and its odour reminiscent of pelargonium [
77]. Its spores show a certain variability, even in the same collection, and are often flattened and sometimes slightly concave to subphaseoliform in profile view (
Figure 8C,E). In frontal view, the spores are ovo-ellipsoid to ellipsoid and broader. According to our observations, the greenish-blue colour at the base of the stipe noted by Kühner in his original diagnosis may be variable, and some collections show a greenish grey to dark dirty-grey at the base, but this colour may be completely absent in some collections (EB 2014080711). Also noteworthy is the pelargonium-like odour in young specimens, which may become fishy later (according to Kühner, the odour is “reminiscent of herring”).
Characteristic of
I. subhirsutum are the pileus and stipe coverings (
Figure 10D,E), which are usually woolly–fibrillose (“mallocyboid”). However, some collections show a more hirsute pileus surface (e.g., EL 11-19,
Figure 10F). The stipe is similar in appearance to the pileus, also woolly–fibrillose, and never hirsute or squarrose. We have observed a variable tendency to reddening of the flesh and, in some collections of young specimens, the presence of a yellowish tinge in the lamellae.
Kühner [
77] provisionally named this species
Inocybe calamistrata var.
latispora, because the spores have a distinct ovoid–ellipsoid outline in frontal view, in contrast to
I. calamistratum and other similar species, where narrower spores with subphaseoliform to phaseoliform outlines predominate. Only
I. gracilentum can show spores of similar width to
I. subhirsutum, but the Q
avg is higher (1.8–2.0), and therefore their appearance is more elongated. Kühner’s paper was published after the validation of the holotype, due to a delay in publication.
The numerous collections studied, especially those from Fennoscandia, where it seems to be common, indicate that the spore morphology of
I. subhirsutum may show some variation in width and appearance. The holotype collection shows a clear dominance of broadly ellipsoidal to ovo-ellipsoidal spores in frontal view, but this feature is variable even within the same collection. Kühner [
77] reported spore dimensions of “9–10.6–13.5–14.7 × 6.2–6.7–8.2–9 µm, Q = 1.4–1.7”, which is in reasonable agreement with our examination of the holotype. The holotype was originally collected in an alpine zone under
Salix reticulata on calcareous soil (“in calcareo solo”, according to Kühner), but we have also studied genetically matching samples from more acidic soils (probably washed) with
Salix herbacea. These latter may also show some variability in spore size and shape, also including spores that are slightly narrower and more ellipsoidal than those reported for the holotype. Unfortunately, we were only able to observe a few cheilocystidia in Kühner’s collection, as the lamellar edge was quite collapsed, and the dimensions given here are only approximate. Very interesting are the data reported by Kuyper [
79] from a collection in Jämtland (Sweden) under
Salix retusa in the boreal zone; although he called it
I. calamistrata, considering it and
I. subhirsuta as synonyms, it seems to correspond to the latter because of its wider spores of 6–6.5 µm and its habitat. Phylogenetic studies support the separation of the two species at the specific level.
As can be observed from the phylogenetic tree (
Figure 1), there is a rather large amount of genetic variation within
I. subhirsutum, and we can genetically regard it as a species complex. There is a tendency to separate the strict Arctic–alpine specimens associated with dwarf
Salix and
Dryas and those from the boreal areas associated with
Populus tremula,
Alnus,
Betula pubescens and mixed
Pinus–
Betula habitats. However, more data are needed to confirm this hypothesis to determine whether genetic data can be correlated with the observed morphological variation in, e.g., spore morphology and habitats.
Figure 8.
Inosperma subhirsutum Holotype Herb. R. Kühner 72-102 (G00052221). (A,B) Voucher material and label. (C) Basidiospores. (D) Cheilocystidia. (E) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C,D); 2 µm (E). Photographs (A,B) by J.C. Zamora.
Figure 8.
Inosperma subhirsutum Holotype Herb. R. Kühner 72-102 (G00052221). (A,B) Voucher material and label. (C) Basidiospores. (D) Cheilocystidia. (E) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C,D); 2 µm (E). Photographs (A,B) by J.C. Zamora.
Figure 9.
Basidiomes of the species of the Calamistratum group. (A) Inosperma calamistratum AH 44420 (ESP). (B) I. calamistratum AH 46636 (POR). (C) I. calamistratum AH 56397 (ESP). (D) I. gracilentum Holotype EL 85-19 (SWE). (E) I. neohirsutum EL 163-15 (SWE). (F) I. neohirsutum AH 24959 (ESP). (G) I. neohirsutum AH 21333 (ESP). (H) I. neohirsutum AH 48235 (FRA). Photograph (C) by E. Rubio, (F) by P. Juste. Scale bars: 10 mm (A–H).
Figure 9.
Basidiomes of the species of the Calamistratum group. (A) Inosperma calamistratum AH 44420 (ESP). (B) I. calamistratum AH 46636 (POR). (C) I. calamistratum AH 56397 (ESP). (D) I. gracilentum Holotype EL 85-19 (SWE). (E) I. neohirsutum EL 163-15 (SWE). (F) I. neohirsutum AH 24959 (ESP). (G) I. neohirsutum AH 21333 (ESP). (H) I. neohirsutum AH 48235 (FRA). Photograph (C) by E. Rubio, (F) by P. Juste. Scale bars: 10 mm (A–H).
Figure 10.
Basidiomes of Calamistratum group. (A) Inosperma praetermissum AH 46960 (ITA). (B) I. praetermissum EB 2005083005 (ITA). (C) I. praetermissum EL 130-19 (SWE). (D) I. subhirsutum EB 2014080711 (ITA). (E) I. subhirsutum EL 76-12 (SWE). (F) I. subhirsutum EL 11-19 (SWE). Scale bars: 10 mm (A–F).
Figure 10.
Basidiomes of Calamistratum group. (A) Inosperma praetermissum AH 46960 (ITA). (B) I. praetermissum EB 2005083005 (ITA). (C) I. praetermissum EL 130-19 (SWE). (D) I. subhirsutum EB 2014080711 (ITA). (E) I. subhirsutum EL 76-12 (SWE). (F) I. subhirsutum EL 11-19 (SWE). Scale bars: 10 mm (A–F).
Inosperma geraniodorum (J. Favre) Matheny & Esteve-Rav., Mycologia 112(1): 102, 2019.
MycoBank No. 830366
≡ Inocybe geraniodora J. Favre, Ergebnisse der Wissenschaftlichen Untersuchungen des Schweiszerischen Nationalparks 5: 200, 1955. MycoBank No. 298912.
Lectotype. Switzerland, Graübunden, Parc National Grisons, Ofen Pass, God dal Fuorn: 46°39′42.78″ N, 10°12′42.66″ E, under
Alnus alnobetula, 1850 m alt., 4 September 1942, Herb. J. Favre Z.A.82b (G 00052203), GenBank accession: ITS1 (PP431545), ITS2 (PP431549). Lectotype designated by Monthoux and Kuyper in Kuyper ([
4]: 37). MBT 10013961.
Description. Favre [
69], Kuyper [
4], Senn-Irlet [
80], Bizio [
81], Bon [
65], Breitenbach and Kränzlin [
57].
Additional microscopic examination of the lectotype of I. geraniodora. Basidiospores (11.0–)11.7–15.5(–16.0) × 7.1–8.8(–9.2) µm, Spavg = 13.6 × 7.9 µm, Q = (1.4–)1.5–1.8(–1.9), Qavg = 1.7 (n = 30), smooth, thick-walled (≈ 1 µm), mostly ellipsoid to broadly ellipsoid, hardly or not phaseoliform in profile, ochraceous brown. Basidia clavate, mostly four-spored, hyaline, often with intracellular red-brownish pigment. Pleurocystidia absent. Cheilocystidia 45.0–55.1(–60.0) × (9.5–)10.1–17.6(–18.1) µm, Chavg = 51.3 × 13.8 µm (n = 8), subcylindrical to subclavate or clavate, with rounded apex, hyaline, thin-walled, often with reddish-brown content. Caulocystidia present near apex, grouped in clusters, similar to cheilocystidia, 40.7–48.8 × 11.4–17.7 µm, Caavg = 44.4 × 13.8 µm. Clamp connections present.
Distribution. Its distribution is confirmed in the boreal and alpine regions of Europe and North America. In Europe, it has been found in the Alps (France, Italy and Switzerland), the Pyrenees (Spain), Finland, Iceland, Norway and Sweden. Three references deposited in GenBank match the lectotype of
I. geraniodorum: KC965816 confirms its presence in Arctic Canada on Banks Island [
82]; the other two are from Svalbard (Norway), JF304334 [
83] and from Sweden, MH310767 [
84]. There are no sequences in the UNITE database that match
I. geraniodorum.
Ecology. Inosperma geraniodorum inhabits alpine and northern boreal ecosystems on calcareous soils, associated with various dwarf willows (
Salix reticulata,
S. retusa, etc.) and
Dryas octopetala, and most probably with
Bistorta vivipara. It can also develop in subalpine ecosystems at the upper limit of coniferous forests, between 1750 and 2000 m alt. [
85]. The lectotype was collected in the subalpine level close to
Alnus alnobetula, with which it can probably establish ectomycorrhizae.
Etymology. From the Latin Geranium, the plant which is commonly called pelargonium or geranium, and odor = smell, because its smell is reminiscent of this plant.
Additional specimens examined. Italy, Veneto, Belluno, Canale d’Agordo, Pian delle Comelle: 46°17′38″ N, 11°51′43″ E, under Dryas octopetala in sandy calcareous soil, 1827 m alt., 10 August 2008, leg. E. Bizio, EB 2008081002 (dupl. AH 56199), GenBank accession: ITS (PP431523). Veneto, Belluno, Cortina d’Ampezzo, Passo Falzarego: 46°30′29″ N, 12°01′50″ E, alpine scrubland in calcareous soil with Salix retusa and Dryas octopetala, 2238 m alt., 3 August 2019, leg. E. Bizio, EB 2019080304 (dupl. AH 46961), GenBank accession: ITS (PP431524). Norway, Oppland, Dovre, Grimsdalen: alpine heath with Salix reticulata on calcareous soil, 21 August 2012, leg. E. Larsson, EL 105-12 (GB 0243141), GenBank accession: ITS-LSU (OR823941). Spain, Cataluña (Catalonia), Lleida, Espot, Muntanya dels Estanyets: 42°32′37.92″ N, 1°4′21.45″ E, in a community of dwarf willows (Salix reticulata) and Dryas octopetala, in calcareous soil, 2240 m alt., 22 August 1999, leg. J. Llistosella, J. Vila, J. Girbal & F. Esteve Raventós, AH 25490 (dupl. JVG 990822-5). Sweden, Torne lappmark, Jukkasjärvi, Kopparåsen: alpine heath with Dryas octopetala and Salix reticulata on calcareous soil, 635 m alt., 17 August 2017, leg. E. Larsson, EL 156-17 (GB 0243140), GenBank accession: ITS-LSU (OR823942), RPB2 (PP092176). Lule lappmark, Jokkmokk, Padjelanta NP: alpine heath with Dryas octopetala and Salix reticulata on calcareous soil, 860 m alt., 14 August 2016, leg. H. Croneborg, EL 126-16 (GB 0243139), GenBank accession: ITS-LSU (OR823943), RPB2 (PP092175).
Notes.
Inosperma geraniodorum can be recognised by the absence of a blue-greenish colour of the stipe, the slender habit (“mycenoid” type, according to Vellinga [
25]), usually with a paraboloid to campanulate, often subumbonate pileus, the dark chocolate-brown colour of the basidiomata, the fibrillose to squamulose surface of the pileus (
Figure 15C,D), and the large ellipsoid spores (
Figure 11E,F). In young specimens, the typical smell of pelargonium is perceptible, although in some cases a fishy or cucumber component is present, especially in mature specimens. Similar in odour to
I. geraniodorum is
I. veliferum, which is slightly smaller and has a convex, not or hardly umbonate pileus, which is finely felted–fibrillose and initially covered with a persistent whitish veil.
Inosperma geminum, with an odour and habitat similar to those of pelargonium, is another small and reddish-brown species, but it does not have a persistent veil and differs from
I. geraniodorum and
I. veliferum in its slightly smaller and narrower spores, with marked phaseoliform to naviculiform tendency in profile, and shorter cheilocystidia on average.
According to the data obtained from our phylogenetic analysis, the species most closely related to I. geraniodorum is I. turietoense. Both have a similar habit, with a slender stipe much longer than the diameter of the pileus, a brown to brownish-red colour, a poorly developed or absent veil, a pileus with a fibrillose scaly surface, and large spores. However, they differ in their ecological preferences, as I. turietoense is a nemoral species in mountainous areas, not reaching subalpine or alpine altitudes, it is larger in size, and the pileus is decorated with a characteristic appressed scaly, tabby ornamentation, which contrasts strongly with the background due to its darker colour. Inosperma turietoense does not have the characteristic pelargonium odour of I. geraniodorum.
Favre [
69] first described
I. geraniodorum based on several collections without designating a holotype (citing several syntypes). He also mentioned its occurrence in the upper subalpine zones of the areas visited [
85]. The collection chosen by Kuyper [
4] as the lectotype has been successfully sequenced and allows us to clarify its taxonomic position. In the herbarium of J. Favre, deposited in G, there are about twenty collections identified by the Swiss mycologist as
Inocybe geraniodora. Apart from the lectotype, molecular data have not yet been obtained for any of them, so it is quite likely that some may correspond to other close or similar species (such as
I. veliferum).
Favre’s original description of
I. geraniodorum was based on the syntypes, and this fact can be confirmed by the various collections mentioned and drawn by him in the protologue. The study of the lectotype has shown spores slightly shorter than those given by Favre [14–18(–19.5) µm], but it cannot be ruled out that some of these collections have bisporic basidia (a common occurrence in high mountain collections), and consequently the basidiospores show some variability in length. However, we did not observe bisporic basidia in the lectotype, as the hymenial elements were collapsed. This is quite common in
Inosperma specimens. In Favre’s iconography ([
69]: 83, Figure 67) the macromorphological variability between different collections and their microscopic characteristics are clearly shown.
Inocybe geraniodora var.
gracilenta was also introduced by Favre [
69] but invalidly published. It was considered a variant of
I. geraniodora in the alpine zone, characterised by its very small size, smaller spores and shorter cheilocystidia, 32–50 × 9–13 µm. The only existing collection in G was successfully sequenced, and the ITS was obtained. It corresponds to a species belonging to the Calamistratum group and is presented in this paper as a new species (see
I. gracilentum). Favre [
69] does not mention the odour of this variant, but, presumably because of its name, it should be like that of
I. geraniodorum and therefore have a pelargonium component. Also, in Favre’s iconography ([
69], pl VI, Figure 5), the specimens do not show a blue-greenish tinge at the base of the stipe, which could lead to misinterpretation (Favre’s iconography could bring to mind specimens of
I. geraniodorum).
Favre [
85] also described
Inocybe geraniodora var.
depauperata, collected from high mountain forests in Switzerland, under conifers (
Pinus and
Larix). It is a small species with a hirsute scaly pileus in the central zone, without significant odour, with large amygdaliform spores (15–17 × 7.5–9 µm) and long cheilocystidia (50–70 µm), generally with a capitate apex (12–19 µm diam.). Two collections exist in the Herbarium G. One of them (God Cumün) was successfully sequenced, and its phylogenetic placement demonstrates that it should be included in the Cervicolor group and not in the Calamistratum and Geraniodorum groups dealt with in this paper.
Figure 11.
Inosperma geraniodorum Lectotype Herb. J. Favre Z.A.82b. (A,B) Voucher material and label. (C) Caulocystidia. (D) Cheilocystidia. (E) Basidiospores. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C–E); 2 µm (F).
Figure 11.
Inosperma geraniodorum Lectotype Herb. J. Favre Z.A.82b. (A,B) Voucher material and label. (C) Caulocystidia. (D) Cheilocystidia. (E) Basidiospores. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C–E); 2 µm (F).
Inosperma geminum E. Larss. & Vauras, sp. nov.
MycoBank No. 850670
Diagnosis. Inosperma geminum differs from other morphologically similar species, such as I. geraniodorum and I. veliferum, by having smaller basidiomata and narrower spores that are adaxially plane to slightly concave or subphaseoliform, often with a navicular appearance in lateral view. They differ in ITS sequence data and are phylogenetically distinct.
Holotype. Sweden, Lule lappmark, Jokkmokk, Padjelanta NP, Tuottar: alpine site, SW slope with Dryas octopetala, Salix reticulata and S. herbacea, on calcareous ground, 980 m alt., 13 August 2016, leg. J. Vauras, JV 31497 (TUR, isotypes in GB 0207615 and AH 56239), GenBank accession: ITS-LSU (OR823936), RPB2 (PP092174).
Description. Pileus 8–25 mm; when young, hemispherical, conical to obtusely conical with incurved margin; later, conico-convex to plano-convex, sometimes with a broad umbo. Surface dry, fibrillose to finely scaly; with age, squamulose at centre of the disc, ochraceous brown to reddish brown, velipellis ephemeral, pale. Lamellae rather sparse, broadly adnate to emarginate (L = 26–36), interspaced with lamellulae; at first, pale beige; with age, ochraceous brown; edge pale, fimbriate. Stipe 15–30 × 1.5–3 mm, dry, equal to slightly bulbous, pale ochraceous brown; later, concolourous with pileus, fibrillose to coarsely fibrillose; at apex, white, flocculose. Context ochraceous brown. Odour distinct of pelargonium but often also of fish. Basidiospores 11.8–12.5(–15.3) × 5.6–6.7(–7.4) Spawg = 12.5 × 6.7 µm, Q = 1.7–2.0, Qawg = 1.9 (n = 150/5), smooth, ochraceous brown, variable in shape, ellipsoid to narrowly ellipsoid in face view, some adaxially plane to slightly concave, quite often subphaseoliform with a navicular appearance, apiculus small, ochraceous brown. Basidia 42–48 × 10–13 µm (n = 45/5), narrowly clavate, mainly four-spored, hyaline. Pleurocystidia absent. Cheilocystidia 20–48(–53) × 11–17(–20) µm (n = 45/3), pyriform, clavate or subcylindrical, hyaline, thin-walled, some with brownish content. Caulocystidia present near the apex, similar to cheilocystidia but generally shorter, abundant, 20–38 × 9–16 µm (n = 20/2). Clamp connections present.
Distribution. So far known from the alpine zone in Sweden and Norway. No additional sequence matching data were available in GenBank, but there is one specimen in the UNITE database that originates from Norway (UDB07673341|NOBAS 1542-15).
Ecology. It seems to be restricted to herb-rich alpine ecosystems on calcareous ground, associated with Dryas octopetala and Salix reticulata.
Etymology. Refers to the Latin word geminum, meaning twin, double, pair, resembling or similar to, because of its similarity to I. veliferum and I. geraniodorum.
Additional specimens examined. Norway, Oppland, Dovre, Kongsvoll: in subalpine Betula forest on calcareous soil, with Salix reticulata, 20 August 1986, leg. L. & A. Stridvall 86/107, GB 0064330, GenBank accession: ITS (OR823940). Sweden, Torne lappmark, Jukkasjärvi, Latnja: alpine cliff ecosystem on calcareous ground, with Dryas octopetala, 925 m alt., 6 August 2006, leg. E. Larsson, EL 63-06 (GB 0207619, dupl. AH 56241), GenBank accession: ITS-LSU (OR823936), RPB2 (PP092172). Torne lappmark, Jukkasjärvi, Orddajohka towards Vilgesgierdu: alpine cliff ecosystem on calcareous ground, with Dryas octopetala, 12 August 2017, leg. E. Larsson, EL 50-17 (GB 0207618), GenBank accession: ITS-LSU (OR823938), RPB2 (PP092173). Härjedalen, Storsjö, Svansjökläppen: alpine area with Dryas otopetala on calcareous ground, 17 August 2006, leg. E. Larsson, EL 106-06 (GB 0207617, dupl. AH 56240), GenBank accession: ITS-LSU (FN550945)—as Inocybe geraniodora. Jämtland, Frostviken, Raavre: alpine heath on calcareous soil with Dryas octopetala and Salix reticulata on calcareous ground, 820 m alt., 23 August 2019, leg. H. Croneborg 96-19, GB 0207616, GenBank accession: ITS-LSU (OR823939).
Notes.
Inosperma geminum is very similar to both
I. geraniodorum and
I. veliferum. It is a small species characterised by an obtusely conical to plano-convex ochraceous-brown to reddish-brown pileus, fibrillose at the margin and distinctly squamulose at the centre of the pileus, with an ochraceous-brown stipe (
Figure 12A and
Figure 15A,B). It has a distinct odour of pelargonium and fish. It is phylogenetically most closely related to
I. veliferum, but the two species form separate, distinctly supported clades (
Figure 1). The two can also be separated based on differing ecologies and geographic distributions, and they differ in ITS sequence data by eight substitutions, five single, one of 2 bp, one of 4 bp and one of 7 bp insertion/deletion events.
Inosperma geminum can be confused with
I. geraniodorum, as they have similar habitat and their geographic distributions and ecologies overlap.
Inosperma geminum seems to be rare and less common than
I. geraniodorum and has on average smaller basidiomata and a smaller pileus diameter than the latter. The two can also be separated in terms of micromorphology, as
I. geminum has, on average, slightly shorter spores (
Figure 12B,F) and a larger Q
avg = 1.9 vs. 1.6.
Figure 12.
Inosperma geminum Holotype JV 31497 (TUR). (A) Basidiomata. (B) Basidiospores. (C) Cheilocystidia. (D) Cheilocystidia and basidia. (E) Caulocistydia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F). Photograph (A) by J. Vauras.
Figure 12.
Inosperma geminum Holotype JV 31497 (TUR). (A) Basidiomata. (B) Basidiospores. (C) Cheilocystidia. (D) Cheilocystidia and basidia. (E) Caulocistydia. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–E); 2 µm (F). Photograph (A) by J. Vauras.
Inosperma turietoense Pancorbo & Esteve-Rav., sp. nov.
MycoBank No. 850671
Diagnosis. Inosperma turietoense is similar to I. geraniodorum, but differs in its larger size, narrower spores and montane rather than alpine habitat. It also has a different odour, with no pelargonium component. Inosperma veliferum and I. geminum are smaller, grow in alpine ecosystems and have a typical pelargonium smell. The four species can also be distinguished by ITS sequence data.
Holotype. Spain, Aragón, Huesca, Torla, Ordesa National Park, Turieto Alto: 42°39′00″ N, 0°4′43″ W, in continental montane mixed forest of Abies alba and Fagus sylvatica, on calcareous soil, 1350 m alt., 26 August 2016, leg. F. Cervera, F. Serrano, F. Mateo, G. Sánchez, F. Tello, F. Pancorbo & F. Esteve-Raventós, AH 47710 (isotypes in FP 16082601 and GB 0266842), GenBank accession: ITS (PP431526), LSU (PP431541), RPB2 (PP478208).
Description. Pileus 20–30 mm, broadly hemispherical to convex when young, later conico-convex to plano-convex, centre subumbonate. Surface dry, fibrillose, finely scaly–fibrillose radially, appressed squamulose especially at centre, scales darker and contrasting with the background, appressed, not recurved, forming a delicate net and forming a virgate to tabby appearance, colour brown to reddish brown, velipellis not observed. Lamellae rather sparse, ventricose, narrowly adnate to annexed (L = 36–48), interspaced with lamellulae (l = 1–2); at first, whitish to pale beige; with age, ochraceous brown; edge pale, fimbriate. Stipe 50–70 × 5–8 mm, dry, equal to tapering downwards, straight to sinuose; at first, dirty white, then pale ochraceous brown; later, concolourous with pileus; fibrillose to coarsely fibrillose; at apex, white, flocculose. Context ochraceous brown, reddening along the stipe and pileus. Odour distinct and complex when cut, earthy to mouldy, sometimes mixed with some aromatic component, reminiscent of a mixture of Inosperma cervicolor/bongardii smells. Basidiospores smooth, ochraceous brown, (9.8–)10.4–13.4(–14.7) × (6.2–)6.4–7.3(–7.6) µm, Spavg = 12.0 × 6.8 µm, Q = (1.45–)1.50–1.94(–2.02), Qavg = 1.7 (n = 209/2), smooth, ellipsoid to subamygdaliform, sometimes subphaseoliform, in lateral view. Basidia long, (46.1–)51.5–61.6(–64.2) × (11.5–)11.8–14.0(–16.3) µm, Bavg = 53.4 × 13.0 µm, narrowly clavate, mainly four-spored, hyaline, some with intracellular brown pigment. Pleurocystidia absent. Cheilocystidia (31.2–)33.7–56.2(–64.5) × (7.5–)8.4–12.8(–15.0) µm, Chavg = 43.5 × 10.7 µm (n = 82/2), subcylindrical with rounded apex, subclavate, sometimes subcapitate, base (multi)septate, thin-walled, some with brownish content. Caulocystidia present near apex, similar to cheilocystidia, multiseptate, but generally larger than hymenia cystidia, abundant, (26.1–)27.9–78.7(–133.3) × (6.8–)8.0–16.8(–17.1) µm, Caavg = 53.3 × 12.4 µm, (n = 42/2). Clamp connections present.
Distribution. So far, known from the type locality in the Central Pyrenees of Spain.
Ecology. On calcareous soils in montane continental areas, in mixed beech (
Fagus sylvatica) and fir (
Abies alba) forests, in the
Buxo–
Fagetum community [
86].
Etymology. Refers to the locality where it was found, called Turieto Alto.
Additional specimens examined. Spain, Aragón, Huesca, Torla, Turieto Alto: 42°38′56″ N, 0°4′12″ W, 1352 m alt., mixed forest of Fagus sylvatica and Abies alba in calcareous soil, 30 August 2015, leg. G. Sánchez, M.A. Ribes, F. Pancorbo & F. Esteve-Raventós, AH 47669 (dupl. FP 15083005), GenBank accession: ITS (PP431525), LSU (PP431540).
Notes.
Inosperma turietoense has certain morphological similarities and is phylogenetically closely related to
I. geraniodorum. The basidiomes are of medium size and slender appearance, but larger than those of
I. geraniodorum (
Figure 13A and
Figure 15G,H). The ellipsoidal spores (
Figure 13B,H) are similar in both, although on average slightly narrower in
I. turietoense (13.7 × 7.9 µm vs. 12.0 × 6.8 µm). It also differs in several notable diagnostic characters: the tabby scaly appearance of the pileus centre, with darker adpressed and delicate scales, the different odour without traces of pelargonium, and a more continental, temperate, montane habitat. In the collections studied,
I. turietoense has a typically attenuated stipe towards the base, and the reddening of the flesh and surface of the stipe is noticeable. Collections made in situ have led us to make an approximate identification with a member of the Cervicolor group, whose species are phylogenetically distant and show some differences in pileus appearance, but it gives an idea of the first impression of the new species.
Inosperma veliferum and
I. geminum have smaller basidiomata with a distinct whitish velipellis, at least when young, a pure pelargonium smell in young basidiomes, and boreal–alpine distribution.
Figure 13.
Inosperma turietoense Holotype AH 47710. (A) Basidiomata. (B) Basidiospores. (C) Cheilocystidia. (D) Laminar edge. (E,F) Caulocystidia. (G) Basidia. (H) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–G); 2 µm (H).
Figure 13.
Inosperma turietoense Holotype AH 47710. (A) Basidiomata. (B) Basidiospores. (C) Cheilocystidia. (D) Laminar edge. (E,F) Caulocystidia. (G) Basidia. (H) Spore SEMs. Scale bars: 10 mm (A); 10 µm (B); 50 µm (C–G); 2 µm (H).
Inosperma veliferum (Kühner) Matheny & Esteve-Rav., Mycologia 112(1): 105, 2019.
MycoBank No. 830407
≡ Inocybe geraniodora var. velifera Kühner, Documents Mycologiques 19(74): 19, 1988. MycoBank No. 135089.
≡ Inocybe velifera (Kühner) Bon, Bulletin Trimestriel de la Fédération Mycologique Dauphiné-Savoie 37(144): 78, 1997. MycoBank No. 436881.
Holotype. France, Savoie, Parc National de la Vanoise, Haute-Maurienne, Le Vallon: 45°25′42″ N, 6°24′17″ E, on almost bare ground with Salix herbacea, 2700 m alt., 10 September 1971, Herb. R. Kühner 71-143 (G 00110853), GenBank accession: ITS (PP431520).
Description. Kühner [
77], Bon [
65].
Additional microscopic examination of the holotype of I. geraniodora var. velifera. Basidiospores (10.8–)11.5–14.5(–15.5) × 7.1–9.0(–9.5) µm, Spavg = 13.2 × 8.0 µm, Q = (1.38–)1.44–1.74(–1.80), Qavg = 1.6 (n = 21), smooth, thick-walled (≈ 0.7 µm), ellipsoid to broadly ellipsoid, ochraceous brown. Basidia mostly collapsed, clavate, four-spored, hyaline, often with intracellular brown pigment. Pleurocystidia absent. Cheilocystidia (26.0–)30.5–50.9(–51.4) × (9.6–)10.0–14.2(–14.5) µm, Chavg = 42.7 × 12.0 µm (n = 7), variable in shape, subcylindrical, clavate, sublageniform, sometimes subcapitate, thin-walled, some with brownish content. Caulocystidia present near apex, in clusters, similar to cheilocystidia, numerous, (22.9–)25.6–67.5(–72.1) × (9.8–)10.3–18.1(–18.8) µm, Caavg = 44.7 × 13.6 µm, (n = 7). Clamp connections present.
Distribution.
Inosperma veliferum is distributed in central and southern alpine areas of the European continent. Except for one matching sequence, we are not aware of any more collections or deposited sequences from boreal areas in either GenBank or UNITE databases. Its current distribution includes France [
65,
77], Italy (GenBank JF908117—as
I. geraniodora) and Spain ([
87]—as
I. geraniodora var.
geraniodora).
Ecology. It seems to be a strictly alpine species that thrives at high altitudes. It was originally described in association with
Salix herbacea, a plant that prefers acidic soils, although it can invade snow patches on calcareous substrates [
88]; the collections studied in Italy and Spain were found in mats of
Salix retusa,
S. reticulata and
Dryas octopetala on calcareous soils. All collections were found above 2100 m alt.
Etymology. Derived from the Latin velifer, from velum = veil and -fer = to carry, referring to the presence of a veil.
Additional specimens examined. Italy, Trentino-Alto Adige, Sen Jan di Fassa (TN), Rifugio Vallaccia: in calcareous soil with Salix retusa, S. reticulata and Dryas octopetala, 2355 m alt., 14 August 1994, leg. E. Bizio, MCVE 4485, GenBank accession: ITS (JF908117)—as I. geraniodora. Veneto, Falcade, Gruppo Marmolada, Passo col Becher: 46°23′54″ N, 11°52′04″ E, in calcareous soil in Salix retusa and S. reticulata scrub, 2292 m alt., 9 August 1993, leg. E. Bizio, EB 1993080906 (dupl. AH 56198 and MCVE 20884), GenBank accession: ITS (PP431519), LSU (PP431539). Veneto, Belluno, Falcade, Forcella Venegia: 46°19′55″ N, 11°46′26″ E, alpine scrub with Salix retusa, S. reticulata, Dryas octopetala, Bistorta vivipara and Kalmia procumbens, in calcareous soil, 2250 m alt., 10 August 2019, leg. E. Bizio, AH 46962, GenBank accession: ITS (PP431521). Spain, Aragón, Huesca, Hoz de Jaca, Sierra de Tendenera, Peña Sabocos: 42°41′14.32″ N, 0°15′40.77″ W, in calcareous bare soil with scattered scrubs of Salix reticulata and Dryas octopetala, 2180 m alt., 27 August 1996, leg. F. Arenal, E. Horak & F. Esteve Raventós, AH 21346, GenBank accession: ITS (PP431522).
Notes. Inosperma veliferum is morphologically very close to I. geminum and I. geraniodorum. These species have initially a pure pelargonium smell and lack blue-greenish pigments in the basidiomata. In addition, the appearance of the surfaces of the pileus and stipe differs markedly between the members of the Geraniodorum group and those of the Calamistratum group, the latter being characterised by a distinct scaly or even coarsely scaly to squarrose appearance of their surfaces. The species of the Calamistratum group show spores that are more elongated and narrower, ellipsoid to subcylindrical or subphaseoliform. Only I. subhirsutum seems to be an exception, as some collections (including the holotype) show broadly ellipsoidal spores and more fibrillose–woolly to squamulose surfaces.
Inosperma geraniodora var.
velifera was described by Kühner [
77] from the French Alps. Bon [
65] raised it to the rank of a separate species because of the presence of the characteristic veil and the lower Q of the spores. It is recognisable by its small size and the more obtuse and convex appearance of the pileus, which is not or hardly umbonate and never has a squamulose surface, but is smooth to fibrillose (
Figure 14A and
Figure 15E,F). A good diagnostic feature is the presence of an abundant whitish veil, especially in young or unwashed specimens, which totally or partially camouflages the reddish-brown colour of the pileus. In
I. geraniodorum and
I. geminum, the veil may be present on the pileus disc in young stages, but in no case is it so abundant or developed, and both have a subsquamose pileus centre. Because of its small size,
I. veliferum can be confused with
I. geminum, the species to which it appears to be the most closely related phylogenetically. The pileus of the latter is sometimes more campanulate and subumbonate and is distinctly squamulose at the centre, and its spores are narrower (13.2 × 8.0 µm vs. 12.5 × 6.7 µm), with a marked subphaseoliform to naviculiform tendency in profile. So far,
Inosperma geminum has been found exclusively in the alpine zone of the Scandinavian mountains, whereas
I. veliferum seems to be restricted to the alpine areas of central Europe and the Pyrenees. However,
I. geraniodorum is widespread in both alpine and boreal regions and can also be found at subalpine altitudes between 1800 and 2000 m [
85]. Comparative studies of the size and shape of spores and cystidia of the samples of
I. geraniodorum and
I. veliferum studied have not shown any remarkable or distinct microscopic differences between the two for diagnostic use. Both species have similar average dimensions in spore size, as well as size and shape of the cystidia. The macroscopic appearance of
I. geraniodorum is slightly different, producing darker and generally larger basidiomes, often with a squamulose conical to subumbonate pileus, and the veil, if present, is more diffuse and transient.
Figure 14.
Inosperma veliferum Holotype Herb. R. Kühner 71-143 (G 00110853). (A,B) Voucher material and label material. (C) Caulocystidia. (D) Cheilocystidia. (E) Spores. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C–E); 2 µm (F). Photographs (A,B) by J.C. Zamora.
Figure 14.
Inosperma veliferum Holotype Herb. R. Kühner 71-143 (G 00110853). (A,B) Voucher material and label material. (C) Caulocystidia. (D) Cheilocystidia. (E) Spores. (F) Spore SEMs. Scale bars: 10 mm (A); 10 µm (C–E); 2 µm (F). Photographs (A,B) by J.C. Zamora.
Figure 15.
Basidiomes of the species of the Geraniodorum group. (A) Inosperma geminum EL 63-06 (SWE). (B) I. geminum EL 106-06 (SWE). (C) I. geraniodorum EB 20100823 (ITA). (D) I. geraniodorum AH 46961 (ITA). (E) I. veliferum AH 46962 (ITA). (F) I. veliferum AH 56198 (ITA). (G,H) I. turietoense AH 47669 (ESP). Scale bars: 10 mm (A–H). Photograph (C) by C. Zoldan.
Figure 15.
Basidiomes of the species of the Geraniodorum group. (A) Inosperma geminum EL 63-06 (SWE). (B) I. geminum EL 106-06 (SWE). (C) I. geraniodorum EB 20100823 (ITA). (D) I. geraniodorum AH 46961 (ITA). (E) I. veliferum AH 46962 (ITA). (F) I. veliferum AH 56198 (ITA). (G,H) I. turietoense AH 47669 (ESP). Scale bars: 10 mm (A–H). Photograph (C) by C. Zoldan.
- 1-
A characteristic blue-green or grey-green colour at the base of the stipe (young, not water-soaked specimens should be observed; in some collections of I. subhirsutum, these colours may sometimes be absent). Spores with Wavg = 5–7 µm—Calamistratum Group............................................................................................................................................... 2
- 1′-
Blue-green colours absent. Spores with Wavg = 6.7–8.0 µm—Geraniodorum Group.............................................................................................................................................. 6
- 2-
Pileus and stipe surfaces strongly hirsute–squamose to squarrose. Habitat Atlantic/continental, nemoral to boreo-nemoral, under conifers, Betulaceae, Fagaceae or mixed coniferous/broadleaved forests..................................................................................................... 3
- 2′-
Pileus and stipe surfaces hirsute–fibrillose, scales not so bristly, more flattened, often tessellated and woolly in appearance, initially covered by an ochraceous veil (observe young specimens). Habitat boreal, alpine or Arctic, sometimes at subalpine levels............................................................................................................................................... 4
- 3-
Pileus and stipe surfaces (observe young specimens) strongly bristly, squarrose. Scales distributed over most of the surface of the pileus at maturity, individualised, rarely welded together. Spores long, ellipsoidal in frontal view, phaseoliform in profile (W = 55.5 µm, Qavg = 2), cheilocystidia mostly subcylindrical to claviform, often two- to three-septate at base, <40 µm in length.................................................................................... I. calamistratum
- 3′-
Pileus surface typically decorated in the centre with broad, often welded scales (tessellated–pyramidal appearance) at maturity, contrasting with the squamulose–fibrillose to smooth margin. Spores ellipsoidal in frontal view, phaseoliform in profile (W = 5.5–6 µm, Qavg = 1.7), cheilocystidia broadly claviform to subspheropedunculate, more rarely subcylindrical, <45 µm in length............................................................... I. neohirsutum
- 4-
Spores very elongate, subcylindrical in frontal view, phaseoliform in lateral view, Qavg = 2.2, cheilocystidia mostly cylindrical to broadly claviform, 40–60(–65) µm long. In boreal and alpine areas, on both acidic and calcareous soils, together with Salix, Dryas, Betula nana and Bistorta, sometimes also occurring in montane/subalpine and hemiboreal forests under conifers................................................................................................................. I. praetermissum
- 4′-
Spores different.............................................................................................................................. 5
- 5-
Spores long, ellipsoid in frontal view, ellipsoid–subphaseoliform in lateral view, Qavg = 1.8, cheilocystidia typically short, mostly pyriform to broadly claviform, <40 µm long. In alpine environments and calcareous soils, under Salix, Dryas and Bistorta....................................................................................................................... I. gracilentum
- 5′-
Spores broadly ellipsoidal to subovoid in frontal view, ellipsoidal and hardly to slightly subreniform in lateral view, Qavg = 1.6, cheilocystidia 35–50 µm, versiform, mostly claviform, broadly claviform to subcylindrical. Pileus and stipe scaly–fibrillose to woolly–fibrillose (“Mallocybe” appearance). Stipe with fibrillose to lanose surface. In alpine and boreal areas, in shrubland associated with Salix, sometimes with Dryas, Betula nana and Bistorta, less often associated with Salix trees in montane–subalpine coniferous forests (Alps)....................................................................................................... I. subhirsutum complex
- 6-
Medium–small to small species, with reddish-brown pileus and stipe, with fibrillose scales concolourous to the pileus or pileus smooth–fibrillose, covered with a distinctive whitish veil when young. In alpine, subalpine and boreal areas................................................................................................................................................. 7
- 6′-
Medium-sized species, reminiscent of I. cervicolor/subrubescens in appearance, with brown to hazel pileus, decorated in the central area with delicate, darker and applied scales (brindled appearance). Cystidia subcylindrical, sometimes with subcapitate apex. Spores broadly ellipsoidal in frontal and lateral view, Qavg = 1.7. In mixed montane Abies/Fagus forests on calcareous soils. Known to date from the Spanish Pyrenees..................................................................................................................... I. turietoense
- 7-
Species with broadly ellipsoid to ellipsoid spores in frontal and lateral view, Qavg = 1.6–1.8..................................................................................................................................................... 8
- 7′-
Spores ellipsoid in frontal view, navicular–phaseoliform in lateral view, Qavg = 1.9, cystidia versiform. Pileus typically squamose at the centre. In calcareous soils associated with Salix and Dryas. Species of exclusively boreal and alpine distribution, known so far from Scandinavia.......................................................................................................... I. geminum
- 8-
Pileus convex and obtuse at maturity; when young, covered by a manifest whitish veil on a smooth to finely fibrillose brownish ground (see unwashed specimens). Flesh reddish at the stipe cortex, not becoming very dark upon age. Spores broadly ellipsoidal to subovoid in both frontal and lateral view. In alpine areas, under Salix and Dryas, on both calcareous and acidic soils.............................................................................................................. I. veliferum
- 8′-
Pileus convex–campanulate at maturity, becoming scaly–fibrillose (sometimes bristly) in the centre, not covered by an apparent veil, or, if present, very ephemeral. Flesh dark reddish in mature basidiomata. Spores ellipsoid, broadly ellipsoid to subovoid in both frontal and lateral views, not or hardly subphaseoliform in profile. In alpine and boreal areas, associated with Dryas, Salix and Bistorta, but also in the upper subalpine level (Alps), probably associated with Alnus alnobetula........................................... I. geraniodorum