Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Growth Conditions
2.2. In Vitro Sporangiospore Germination Analysis
2.3. Animals
2.4. Inocula Preparations
2.5. Blast Overpressure Exposure, Surgery, Infection, and Treatment
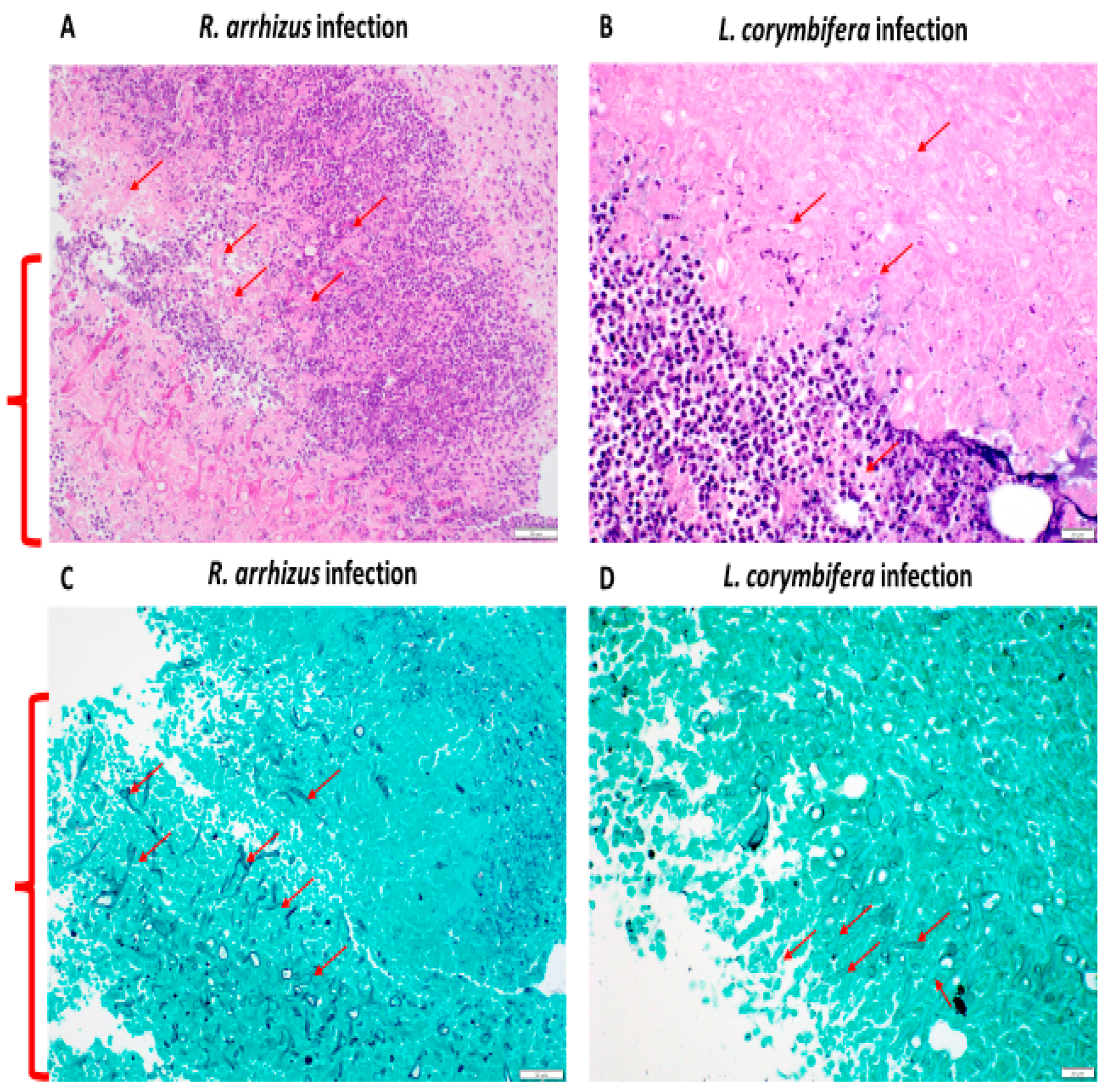
2.6. Wound Assessment, Fungal Burden Semi-Quantification, and Histopathological Analysis
2.7. Statistical Analysis
3. Results
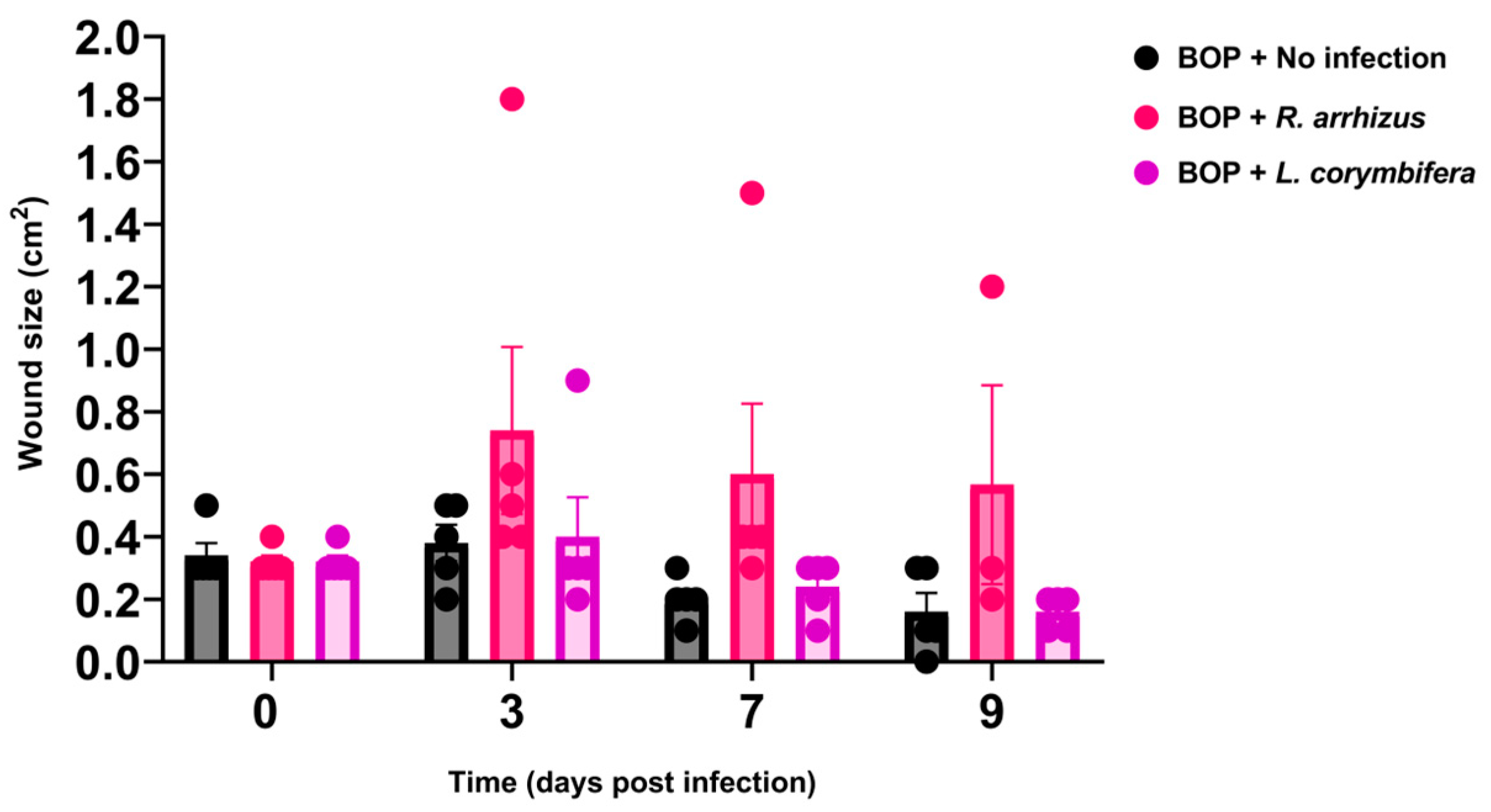
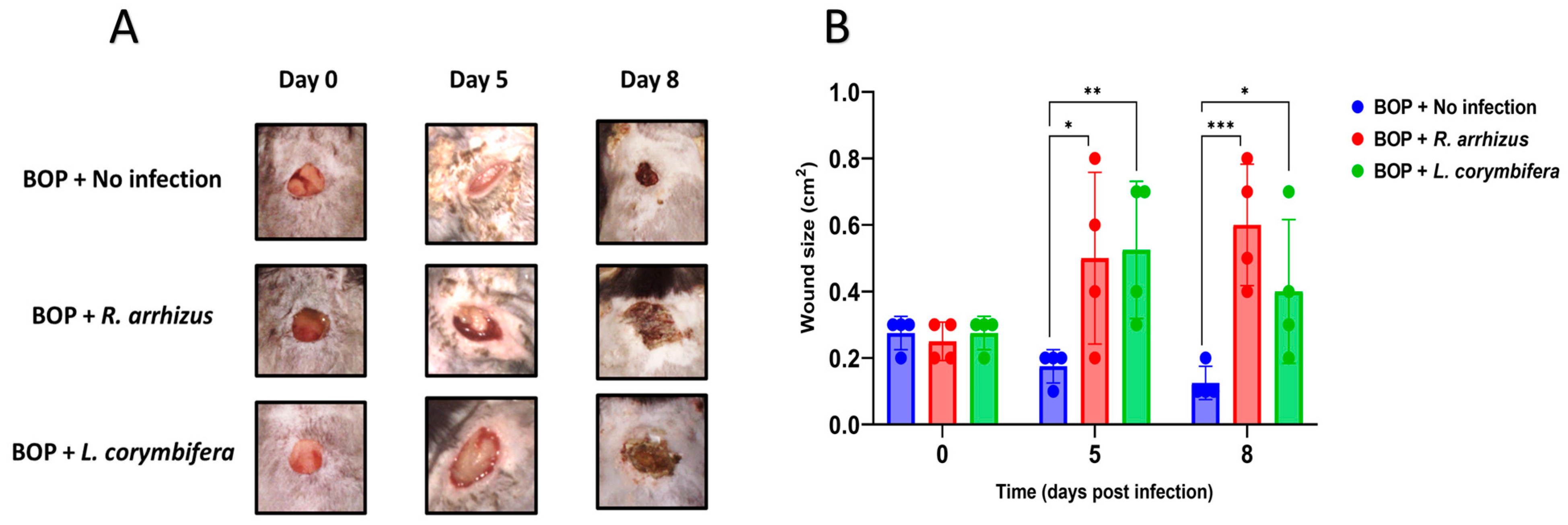
3.1. Hyphae of R. arrhizus and L. corymbifera Cause Sustained Wound Infection in Blast-Exposed and Cyclophosphamide-Treated Mice
3.2. Higher Hyphal Inocula of R. arrhizus and L. corymbifera Result in Larger Wounds and More Extensive Tissue Necrosis in Blast-Exposed Mice
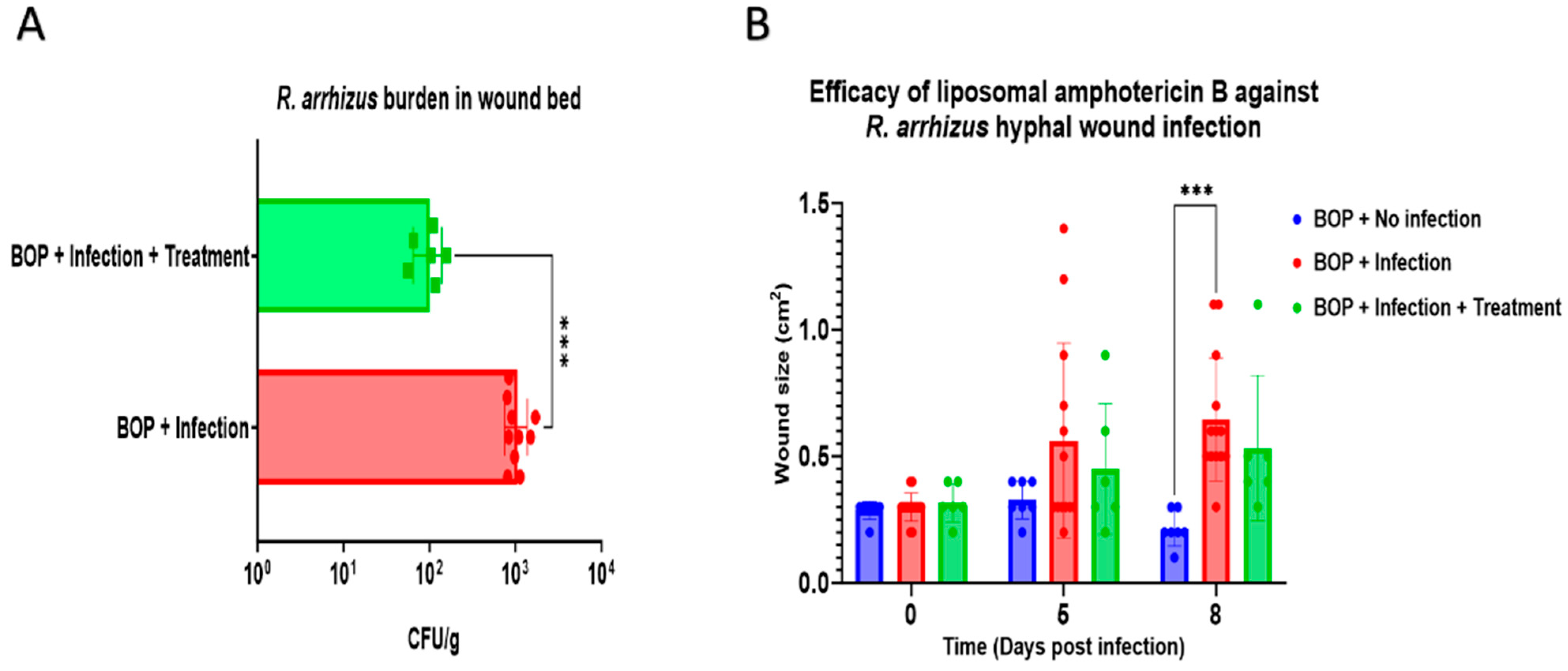
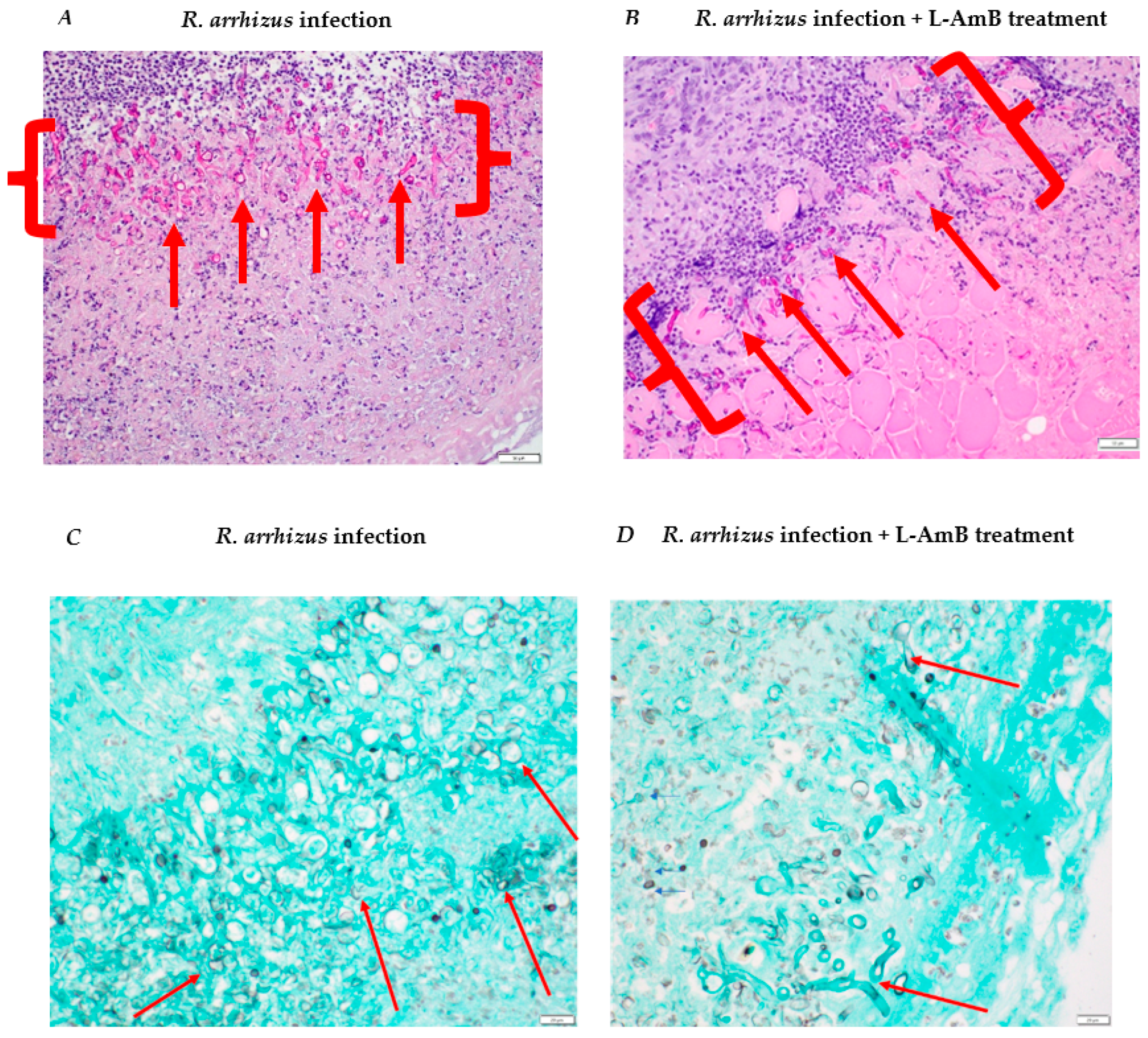
3.3. Assessment of Liposomal Amphotericin B Efficacy against R. arrhizus Hyphae Infection in a Combat-Relevant Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
References
- Petersen, K.; Riddle, M.S.; Danko, J.R.; Blazes, D.L.; Hayden, R.; Tasker, S.A.; Dunne, J.R. Trauma-related infections in battlefield casualties from Iraq. Ann. Surg. 2007, 245, 803–811. [Google Scholar] [CrossRef]
- Tribble, D.R.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Mende, K.; Blyth, D.M.; Petfield, J.L.; McDonald, J. After the Battlefield: Infectious Complications among Wounded Warriors in the Trauma Infectious Disease Outcomes Study. Mil. Med. 2019, 184, 18–25. [Google Scholar] [CrossRef]
- Greer, N.; Sayer, N.; Kramer, M.; Koeller, E.; Velasquez, T. Prevalence and Epidemiology of Combat Blast Injuries from the Military Cohort 2001–2014; VA Evidence-Based Synthesis Program Reports; ESP Project #09-009; Department of Veterans Affairs (US): Washington, DC, USA, 2016. [Google Scholar]
- Ritenour, A.E.; Blackbourne, L.H.; Kelly, J.F.; McLaughlin, D.F.; Pearse, L.A.; Holcomb, J.B.; Wade, C.E. Incidence of primary blast injury in US military overseas contingency operations: A retrospective study. Ann. Surg. 2010, 251, 1140–1144. [Google Scholar] [CrossRef]
- Weintrob, A.C.; Murray, C.K.; Xu, J.; Krauss, M.; Bradley, W.; Warkentien, T.E.; Lloyd, B.A.; Tribble, D.R. Early Infections Complicating the Care of Combat Casualties from Iraq and Afghanistan. Surg. Infect. 2018, 19, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Surbatovic, M.; Filipovic, N.; Radakovic, S.; Stankovic, N.; Slavkovic, Z. Immune cytokine response in combat casualties: Blast or explosive trauma with or without secondary sepsis. Mil. Med. 2007, 172, 190–195. [Google Scholar] [CrossRef]
- Thompson, K.B.; Krispinsky, L.T.; Stark, R.J. Late immune consequences of combat trauma: A review of trauma-related immune dysfunction and potential therapies. Mil. Med. Res. 2019, 6, 11. [Google Scholar] [CrossRef]
- Phipps, H.; Mondello, S.; Wilson, A.; Dittmer, T.; Rohde, N.N.; Schroeder, P.J.; Nichols, J.; McGirt, C.; Hoffman, J.; Tanksley, K.; et al. Characteristics and Impact of U.S. Military Blast-Related Mild Traumatic Brain Injury: A Systematic Review. Front. Neurol. 2020, 11, 559318. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.C.J.; Ganesan, A.; Shaikh, F.; Carson, M.L.; Bradley, W.; Warkentien, T.E.; Tribble, D.R. Combat-Related Invasive Fungal Wound Infections. Mil. Med. 2022, 187, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J.; et al. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef]
- Warkentien, T.E.; Shaikh, F.; Weintrob, A.C.; Rodriguez, C.J.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Aggarwal, D.; Carson, M.L.; Tribble, D.R.; et al. Impact of Mucorales and Other Invasive Molds on Clinical Outcomes of Polymicrobial Traumatic Wound Infections. J. Clin. Microbiol. 2015, 53, 2262–2270. [Google Scholar] [CrossRef]
- Weintrob, A.C.; Weisbrod, A.B.; Dunne, J.R.; Rodriguez, C.J.; Malone, D.; Lloyd, B.A.; Warkentien, T.E.; Wells, J.; Murray, C.K.; Bradley, W.; et al. Combat trauma-associated invasive fungal wound infections: Epidemiology and clinical classification. Epidemiol. Infect. 2015, 143, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kronen, R.; Liang, S.Y.; Bochicchio, G.; Bochicchio, K.; Powderly, W.G.; Spec, A. Invasive Fungal Infections Secondary to Traumatic Injury. Int. J. Infect. Dis. 2017, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.R.; Ganesan, A.; Rodriguez, C.J. Combat trauma-related invasive fungal wound infections. Curr. Fungal Infect. Rep. 2020, 14, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Hospenthal, D.R.; Petraitis, V.; Kontoyiannis, D.P. Necrotizing Mucormycosis of Wounds following Combat Injuries, Natural Disasters, Burns, and Other Trauma. J. Fungi 2019, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, B.; Kent, S.; Wall, D. Cutaneous mucormycosis secondary to penetrative trauma. Injury 2016, 47, 1383–1387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sipsas, N.V.; Gamaletsou, M.N.; Anastasopoulou, A.; Kontoyiannis, D.P. Therapy of Mucormycosis. J. Fungi 2018, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- McKeny, P.T.; Nessel, T.A.; Zito, P.M. Antifungal Antibiotics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jensen, H.E. Murine subcutaneous granulomatous zygomycosis induced by Absidia corymbifera. Mycoses 1992, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, Z.; Li, R.; Yu, J. Impairment of Th cell response in Card9 knockout mice with cutaneous mucormycosis caused by Rhizopus arrhizus. Exp. Dermatol. 2019, 28, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, S.; Wan, Z.; Li, R.; Yu, J. In Vivo and In Vitro Impairments in T Helper Cell and Neutrophil Responses against Mucor irregularis in Card9 Knockout Mice. Infect. Immun. 2021, 89, e00040-21. [Google Scholar] [CrossRef]
- Xu, W.; Liang, G.; Peng, J.; Long, Z.; Li, D.; Fu, M.; Wang, Q.; Shen, Y.; Lv, G.; Mei, H.; et al. The influence of the mating type on virulence of Mucor irregularis. Sci. Rep. 2017, 7, 10629. [Google Scholar] [CrossRef]
- Sheldon, W.H.; Bauer, H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J. Exp. Med. 1959, 110, 845–852. [Google Scholar] [CrossRef]
- Sheldon, W.H.; Bauer, H. Activation of quiescent mucormycotic granulomata in rabbits by induction of acute alloxan diabetes. J. Exp. Med. 1958, 108, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, W.H.; Bauer, H. Tissue Mast Cells and Acute Inflammation in Experimental Cutaneous Mucormycosis of Normal, 48/80-Treated, and Diabetic Rats. J. Exp. Med. 1960, 112, 1069–1084. [Google Scholar] [CrossRef]
- Paplanus, S.H.; Sheldon, W.H. Acute Inflammation and Tissue Mast Cells in Adrenalectomized Rats with Cutaneous Mucormycosis. J. Exp. Med. 1963, 118, 165–174. [Google Scholar] [CrossRef]
- Bao, W.; Jin, L.; Fu, H.J.; Shen, Y.N.; Lu, G.X.; Mei, H.; Cao, X.Z.; Wang, H.S.; Liu, W.D. Interleukin-22 mediates early host defense against Rhizomucor pusilluscan pathogens. PLoS ONE 2013, 8, e65065. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Ben-Ami, R.; Best, L.; Albert, N.; Walsh, T.J.; Kontoyiannis, D.P. Tacrolimus enhances the potency of posaconazole against Rhizopus oryzae in vitro and in an experimental model of mucormycosis. J. Infect. Dis. 2013, 207, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Bobrov, A.G.; Bolton, J.S.; Rouse, M.D.; Heyburn, L.; Pavlovic, R.; Garry, B.I.; Alamneh, Y.; Long, J.; Swierczewski, B.; et al. Blast Waves Cause Immune System Dysfunction and Transient Bone Marrow Failure in a Mouse Model. Front. Bioeng. Biotechnol. 2022, 10, 821169. [Google Scholar] [CrossRef]
- Sajja, V.S.; Tenn, C.; McLaws, L.J.; Vandevord, P.J. A temporal evaluation of cytokines in rats after blast exposure. Biomed. Sci. Instrum. 2012, 48, 374–379. [Google Scholar]
- Giacobbe, D.R.; Riccardi, N.; Vena, A.; Bassetti, M. Mould Infections of Traumatic Wounds: A Brief Narrative Review. Infect. Dis. Ther. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.C.; Lantieri, L.; Lortholary, O.; Lanternier, F.; et al. Posttraumatic mucormycosis: A nationwide study in France and review of the literature. Medicine 2014, 93, 395–404. [Google Scholar] [CrossRef]
- Samdavid Thanapaul, R.J.R.; Roberds, A.; Rios, K.E.; Walsh, T.J.; Bobrov, A.G. Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents than Sporangiospores In Vitro and in Galleria mellonella. J. Fungi 2023, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.R.; Fraga-Silva, T.F.; Almeida, D.F.; Dos Santos, R.F.; Finato, A.C.; Amorim, B.C.; Andrade, M.I.; Soares, C.T.; Lara, V.S.; Almeida, N.L.; et al. Rhizopus-host interplay of disseminated mucormycosis in immunocompetent mice. Future Microbiol. 2020, 15, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E., Jr.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Wells, J.; Shaikh, F.; Peterson, P.; Bradley, W.; Carson, M.L.; Petfield, J.L.; Klassen-Fischer, M.; Akers, K.S.; Downing, K.; et al. Molecular Detection of Filamentous Fungi in Formalin-Fixed Paraffin-Embedded Specimens in Invasive Fungal Wound Infections Is Feasible with High Specificity. J. Clin. Microbiol. 2019, 58, e01259-19. [Google Scholar] [CrossRef] [PubMed]
- Padhye, A.A.; Ajello, L. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 1988, 26, 1861–1863. [Google Scholar] [CrossRef]
- Brabcova, V.; Novakova, M.; Davidova, A.; Baldrian, P. Dead fungal mycelium in forest soil represents a decomposition hotspot and a habitat for a specific microbial community. New Phytol. 2016, 210, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Stursova, M.; Kohout, P.; Human, Z.R.; Baldrian, P. Production of Fungal Mycelia in a Temperate Coniferous Forest Shows Distinct Seasonal Patterns. J. Fungi 2020, 6, 190. [Google Scholar] [CrossRef]
- Simon, A.; Herve, V.; Al-Dourobi, A.; Verrecchia, E.; Junier, P. An in situ inventory of fungi and their associated migrating bacteria in forest soils using fungal highway columns. FEMS Microbiol. Ecol. 2017, 93, fiw217. [Google Scholar] [CrossRef]
- Mousavi, B.; Costa, J.M.; Arne, P.; Guillot, J.; Chermette, R.; Botterel, F.; Dannaoui, E. Occurrence and species distribution of pathogenic Mucorales in unselected soil samples from France. Med. Mycol. 2018, 56, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Mucor Mould Mycelium Growing in Soil, Timelapse—Stock Video Clip—K006/8792—Science Photo Library. Available online: https://www.sciencephoto.com/media/852797/view/mucor-mould-mycelium-growing-in-soil-timelapse (accessed on 12 May 2024).
- Loussert, C.; Schmitt, C.; Prevost, M.C.; Balloy, V.; Fadel, E.; Philippe, B.; Kauffmann-Lacroix, C.; Latge, J.P.; Beauvais, A. In vivo biofilm composition of Aspergillus fumigatus. Cell. Microbiol. 2010, 12, 405–410. [Google Scholar] [CrossRef]
- Motaung, T.E.; Peremore, C.; Wingfield, B.; Steenkamp, E. Plant-associated fungal biofilms-knowns and unknowns. FEMS Microbiol. Ecol. 2020, 96, fiaa224. [Google Scholar] [CrossRef] [PubMed]
- Peiqian, L.; Xiaoming, P.; Huifang, S.; Jingxin, Z.; Ning, H.; Birun, L. Biofilm formation by Fusarium oxysporum f. sp. cucumerinum and susceptibility to environmental stress. FEMS Microbiol. Lett. 2014, 350, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Williams, C. The clinical importance of fungal biofilms. Adv. Appl. Microbiol. 2013, 84, 27–83. [Google Scholar] [CrossRef]
- Singh, R.; Shivaprakash, M.R.; Chakrabarti, A. Biofilm formation by zygomycetes: Quantification, structure and matrix composition. Microbiology 2011, 157, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Cooter, R.D.; Lim, I.S.; Ellis, D.H.; Leitch, I.O. Burn wound zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 1990, 28, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; Porto, E.; Tayah, M.; Valente, N.Y.; Lacaz Cda, S.; Maranhao, W.M.; Rodrigues, M.C. Subcutaneous mucormycosis caused by Mucor hiemalis Wehmer f.luteus (Linnemann) Schipper 1973. Mycoses 1990, 33, 241–246. [Google Scholar] [CrossRef]
- Rodrigues Hoffmann, A.; Ramos, M.G.; Walker, R.T.; Stranahan, L.W. Hyphae, pseudohyphae, yeasts, spherules, spores, and more: A review on the morphology and pathology of fungal and oomycete infections in the skin of domestic animals. Vet. Pathol. 2023, 60, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.J.; Simpson, I.; Khuu, M.H.; Kidd, S.E.; Lo, C.H.; Jenkin, G.A. An unusual ulcer: A case of cutaneous mucormycosis caused by Rhizopus oryzae. Med. Mycol. Case Rep. 2015, 7, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Shivananda, P.; Mahabala, C.; Kausalya, S.; Suchitra, S.; Anand, K.U. Cutaneous mucormycosis with necrotising fasciitis in a young immunocompetent individual. Trop. Dr. 2011, 41, 183–184. [Google Scholar] [CrossRef]
- Lloyd, B.; Weintrob, A.C.; Rodriguez, C.; Dunne, J.R.; Weisbrod, A.B.; Hinkle, M.; Warkentien, T.; Murray, C.K.; Oh, J.; Millar, E.V.; et al. Effect of early screening for invasive fungal infections in U.S. service members with explosive blast injuries. Surg. Infect. 2014, 15, 619–626. [Google Scholar] [CrossRef]
- Rodriguez, C.J.; Weintrob, A.C.; Shah, J.; Malone, D.; Dunne, J.R.; Weisbrod, A.B.; Lloyd, B.A.; Warkentien, T.E.; Murray, C.K.; Wilkins, K.; et al. Risk factors associated with invasive fungal infections in combat trauma. Surg. Infect. 2014, 15, 521–526. [Google Scholar] [CrossRef] [PubMed]
| Mouse Groups | Proportion of Infected Mice Detected by | Histological Analysis of Wound Tissues | |
|---|---|---|---|
| CFUs | Histology | ||
| BOP + R. arrhizus | 2/3 | 1/3 |
|
| CP + R. arrhizus | 5/5 | 1/3 |
|
| BOP + L. corymbifera | 5/5 | 2/3 |
|
| CP + L. corymbifera | 4/4 | 1/3 |
|
| Organism | Proportion of Mice with Detectable CFUs/Tissues Infected | Histological Analysis of Wound Tissues | ||
|---|---|---|---|---|
| With Homogenization | Without Homogenization | Histology | ||
| R. arrhizus | 1/4 | 4/4 | 4/4 |
|
| L. corymbifera | 3/4 | 3/4 | 2/4 |
|
| Mouse Groups | Proportion of Mice with Detectable CFUs/Tissues Infected | Histological Analysis of Wound Tissues | |
|---|---|---|---|
| CFUs | Histology | ||
| BOP + Infection | 10/10 | 6/6 |
|
| BOP + Infection + Treatment | 6/6 | 6/6 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samdavid Thanapaul, R.J.R.; Alamneh, Y.A.; Finnegan, D.K.; Antonic, V.; Abu-Taleb, R.; Czintos, C.; Boone, D.; Su, W.; Sajja, V.S.; Getnet, D.; et al. Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases. J. Fungi 2024, 10, 364. https://doi.org/10.3390/jof10050364
Samdavid Thanapaul RJR, Alamneh YA, Finnegan DK, Antonic V, Abu-Taleb R, Czintos C, Boone D, Su W, Sajja VS, Getnet D, et al. Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases. Journal of Fungi. 2024; 10(5):364. https://doi.org/10.3390/jof10050364
Chicago/Turabian StyleSamdavid Thanapaul, Rex J. R., Yonas A. Alamneh, Daniel K. Finnegan, Vlado Antonic, Rania Abu-Taleb, Christine Czintos, Dylan Boone, Wanwen Su, Venkatasivasai S. Sajja, Derese Getnet, and et al. 2024. "Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases" Journal of Fungi 10, no. 5: 364. https://doi.org/10.3390/jof10050364
APA StyleSamdavid Thanapaul, R. J. R., Alamneh, Y. A., Finnegan, D. K., Antonic, V., Abu-Taleb, R., Czintos, C., Boone, D., Su, W., Sajja, V. S., Getnet, D., Roberds, A., Walsh, T. J., & Bobrov, A. G. (2024). Development of a Combat-Relevant Murine Model of Wound Mucormycosis: A Platform for the Pre-Clinical Investigation of Novel Therapeutics for Wound-Invasive Fungal Diseases. Journal of Fungi, 10(5), 364. https://doi.org/10.3390/jof10050364






