Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform
Abstract
1. Introduction
2. Methods
3. Results
3.1. Limit of Detection (LoD)
3.2. Accuracy
3.3. Analytical Specificity
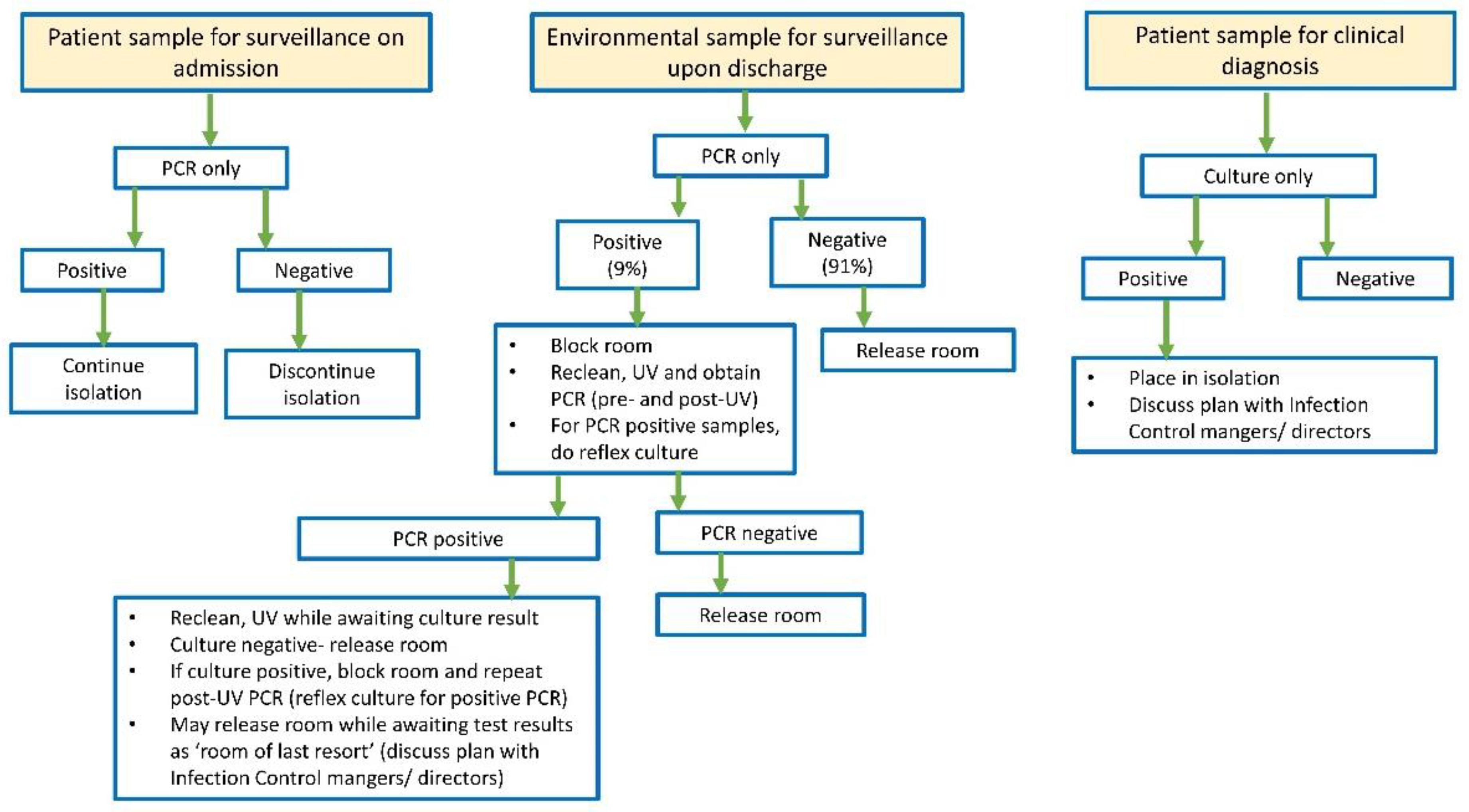
3.4. Implementation of CAURIS-PCR for Surveillance
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Carbone, A.; Oliva, M.; Schinca, E.; Boni, S.; Pontali, E. An Overview on Candida auris in Healthcare Settings. J. Fungi 2023, 9, 913. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an Agent of Hospital-Associated Outbreaks: Which Challenging Issues Do We Need to Have in Mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef]
- Ferrer Gómez, C.; Solís Albamonte, P.; Delgado Navarro, C.; Salvador García, C.; Tormo Palop, N.; Andrés Ibáñez, J.A. Analysis of Candida auris candidemia cases in an Intensive Care Unit of a tertiary hospital. Rev. Esp. Anestesiol. Reanim. 2021, 68, 431–436. [Google Scholar] [CrossRef]
- Ortiz-Roa, C.; Valderrama-Rios, M.C.; Sierra-Umaña, S.F.; Rodríguez, J.Y.; Muñetón-López, G.A.; Solórzano-Ramos, C.A.; Escandón, P.; Alvarez-Moreno, C.A.; Cortés, J.A. Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort. J. Fungi 2023, 9, 715. [Google Scholar] [CrossRef]
- Pandya, N.; Cag, Y.; Pandak, N.; Pekok, A.U.; Poojary, A.; Ayoade, F.; Fasciana, T.; Giammanco, A.; Caskurlu, H.; Rajani, D.P.; et al. International Multicentre Study of Candida auris Infections. J. Fungi 2021, 7, 878. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef]
- Dire, O.; Ahmad, A.; Duze, S.; Patel, M. Survival of Candida auris on environmental surface materials and low-level resistance to disinfectant. J. Hosp. Infect. 2023, 137, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wickes, B.L. Analysis of a Candida auris Outbreak Provides New Insights into an Emerging Pathogen. J. Clin. Microbiol. 2020, 58, e02083-19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2020, 58, e01503-19. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Dangana, T.; Sexton, D.J.; Fukuda, C.; Yelin, R.D.; Stanley, M.; Bell, P.B.; Baskaran, S.; Deming, C.; Chen, Q.; et al. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat. Med. 2021, 27, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiotics 2020, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Leach, L.; Zhu, Y.; Chaturvedi, S. Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples. J. Clin. Microbiol. 2018, 56, e01223-17. [Google Scholar] [CrossRef] [PubMed]
- Leach, L.; Russell, A.; Zhu, Y.; Chaturvedi, S.; Chaturvedi, V. A Rapid and Automated Sample-to-Result Candida auris Real-Time PCR Assay for High-Throughput Testing of Surveillance Samples with the BD Max Open System. J. Clin. Microbiol. 2019, 57, e00630-19. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Widen, R.; Vestal, G.; Uy, D.; Silbert, S. A TaqMan Probe-Based Real-Time PCR Assay for the Rapid Identification of the Emerging Multidrug-Resistant Pathogen Candida auris on the BD Max System. J. Clin. Microbiol. 2019, 57, e01604-18. [Google Scholar] [CrossRef]
- Sorenson, N.; Alexander, J.; Moghaddasi, A.; Hill, A.; Harris, J.; McCune, S.; Gick, E.; Blosser, S.; Lewinski, M.; Cady, K. High-Throughput C. auris (RUO) Assay on the Cobas 5800/6800/8800 System. 2023. Available online: https://medically.roche.com/global/en/infectious-disease/asm-microbe-2023/medical-material/ASM-Microbe-2023-poster-sorenson-high-throughput-c-auris-screening-assay-pdf.html (accessed on 6 November 2023).
- Sathyapalan, D.T.; Antony, R.; Nampoothiri, V.; Kumar, A.; Shashindran, N.; James, J.; Thomas, J.; Prasanna, P.; Sudhir, A.S.; Philip, J.M.; et al. Evaluating the measures taken to contain a Candida auris outbreak in a tertiary care hospital in South India: An outbreak investigational study. BMC Infect. Dis. 2021, 21, 425. [Google Scholar] [CrossRef]
- Freitas, B.L.; Leach, L.; Chaturvedi, V.; Chaturvedi, S. Reverse Transcription-Quantitative Real-Time PCR (RT-qPCR) Assay for the Rapid Enumeration of Live Candida auris Cells from the Health Care Environment. J. Clin. Microbiol. 2022, 60, e0077921. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; De Maeyer, H.; Allen, M. Evaluation of different cleaning strategies for removal of contaminating DNA molecules. Genes 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.P.; Menz, J.; Dusza, S.; Montecalvo, M.A. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am. J. Infect. Control 2014, 42, 586–590. [Google Scholar] [CrossRef]
- Ponnachan, P.; Vinod, V.; Pullanhi, U.; Varma, P.; Singh, S.; Biswas, R.; Kumar, A. Antifungal activity of octenidine dihydrochloride and ultraviolet-C light against multidrug-resistant Candida auris. J. Hosp. Infect. 2019, 102, 120–124. [Google Scholar] [CrossRef] [PubMed]
- De Groot, T.; Chowdhary, A.; Meis, J.F.; Voss, A. Killing of Candida auris by UV-C: Importance of exposure time and distance. Mycoses 2019, 62, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Stelfox, H.T.; Bates, D.W.; Redelmeier, D.A. Safety of patients isolated for infection control. JAMA 2003, 290, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Perencevich, E.N.; Goto, M.; Livorsi, D.J.; Balkenende, E.; Kiscaden, E.; Schweizer, M.L. Patient care experience with utilization of isolation precautions: Systematic literature review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Bell, C.; Stall, N.; Tomlinson, G.; McGeer, A.; Morris, A.; Gardam, M.; Abrams, H.B. The Effect of Hospital Isolation Precautions on Patient Outcomes and Cost of Care: A Multi-Site, Retrospective, Propensity Score-Matched Cohort Study. J. Gen. Intern. Med. 2017, 32, 262–268. [Google Scholar] [CrossRef]
- Kean, R.; Sherry, L.; Townsend, E.; McKloud, E.; Short, B.; Akinbobola, A.; Mackay, W.G.; Williams, C.; Jones, B.L.; Ramage, G. Surface disinfection challenges for Candida auris: An in-vitro study. J. Hosp. Infect. 2018, 98, 433–436. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Malhotra, R.; Mathur, P. Identification of Candida auris by PCR and assessment of biofilm formation by crystal violet assay. Indian J. Med. Microbiol. 2023, 46, 100421. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed]
| Accession Number | Clade |
|---|---|
| SAMN18754594 | African |
| SAMN05379609 | African |
| SAMN05379619 | South American |
| SAMN05379608 | East Asian |
| SAMN05379624 | South Asian |
| Matrices | LoDs (CFU/mL) | Ct * |
|---|---|---|
| Sterile Amies media | 6.77 × 10−4 | 35.7 ± 1.2 |
| CAURIS-PCR negative axilla/groin samples | 0.47 | 35.6 ± 2.0 |
| CAURIS-PCR negative environmental samples | 0.39 | 33.7 ± 1.9 |
| Culture | |||||||
|---|---|---|---|---|---|---|---|
| Axilla/Groin | Environment | Total | |||||
| Positive | Negative | Positive | Negative | ||||
| CAURIS-PCR | Axilla/Groin | Positive | 7 | 1 * | - | - | 8 |
| Negative | 0 | 48 | - | - | 48 | ||
| Environment | Positive | - | - | 2 | 8 * | 10 | |
| Negative | - | - | 0 | 82 | 82 | ||
| Total | 7 | 49 | 2 | 90 | 148 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, F.M.; Bertsch, J.; DeMaet, M.A.; York, T.; McDougal, A.; Patel, J.A.; Ren, P. Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform. J. Fungi 2024, 10, 285. https://doi.org/10.3390/jof10040285
Cerqueira FM, Bertsch J, DeMaet MA, York T, McDougal A, Patel JA, Ren P. Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform. Journal of Fungi. 2024; 10(4):285. https://doi.org/10.3390/jof10040285
Chicago/Turabian StyleCerqueira, Filipe M., Jennifer Bertsch, Mary Ann DeMaet, Teresa York, April McDougal, Janak A. Patel, and Ping Ren. 2024. "Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform" Journal of Fungi 10, no. 4: 285. https://doi.org/10.3390/jof10040285
APA StyleCerqueira, F. M., Bertsch, J., DeMaet, M. A., York, T., McDougal, A., Patel, J. A., & Ren, P. (2024). Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform. Journal of Fungi, 10(4), 285. https://doi.org/10.3390/jof10040285





