Description and Pathogenicity of Colletotrichum kapreanum sp. nov, a Cherelle Wilt Pathogen Belonging to the Gigasporum Species Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. Morphological Observation
2.3. Genomic DNA Extraction, PCR, and Sequencing
2.4. Molecular Phylogenetic Analyses
2.5. Pathogenicity Test
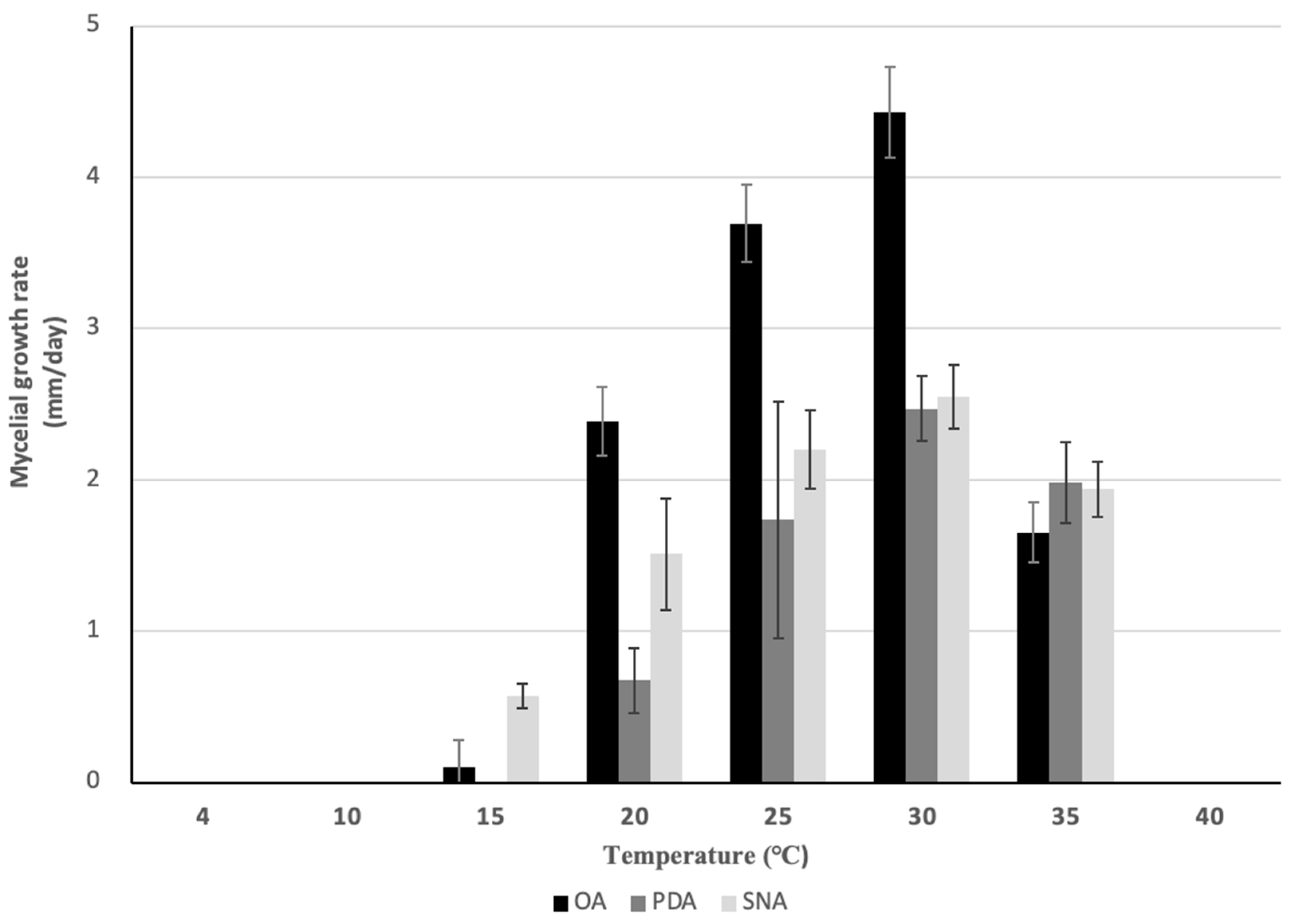
2.6. Mycelial Growth Test
3. Results
3.1. Molecular Phylogenetic Analyses
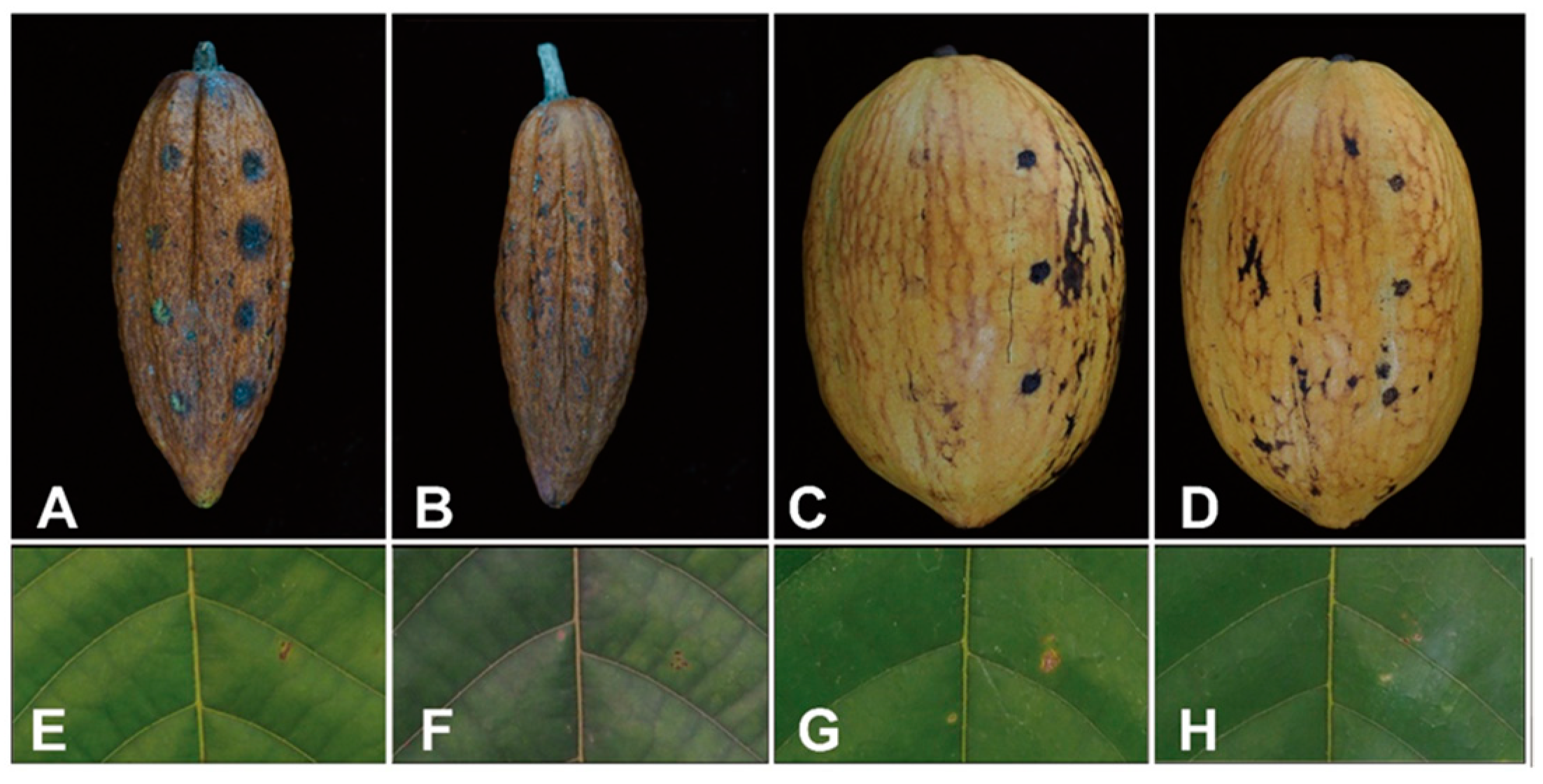
3.2. Pathogenicity Test
3.3. Taxonomy
4. Discussion
4.1. Identification of Pathogenic Isolate
4.2. Pathogenicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, B.A.; Meinhardt, L.W. Cherelle wilt of cacao: A physiological condition. In Cacao Diseases; Bryan, A.B., Lyndel, W.M., Eds.; Springer: Cham, Switzerland, 2016; pp. 483–499. [Google Scholar]
- Nair, K.P. Chapter 5 Cocoa (Theobroma Cacao L.). In Tree Crops; Margaret, D., Ed.; Springer International Publishing: Heidelberg, Germany, 2020; pp. 153–213. [Google Scholar]
- Philippine Cacao Industry Roadmap 2021–2025. Available online: https://www.da.gov.ph/wp-content/uploads/2023/05/Philippine-Cacao-Industry-Roadmap.pdf (accessed on 11 November 2023).
- Serrato-Diaz, L.M.; Mariño, Y.A.; Guadalupe, I.; Bayman, P.; Goenaga, R. First report of Lasiodiplodia pseudotheobromae and Colletotrichum siamense causing cacao pod rot, and first report of C. tropicale causing cacao pod rot in Puerto Rico. Plant Dis. 2020, 104, 592. [Google Scholar] [CrossRef]
- Shibata, A.; Minoshima, A.; Sugawara, Y.; Hirooka, K.Y.; Kagiwada, S. The first record of anthracnose on cacao pods caused by Colletotrichum tropicale in Japan. Annu. Rep. Kanto-Tosan Plant Prot. Soc. 2015, 62, 78–82. [Google Scholar]
- Huda-Shakirah, A.R.; Mohd, M.H. Morphology, Phylogeny, and Pathogenicity of Colletotrichum siamense associated with leaf blight and pod rot of Theobroma cacao in Malaysia. Trop. Plant Pathol. 2023, 48, 319–331. [Google Scholar] [CrossRef]
- James, R.S.; Ray, J.; Tan, Y.P.; Shivas, R.G. Colletotrichum siamense, C. theobromicola and C. queenslandicum from several plant species and the identification of C. asianum in the Northern Territory, Australia. Australas. Plant Dis. Notes 2014, 9, 138. [Google Scholar] [CrossRef]
- Nascimento, A.D.; Lima, M.O.; Feijó, F.M.; Júnior, J.H.; Sobrinho, R.R.; Assunção, I.P.; Lima, G.S.A. First report of Colletotrichum aeschynomenes causing anthracnose in cacao (Theobroma cacao) in Brazil. Plant Dis. 2019, 103, 3284. [Google Scholar] [CrossRef]
- Rojas, E.I.; Rehner, S.A.; Samuels, G.J.; Van Bael, S.A.; Herre, E.A.; Cannon, P.; Chen, R.; Pang, J.; Wang, R.; Zhang, Y.; et al. Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panama: Multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 2010, 102, 1318–1338. [Google Scholar] [CrossRef]
- González, E.; Sutton, T.B.; Correll, J.C. Clarification of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology 2006, 96, 982–992. [Google Scholar] [CrossRef]
- Cheng, B.P.; Huang, Y.H.; Peng, A.T.; Ling, J.F.; Song, X.B.; Chen, X. First report of leaf and fruit spot of Citrus reticulata Blanco cv. Nian Ju caused by Colletotrichum truncatum in China. Plant Dis. 2014, 98, 422. [Google Scholar] [CrossRef]
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correll, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef]
- Réblová, M.; Gams, W.; Seifert, K.A. Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud. Mycol. 2011, 68, 163–191. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS Primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Templeton, M.D.; Rikkerink, E.H.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Takata, Y.; Komine, M.; Uchikawa, K.; Nozawa, S.; Watanabe, K. Pathogenicity of Colletotrichum species isolated from rotten fruit and asymptomatic flowers of loquat in Nagasaki Prefecture, Japan and characterization of C. nagasakiense Takata & Kyoko Watan., sp. nov. J. Gen. Plant Pathol. 2024, 90, 69–97. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia 2014, 33, 83–97. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 1, 2–3. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2; Distributed by the Author; Evolutionary Biology Center, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Swofford, D. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Phytotaxa 2002, 218, 227–240. [Google Scholar]
- Rakotoniriana, E.F.; Scauflaire, J.; Rabemanantsoa, C.; Urveg-Ratsimamanga, S.; Munaut, F. Colletotrichum gigasporum sp. nov., a new species of Colletotrichum producing long straight conidia. Mycol. Prog. 2012, 12, 403–412. [Google Scholar] [CrossRef]
- Sivanesan, A.; Hsieh, W.H. A new ascomycete, Glomerella septospora sp. nov. and its coelomycete anamorph, Colletotrichum taiwanense sp. nov. from Taiwan. Mycol. Res. 1993, 97, 1523–1529. [Google Scholar] [CrossRef]
- Paguntalan, D.P.; Aggangan, N.S.; Buot, I.E., Jr.; Sotto, R.C.; Pangga, I.B. Effect of AMF-cacao association on pod disease incidence in three-year-old cacao trees in an agroforest in Calauan, Laguna, Philippines. Philipp. J. Sci. 2022, 151, 383–395. [Google Scholar] [CrossRef]


| Species | Strain | Species Complex | Host | GenBank Accession Number | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | GAPDH | CHS1 | ACT | TUB2 | ||||
| C. arxii | CBS169.59 | Gigasporum | Oncidium excavatum | KF687717 | KF687824 | KF687781 | KF687784 | KF687868 |
| C. arxii | Paphi 2-1 * | Gigasporum | Paphiopedilum sp. | KF687716 | KF687843 | KF687780 | KF687802 | KF687881 |
| C. gigasporum | CBS101881 | Gigasporum | Solanum betaceum | KF687736 | KF687841 | KF687777 | KF687797 | KF687886 |
| C. gigasporum | CBS124947 | Gigasporum | Theobroma cacao | KF687731 | KF687828 | KF687763 | KF687786 | KF687871 |
| C. gigasporum | CBS125385 | Gigasporum | Theobroma cacao | KF687733 | KF687834 | KF687765 | KF687788 | KF687873 |
| C. gigasporum | CBS125730 | Gigasporum | Theobroma cacao | KF687735 | KF687840 | KF687770 | KF687793 | KF687878 |
| C. gigasporum | CBS181.52 | Gigasporum | Theobroma cacao | KF687734 | KF687838 | KF687775 | KF687799 | KF687885 |
| C. magnisporum | CBS398.84 * | Gigasporum | Unknown | KF687718 | KF687842 | KF687782 | KF687803 | KF687882 |
| C. pseudomajus | CBS571.88 * | Gigasporum | Camellia sinensis | KF687722 | KF687826 | KF687779 | KF687801 | KF687883 |
| C. radicis | CBS529.93 * | Gigasporum | Unknown | KF687719 | KF687825 | KF687762 | KF687785 | KF687869 |
| C. subvariabile | NN040649 * | Gigasporum | Unknown plant | MZ595883 | MZ664054 | MZ799343 | MZ664181 | MZ674001 |
| C. variabile | NN040656 * | Gigasporum | Unknown plant | MZ595884 | MZ664055 | MZ799344 | MZ664182 | MZ674002 |
| C. vietnamense | CBS125477 | Gigasporum | Coffea sp. | KF687720 | KF687831 | KF687768 | KF687791 | KF687876 |
| C. vietnamense | LD16(L2) * | Gigasporum | Coffea sp. | KF687721 | KF687832 | KF687769 | KF687792 | KF687877 |
| C. zhaoqingense | NN058985 * | Gigasporum | Dead petiole of palm | MZ595905 | MZ664065 | MZ799304 | MZ664203 | MZ674023 |
| C. zhaoqingense | NN071035 | Gigasporum | Dead petiole of palm | MZ595906 | MZ664066 | MZ799305 | MZ664204 | MZ674024 |
| C. zhaoqingense | NN071036 | Gigasporum | Dead petiole of palm | MZ595907 | MZ664067 | MZ799306 | MZ664205 | MZ674025 |
| C. kapreanum | PH22T135 * | Gigasporum | Theobroma cacao, cherelle | LC750347 | LC750348 | LC750349 | LC750350 | LC750351 |
| Colletotrichum sp. | CBS159.50 | Gigasporum | Derris sp. | KF687724 | KF687823 | KF687778 | KF687800 | KF687867 |
| C. acutatum | CBS112996 * | Acutatum | Carica papaya | JQ005776 | JQ948677 | JQ005797 | JQ005839 | JQ005860 |
| C. gloeosporioides | ICMP17821 * | Gloeosporioides | Citrus sinensis | JX010152 | JX010056 | JX009818 | JX009531 | JX010445 |
| Species | Strain | Conidial Size (µm) | Medium | ||
|---|---|---|---|---|---|
| Size (µm) | Ave. ± SD (µm) | L/W Ratio | |||
| C. kapreanum | TAP22T135 | 22.9–28.7 × 7.1–8.7 | 26.2 ± 1.7 × 8.1 ± 0.4 | 3.2 | OA |
| (18.2–)21.2–25.5 × 6.7–7.1 | 22.2 ±1.6 × 6.9 ± 0.4 | 3.0 | Pine needle | ||
| C. gigasporum a | CBS133266 | (22–)25–29(–32) × (6–)7–9 | NR | NR | PDA |
| C. zhaoqingense b | NN058985 | 20–24 × 5.5–7 | 21.3 ± 0.9 × 6.3 ± 0.6 | 3.1 | Pine needle |
| C. taiwanense c | IMI353024 | 22–35(–45) × 5–8 | NR | NR | PDA |
| Species | Strain | Ascospores | Medium | |
|---|---|---|---|---|
| Size (µm) | No. of Septa | |||
| C. kapreanum | TAP22T135 | (44.5–)47.5–68.6(–69.5) × 5.2–7(–7.4) | 0–3 (mainly 1) | OA |
| C. gigasporum a | CBS133266 | (56–)60–84(–92) × 5–7 | 0–1 | PDA |
| C. taiwanense b | IMI353024 | 50–58 × 5–8 | 4–5 | Rice straw |
| Species | Strain | Appressoria | ||
|---|---|---|---|---|
| Size (µm) | Color | Shape | ||
| C. kapreanum | TAP22T135 | 4.9–9.6(–10.8) × (2.9–)3.1–5.1(–7.6) | pale to medium brown | irregular, ovoid |
| C. gigasporum a | CBS133266 | 14–16 × 10–12 | pale to medium brown | clavate, irregular |
| C. zhaoqingense b | NN058985 | 5.5–18 × 3.5–6.5 | pale brown | globose, subglobose, ovoid, irregular |
| C. taiwanense c | IMI353024 | 7.0–12.5 × 7.5–15.5 | brown | globose, clavate, irregular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, Y.; Ocampo-Padilla, C.; Malonzo, M.A.C.; Pangilinan, D.C.J.C.; Nozawa, S.; Watanabe, K. Description and Pathogenicity of Colletotrichum kapreanum sp. nov, a Cherelle Wilt Pathogen Belonging to the Gigasporum Species Complex. J. Fungi 2024, 10, 204. https://doi.org/10.3390/jof10030204
Takata Y, Ocampo-Padilla C, Malonzo MAC, Pangilinan DCJC, Nozawa S, Watanabe K. Description and Pathogenicity of Colletotrichum kapreanum sp. nov, a Cherelle Wilt Pathogen Belonging to the Gigasporum Species Complex. Journal of Fungi. 2024; 10(3):204. https://doi.org/10.3390/jof10030204
Chicago/Turabian StyleTakata, Yoshiki, Celynne Ocampo-Padilla, Mike Andre C. Malonzo, Dan Charlie Joy Camara Pangilinan, Shunsuke Nozawa, and Kyoko Watanabe. 2024. "Description and Pathogenicity of Colletotrichum kapreanum sp. nov, a Cherelle Wilt Pathogen Belonging to the Gigasporum Species Complex" Journal of Fungi 10, no. 3: 204. https://doi.org/10.3390/jof10030204
APA StyleTakata, Y., Ocampo-Padilla, C., Malonzo, M. A. C., Pangilinan, D. C. J. C., Nozawa, S., & Watanabe, K. (2024). Description and Pathogenicity of Colletotrichum kapreanum sp. nov, a Cherelle Wilt Pathogen Belonging to the Gigasporum Species Complex. Journal of Fungi, 10(3), 204. https://doi.org/10.3390/jof10030204






