Ozone Application Suppressed the Blue Mold Development and Maintained the Main Active Ingredients Content of Postharvest Fresh Codonopsis pilosula during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Spore Suspension
2.2.2. Ozone Treatment Method
2.2.3. Analysis of Incidence of the Blue Mold and Patulin Accumulation of C. pilosula during Different Storage Periods
level)/(Total number of plants × Highest disease level)] × 100%,
× 100%,
2.2.4. Determination of Weight Loss Rate of C. pilosula during Different Storage Periods
weight(W2)]/[Initial weight(W1)] × 100%,
2.2.5. Analysis of Main Active Ingredients in C. pilosula Inoculated with P. expansum during Different Storage Periods
2.2.6. Analysis of ROS Metabolism in C. pilosula Inoculated with P. expansum during Different Storage Periods
2.2.7. Determination of Cell Membrane Permeability
2.2.8. Determination of Malondialdehyde Content
2.2.9. Data Statistics and Analysis
3. Results
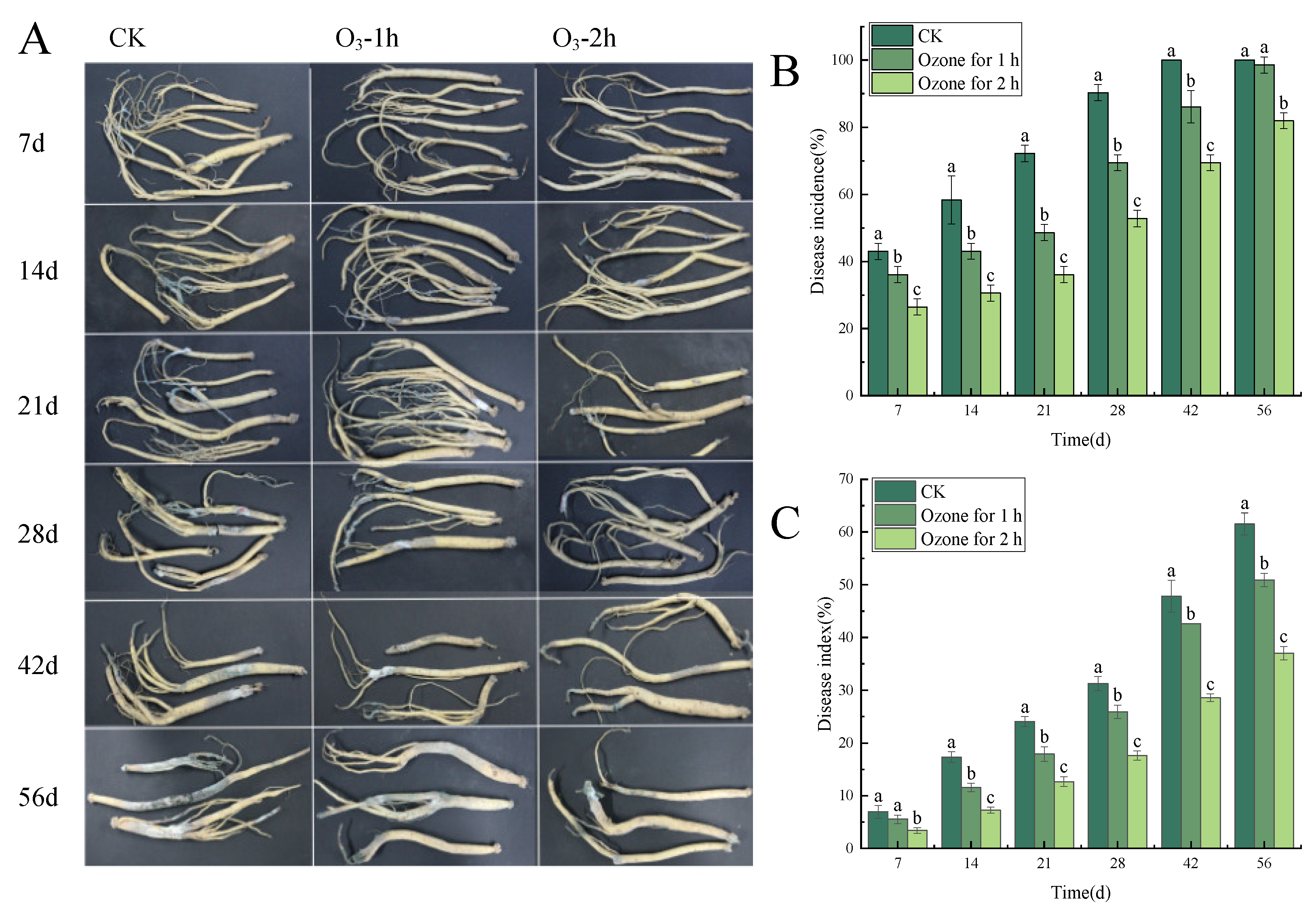
3.1. Ozone Treatment Controlled the Development of the Blue Mold of C. pilosula
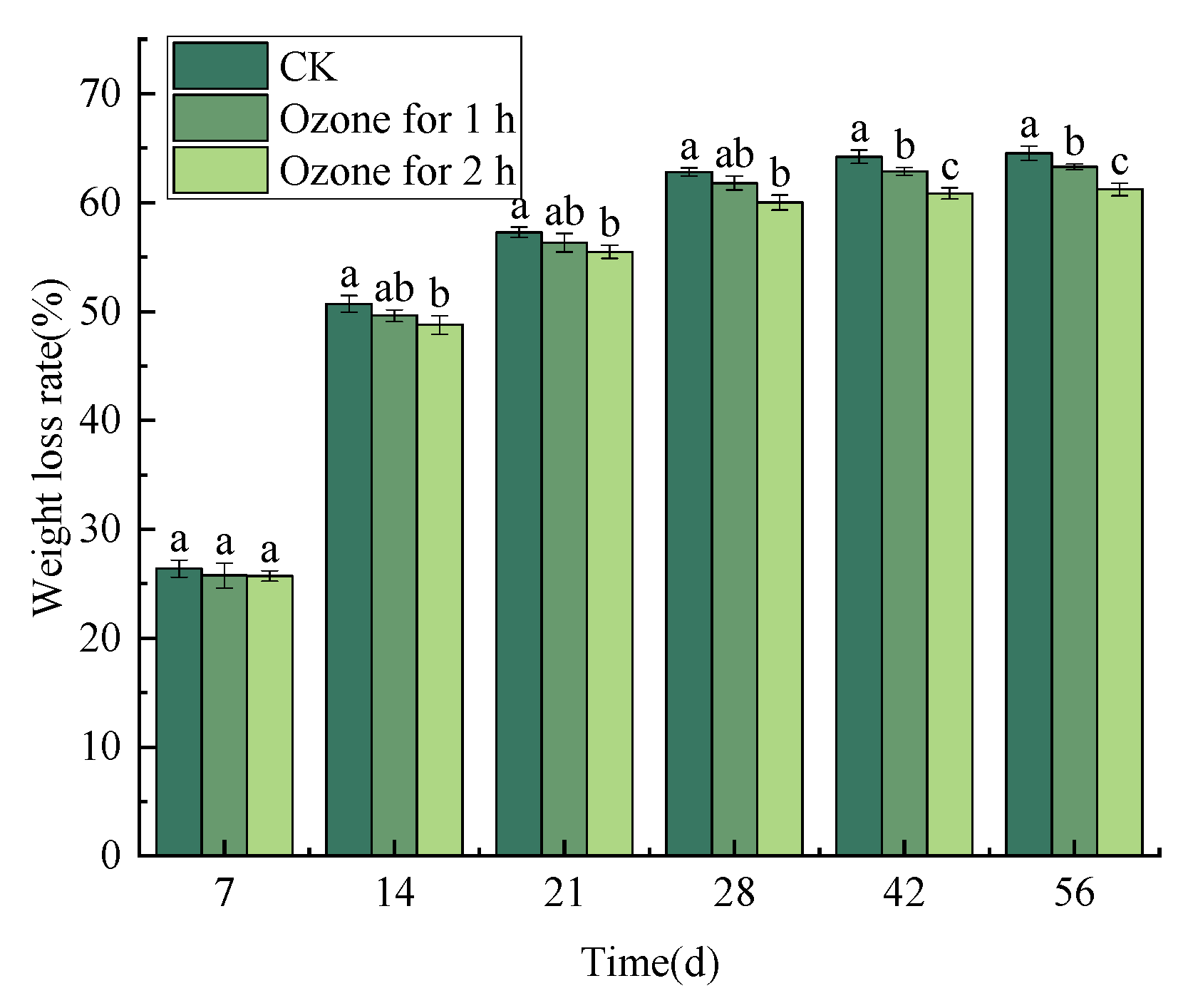
3.2. Ozone Treatment Suppressed the Weight Loss Rate of C. pilosula Inoculated with P. expansum
3.3. Effect of Ozone Treatment on Patulin Accumulation in C. pilosula Inoculated with P. expansum
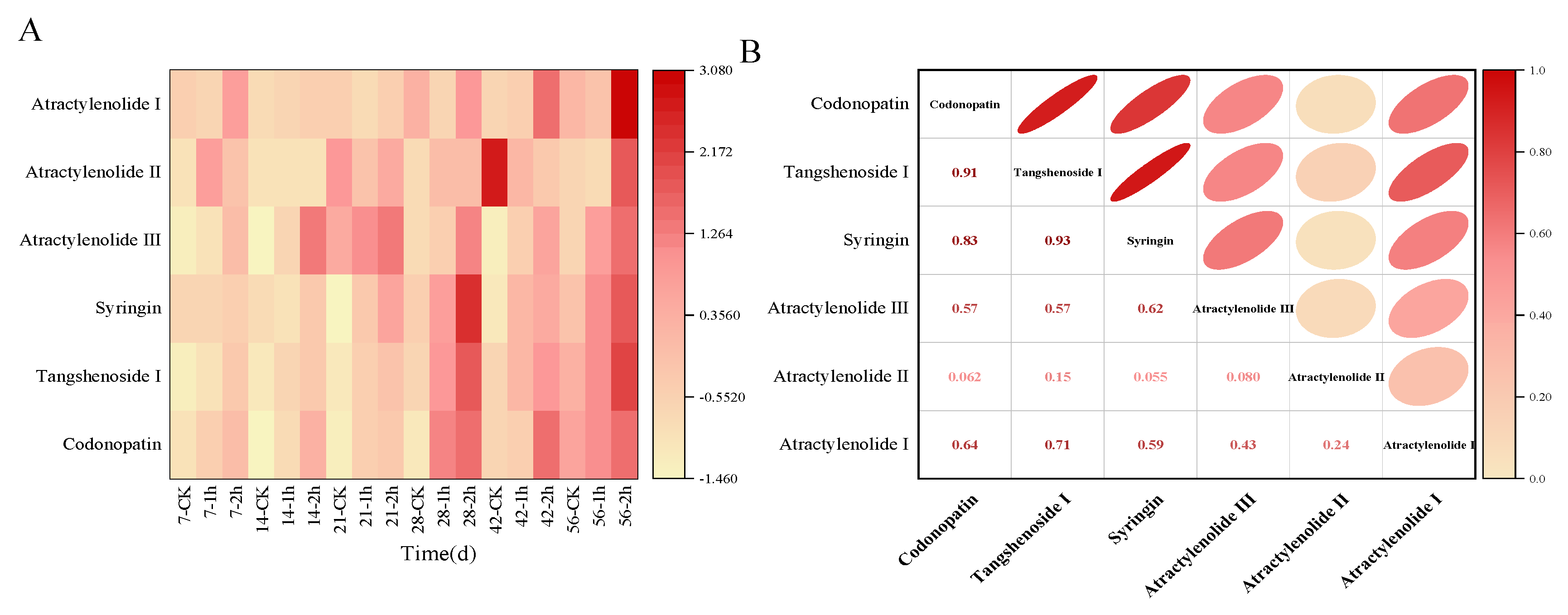
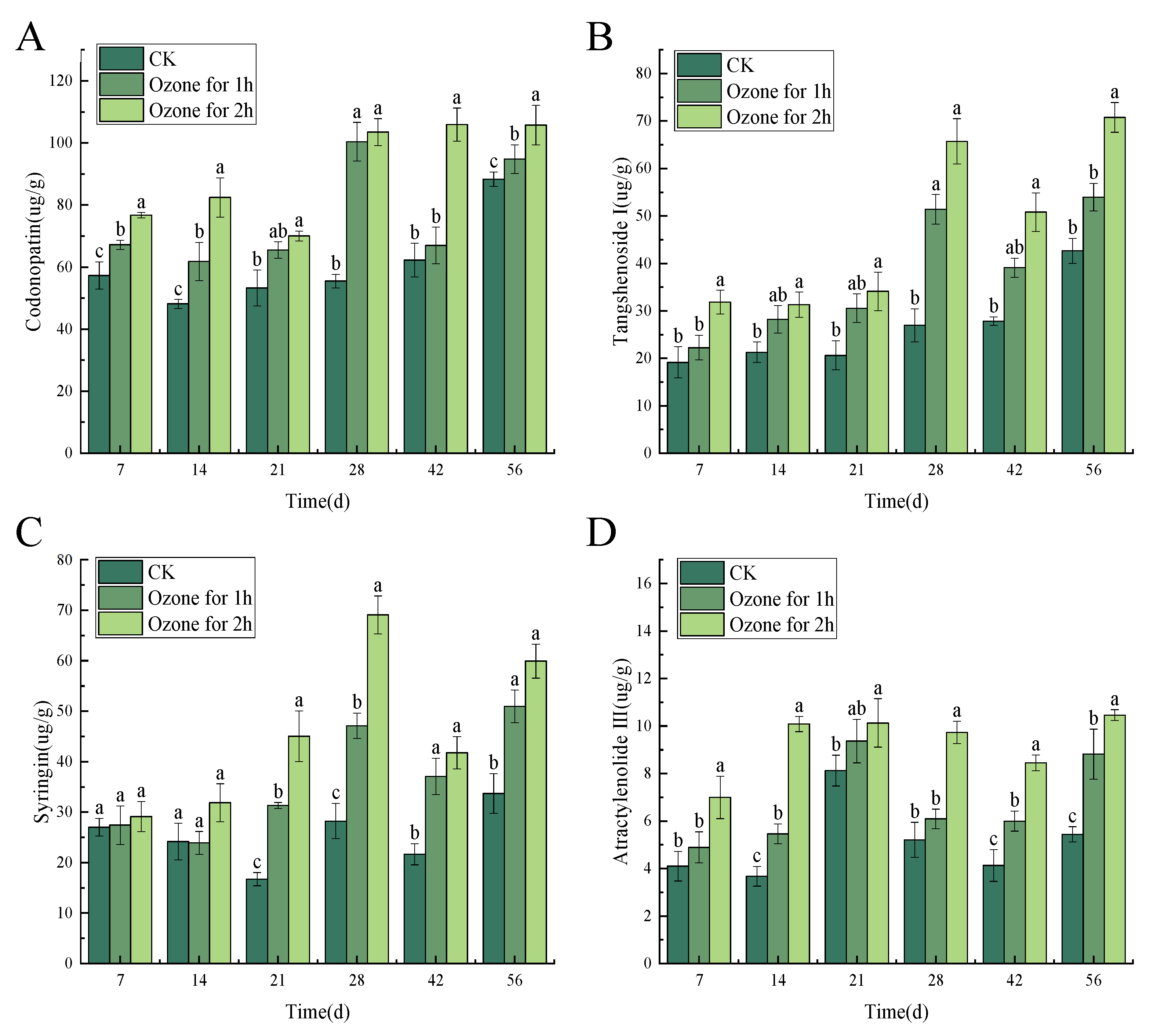
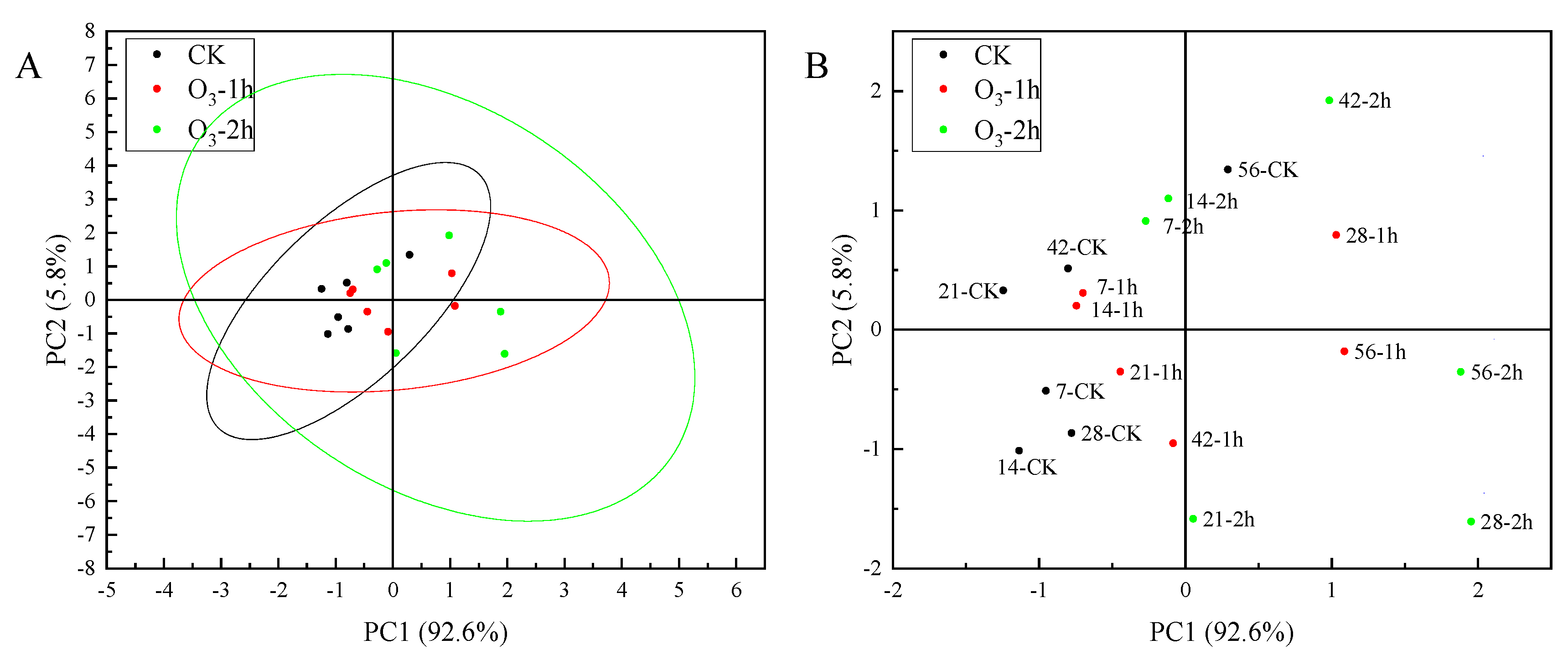
3.4. Ozone Treatment Maintained the Content of Main Active Ingredients in C. pilosula Inoculated with P. expansum
3.5. Ozone Treatment Activated ROS Metabolism in C. pilosula Inoculated with P. expansum
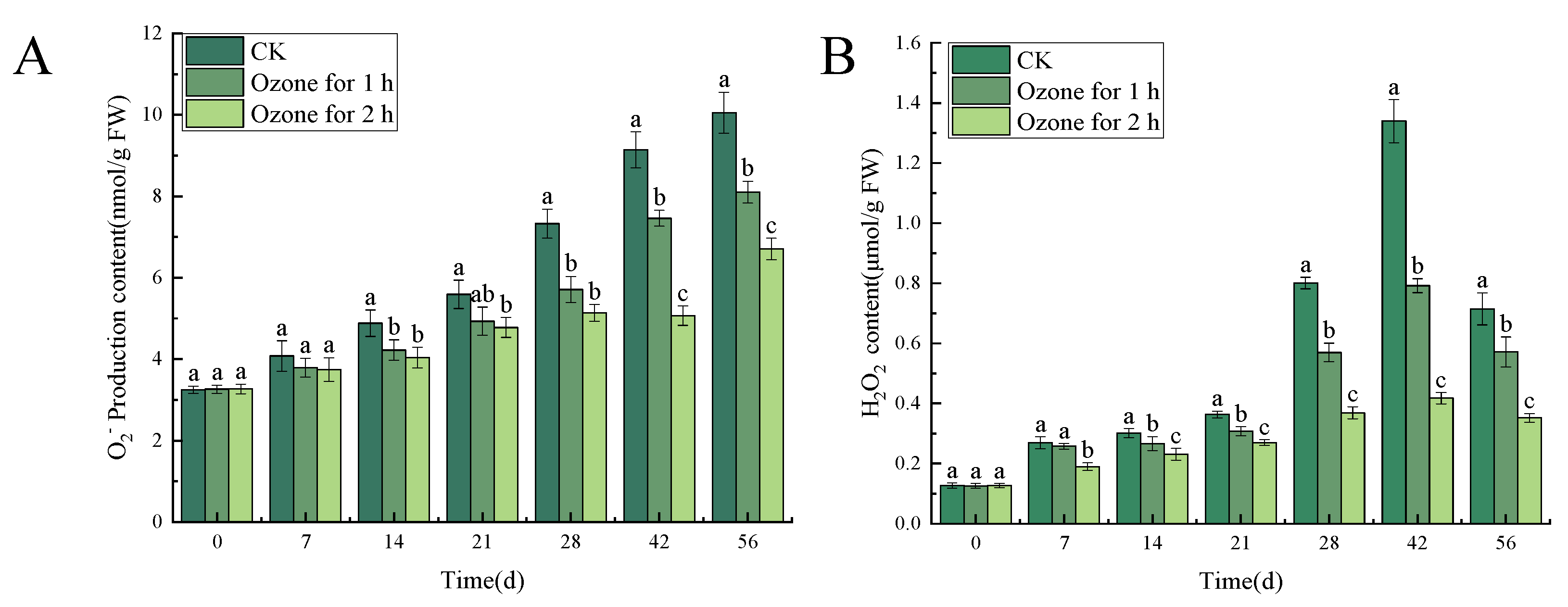
3.5.1. Ozone Treatment Suppressed the Accumulation of O2−. and H2O2 in the Inoculated C. pilosula
3.5.2. Ozone Treatment Increased the Activities of NOX, SOD, CAT and POD in C. pilosula
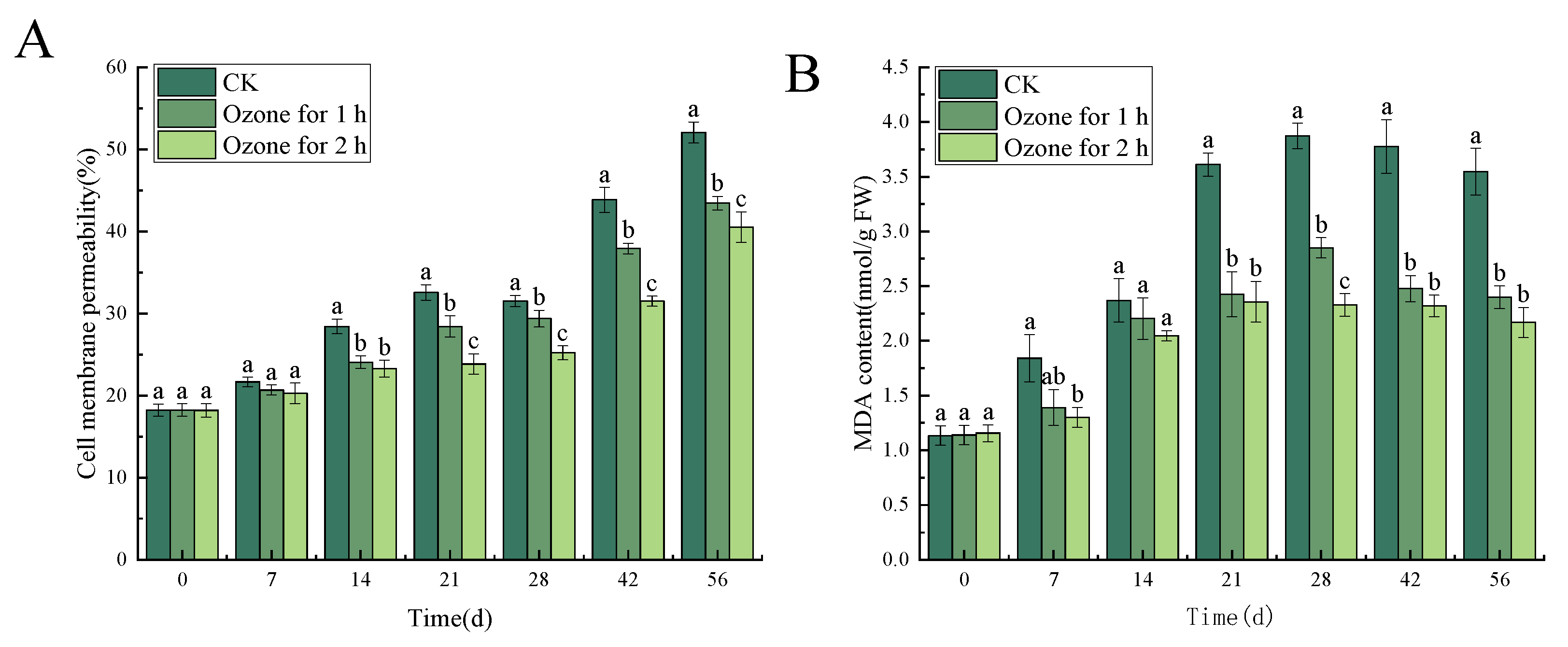
3.6. Effects of Ozone Treatment on Cell Membrane Permeability and MDA Content in C. pilosula
4. Discussion
4.1. Ozone Treatment Inhibits the Occurrence of the Blue Mold of C. pilosula
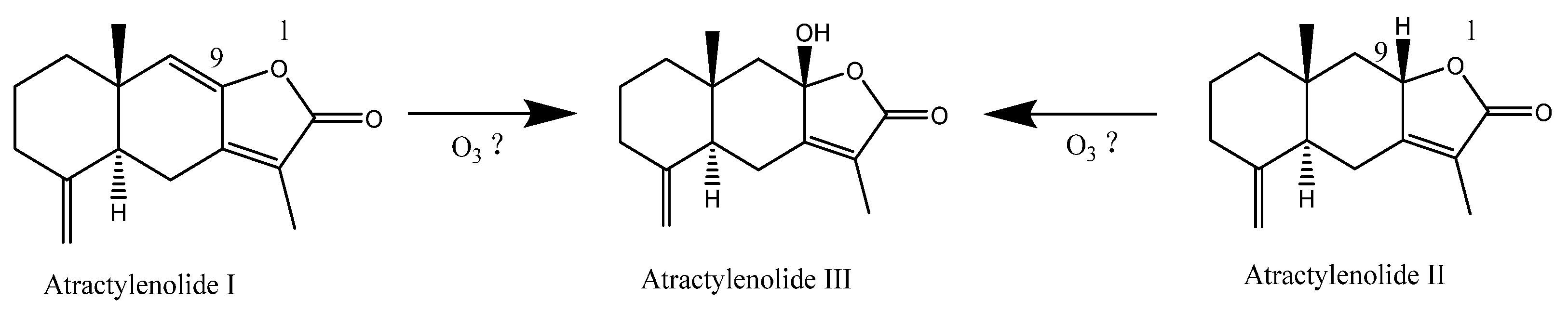
4.2. Effect of Ozone Treatment on the Content of Main Effective Components in C. pilosula Inoculated with P. expansum
4.3. Effects of Ozone Treatment on ROS Metabolism in C. pilosula Tissue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lodi, R.S.; Dong, X.; Jiang, C.; Sun, Z.; Deng, P.; Sun, S.; Wang, X.; Wang, H.; Mesa, A.; Huang, X.; et al. Antimicrobial activity and enzymatic analysis of endophytes isolated from Codonopsis pilosula. FEMS Microbiol. Ecol. 2023, 99, fiad071. [Google Scholar] [CrossRef]
- Du, Y.E.; Lee, J.S.; Kim, H.M.; Ahn, J.-H.; Jung, I.H.; Ryu, J.H.; Choi, J.-H.; Jang, D.S. Chemical constituents of the roots of Codonopsis lanceolata. Arch. Pharm. Res. 2018, 41, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Li, B.; Qi, Y.; Wei, X.; Liu, H.; Zhang, B.; Xiao, P. The effective approach for the quality control of Codonopsis radix based on quality markers of immune activity. J. Sep. Sci. 2022, 45, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Z.; Zhang, C.; Jing, L.R.; Feng, M.; Yang, Y. The structural characterization and immune modulation activities comparison of Codonopsis pilosula polysaccharide (CPPS) and Selenizing cpps (sCPPS) on mouse in vitro and vivo. Int. J. Biol. Macromol. 2020, 160, 814–822. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, Y.; Jia, X.; Fan, J.; Hou, X.; Wang, Y.; Li, X.; Han, J.; Hu, F. Isolation, characterization and immunomodulatory activity of oligosaccharides from Codonopsis pilosula. J. Funct. Foods. 2020, 72, 104070. [Google Scholar] [CrossRef]
- Guo, L.Z. Study on the pharmacological effects and clinical application of Dangshen Tonic. China Health Stand. Manag. 2015, 22, 130–131. [Google Scholar]
- Gao, S.M.; Liu, J.S.; Wang, M.; Cao, T.T.; Qi, Y.D.; Zhang, B.G.; Liu, H.T.; Sun, X.B.; Xiao, P.G. Quantitative and HPLC fingerprint analysis combined with chemometrics for quality evaluation of Codonopsis Radix processed with different methods. Chinese Herb. Med. 2019, 11, 160–168. [Google Scholar] [CrossRef]
- Nam, M.; Jo, S.R.; Kim, Y.C.; Kim, M.S. UPLC-QTOF-MS-Based metabolomics and antioxidant capacity of Codonopsis lanceolata from different geographical origins. Foods 2023, 12, 267. [Google Scholar] [CrossRef]
- Wang, K.; Ngea, G.L.N.; Godana, E.A.; Shi, Y.; Lanhuang, B.; Zhang, X.; Zhao, L.; Yang, Q.; Wang, S.; Zhang, H. Recent advances in Penicillium expansum infection mechanisms and current methods in controlling P. expansum in postharvest apples. Crit. Rev. Food. Sci. 2023, 63, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cui, R.; Zhang, F.; Meng, X.H.; Liu, B.J. Current situation and future challenges of patulin reduction—A review. Food Control 2022, 138, 108996. [Google Scholar] [CrossRef]
- Zheng, X.F.; Wei, W.N.; Zhou, W.Y.; Li, H.X.; Rao, S.Q.; Gao, L.; Yang, Z.Q. Prevention and detoxification of patulin in apple and its products: A review. Food Res. Int. 2021, 140, 110034. [Google Scholar] [CrossRef]
- Sarron, E.; Pascale, G.W.; Thierry, A. Ozone treatments for preserving fresh vegetables quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.Y.; Wang, T.Y.; Li, C.; Wu, Z.X. Effects of ozone treatment on pesticide residues in food: A review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Kaur, K.; Pandiselvam, R.; Kothakota, A.; Ishwarya, S.P.; Zalpouri, R.; Mahanti, N.K. Impact of ozone treatment on food polyphenols—A comprehensive review. Food Control 2022, 142, 109207. [Google Scholar] [CrossRef]
- Luo, A.W.; Bai, J.Q.; Li, R.; Fang, Y.M.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.Z.; Kou, L.P. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Vedhanayakisri, K.A.; Chang, W.-S.; Senthilkumar, P.; Choi, K.-M.; Lee, J.-H.; Oh, B.-T. Molecular and phenotypic characterization of pathogenic fungal strains isolated from ginseng root rot. Physiol. Mol. Plant Pathol. 2018, 104, 141–146. [Google Scholar] [CrossRef]
- Liu, Q.L.; Zhang, R.; Xue, H.L.; Bi, Y. Ozone controls potato dry rot development and diacetoxyscirpenol accumulation by targeting the cell membrane and affecting the growth of Fusarium sulphureus. Physiol. Mol. Plant Pathol. 2023, 199, 112222. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Jia, Q.; Fan, X.K.; Shu, F.X.; Chen, Z.W.; Zhou, Q.N.; Shi, J.H.; Chen, J.S. Molecular identification of endophytic fungi and their pathogenicity evaluation against dendrobium nobile and dendrobium officinale. Int. J. Mol. Sci. 2020, 21, 316. [Google Scholar] [CrossRef]
- Lv, B.; Yang, X.; Xue, H.; Nan, M.; Zhang, Y.; Liu, Z.; Bi, Y.; Shang, S. Isolation of main pathogens causing postharvest disease in fresh Codonopsis pilosula during different storage stages and ozone control against disease and mycotoxin accumulation. J. Fungi 2023, 9, 146. [Google Scholar] [CrossRef]
- Bao, G.; Bi, Y.; Li, Y.; Kou, Z.; Hu, L.; Ge, Y.; Wang, D. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiol. Mol. Plant Pathol. 2014, 86, 35–42. [Google Scholar] [CrossRef]
- Fan, M.C.; Li, W.X.; Hu, X.L. Effect of micro-vacuum storage on active oxygen metabolism, internal browning and related enzyme activities in Laiyang pear (Pyrus bretschneideri Reld). LWT-Food Sci. Technol. 2016, 72, 467–474. [Google Scholar] [CrossRef]
- Venisse, J.; Gullner, S.G.; Brisset, M.N. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.-C. Salicylic acid treatment suppresses Phomopsis longanae Chi-induced disease development of postharvest longan fruit by modulating membrane lipid metabolism. Postharvest Biol. Technol. 2020, 164, 111168. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Wang, B.; Zhang, J.; Nie, J. Dynamic degradation of penconazole and its effect on antioxidant enzyme activity and malondialdehyde content in apple fruit. Sci. Hortic. 2022, 300, 111053. [Google Scholar] [CrossRef]
- Wang, M.; Du, Y.M.; Jiao, W.X.; Fu, M.R. Effects of fruit tissue pH value on the Penicillium expansum growth, patulin accumulation and distribution. J. Food Process. Preserv. 2022, 46, 16881. [Google Scholar] [CrossRef]
- Simla, S.; Boontang, B.; Harakotr, B. Anthocyanin content, total phenolic content, and antiradical capacity in different ear components of purple waxy corn at two maturation stages. Aust. J. Crop Sci. 2016, 10, 675–682. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.C.; Meng, J.L.; Liu, J.Y.; Cheng, Y.F.; Feng, C.P. Effect of ozone treatment on the quality and enzyme activity of Lentinus edodes during cold storage. J. Food Process. Preserv. 2020, 44, 14557. [Google Scholar] [CrossRef]
- Li, B.Q.; Chen, Y.; Zong, Y.Y.; Xu, X.D.; Tian, S.P. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef]
- Xi, J.H.; Yang, D.Y.; Xue, H.L.; Liu, Z.G.; Bi, Y.; Zhang, Y.; Yang, X.; Shang, S. Isolation of the main pathogens causing postharvest disease in fresh Angelica sinensis during different storage stages and impacts of ozone treatment on disease development and mycotoxin production. Toxins 2023, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Kong, W.J.; Liu, Q.T.; Liu, H.M.; Zhao, G.; Yang, M.H. Study on the influence of Aspergillus flavus contamination on the quality of Radix Astragali and its storage condition optimization. Mod. Chin. Med. 2015, 17, 1133–1138. [Google Scholar]
- Shetty, N.P.; Jørgensen, H.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, C.K.; Luo, H.X.; Xu, W.T.; Zhang, H.J.; Tian, J.J.; Ju, R.H.; Wang, L.Q. The combined effect of ozone treatment and polyethylene packaging on postharvest quality and biodiversity of Toona sinensis (A.Juss.) M.Roem. Postharvest Biol. Technol. 2019, 154, 1–10. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Ji, L.L.; Chen, C.K.; Wang, C.R. Effects of ozone treatment on the storage quality of post-harvest tomato. Int. J. Food Eng. 2018, 14, 7–8. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Y.X.; Yang, S.H.; Li, C.; Li, L.; Gao, S.Y.; Wu, Z.X. Mechanism of ozone treatment in delayed softening of fresh-cut kiwifruit during storage. Postharvest Bio. Technol. 2023, 201, 112469. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, Z.; Liu, Q.; Zhang, D.; Xue, H.; Shang, S.; Bi, Y. Ozone Application Suppressed the Blue Mold Development and Maintained the Main Active Ingredients Content of Postharvest Fresh Codonopsis pilosula during Storage. J. Fungi 2024, 10, 163. https://doi.org/10.3390/jof10030163
Chen J, Liu Z, Liu Q, Zhang D, Xue H, Shang S, Bi Y. Ozone Application Suppressed the Blue Mold Development and Maintained the Main Active Ingredients Content of Postharvest Fresh Codonopsis pilosula during Storage. Journal of Fungi. 2024; 10(3):163. https://doi.org/10.3390/jof10030163
Chicago/Turabian StyleChen, Jiangyang, Zhiguang Liu, Qili Liu, Dan Zhang, Huali Xue, Suqin Shang, and Yang Bi. 2024. "Ozone Application Suppressed the Blue Mold Development and Maintained the Main Active Ingredients Content of Postharvest Fresh Codonopsis pilosula during Storage" Journal of Fungi 10, no. 3: 163. https://doi.org/10.3390/jof10030163
APA StyleChen, J., Liu, Z., Liu, Q., Zhang, D., Xue, H., Shang, S., & Bi, Y. (2024). Ozone Application Suppressed the Blue Mold Development and Maintained the Main Active Ingredients Content of Postharvest Fresh Codonopsis pilosula during Storage. Journal of Fungi, 10(3), 163. https://doi.org/10.3390/jof10030163







