Abstract
Banana is one of the most important fruits in the world due to its status as a major food source for more than 400 million people. Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4) causes substantial losses of banana crops every year, and molecular host resistance mechanisms are currently unknown. We here performed a genomewide analysis of the autophagy-related protein 8 (ATG8) family in a wild banana species. The banana genome was found to contain 10 MaATG8 genes. Four MaATG8s formed a gene cluster in the distal part of chromosome 4. Phylogenetic analysis of ATG8 families in banana, Arabidopsis thaliana, citrus, rice, and ginger revealed five major phylogenetic clades shared by all of these plant species, demonstrating evolutionary conservation of the MaATG8 families. The transcriptomic analysis of plants infected with Foc TR4 showed that nine of the MaATG8 genes were more highly induced in resistant cultivars than in susceptible cultivars. Finally, MaATG8F was found to interact with MaATG4B in vitro (with yeast two-hybrid assays), and MaATG8F and MaATG4B all positively regulated banana resistance to Foc TR4. Our study provides novel insights into the structure, distribution, evolution, and expression of the MaATG8 family in bananas. Furthermore, the discovery of interactions between MaATG8F and MaATG4B could facilitate future research of disease resistance genes for the genetic improvement of bananas.
1. Introduction
Banana (Musa spp.) is a major staple food crop with great economic importance; globally, ~124.98 million tons of bananas are produced annually [1]. Banana plants are widely distributed in tropical and subtropical regions, including Africa, Latin America, the Caribbean, Asia, and the Pacific. In some parts of Africa, bananas provide up to 25–35% of daily caloric intake to inhabitants [2]. However, banana is highly susceptible to Fusarium wilt, which is caused by Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Fusarium wilt has a devastating economic impact on the banana industry and has gradually spread to major banana-producing countries; it was identified in Mozambique in 2013, Colombia in 2019, and Peru in 2021 (ProMusa, https://www.promusa.org/, access on 1 December 2023).
Efficient strategies to control Fusarium wilt have not yet been identified. Chemical controls are ineffective because the fungi are soil-borne and affect the vascular bundle of host plants. The repeated use of chemical fungicides also has negative impacts on the environment and human health. However, biological controls have proven unsatisfactory in field experiments [3]. Therefore, disease resistance breeding is the most promising strategy for Foc TR4 management. This approach will require additional research into host species’ Foc TR4 resistance mechanisms, particularly those involving autophagy-related proteins.
The eukaryotic autophagy pathway is a system of controlled cellular degradation that is highly conserved among animals, fungi, and plants [4,5,6]. Autophagy in plants involves a series of five steps: induction, elongation, completion, fusing, and degradation [7]. Kinase signaling activates the ATG1/ATG13 complex to form autophagosomes [8,9,10]. Autophagosomes then transport intracellular components to the vacuoles for degradation by various autophagy-related proteins at different stages of the process [11,12].
The key gene ATG1 was the first autophagy-related gene discovered [13]. It regulates autophagy initiation and autophagosome formation [14,15]. In Arabidopsis thaliana and Camellia sinensis, at least 49 ATG genes have been discovered thus far [16]. Proteins involved in autophagy tend to be highly conserved across many eukaryotic species [17,18]. One such protein, ATG8, is initially processed by a cysteine protease (ATG4) to expose a glycine residue at the C-terminus [19]. Phosphatidylethanolamine (PE) is then attached to this residue by ATG7 and the E2-like enzyme ATG3. Finally, PE is removed from ATG8 by ATG4, leaving free ATG8 available to form autophagosomes [14]. Many reports have indicated that autophagosome formation fails in the absence of ATG8 [20].
Plant biotic stress responses to pathogens involve activation of numerous genes, including those encoding autophagy-related proteins [21,22,23]. Some pathogens suppress host defenses by interfering with autophagy. For example, SDE3 effector in Candidatus Liberibacter undermines autophagy-mediated immunity through the specific degradation of citrus ATG8 family proteins [24]. ATG8 also participates in regulating host plant disease resistance, and disruption of normal ATG8 function can therefore reduce plant defenses and promote infection. For example, the γb protein of barley stripe mosaic virus (BSMV) inhibits autophagy in the host plant Hordeum vulgare by directly interacting with ATG7, preventing ATG–ATG8 interactions and thus promoting successful infection [25]. In apple (Malus domestica), MdATG8i decreases pathogen sensitivity by interacting with the target protein MdEF-Tu. The Valsa Mali effector Vm1G-1794 inhibits autophagy by competitively binding to MdATG8i, thus weakening plant resistance. In contrast, MdATG8i overexpression significantly improves pathogen resistance [21]. Prior studies suggested that the causative agent of apple rot manipulates the apple autophagy pathway through secretion of specific effector proteins. ATG4 and ATG6/Beclin-1 were also shown to participate in autophagy induced by biotic and abiotic stressors [26]. Such studies demonstrated the important roles of ATG8s in host plant infection resistance. Previous reports only simply identified banana ATG genes at the genome scale or characterized their roles in Foc TR4 resistance [27]. However, the molecular-level differences between resistant and susceptible cultivars are still unknown.
In the present study, pathogen-induced ATG8 family members were identified throughout the banana genome. Gene structures, conserved domains, phylogenetic relationships, chromosomal locations, and expression patterns of MaATG8 genes in response to Foc TR4 infection were analyzed. Furthermore, MaATG8 functions were established through the assessment of interactions with other ATGs and the effects of gene silencing in banana during Foc TR4 infection. This study provides valuable new insights that will inform future research into the molecular mechanisms of autophagy and resistance breeding in banana.
2. Materials and Methods
2.1. Culture Conditions for Plants and Fungi
The Cavendish banana (Musa spp. AAA group) cultivars ‘ZhongJiao No. 6’ (ZJ6) and ‘Brazilian’ (BX) were used as resistant cultivar and susceptible cultivar in this research. ZJ6 (from Fruit Research Institute, Guangdong Academy of Agricultural Sciences, China) is artificially bred from BX. The banana plantlets at the five- to six-leaf stage were used in a controlled greenhouse at 28 °C under a 14/10 h light/dark cycle. Nicotiana benthamiana plants, a plant model with a clear genetic background, were grown for ~28 d in a greenhouse at 24 °C under a 16/8 h light/dark cycle. Prior to plant inoculation, Foc TR4 strain II5 (NRRL#54006), a model strain with a clear genetic background in research studies of the banana host and Foc, was cultured on potato dextrose agar (PDA) for 6 d or in potato dextrose broth (PDB) for 3 d.
2.2. Identification of ATG8 Genes in Multiple Species
The amino acid (aa), genomic DNA (gDNA), and coding sequences (CDS) of Musa. acuminata were downloaded from the Banana Genome Database (http://banana-genome-hub.southgreen.fr/, access on 7 October 2023). A hidden Markov model (HMM) for ATG8 (PF02991) was downloaded from the Pfam database (http://pfam.xfam.org/, access on 7 October 2023). The M. acuminata genome was then searched using PF02991 as a query with the Simple HMM Search in TBtools. NCBI CDD search (https://www.ncbi.nlm. nih.gov/cdd, access on 7 October 2023) was used for domain validation in the resulting sequences. Using candidate protein IDs, the aa, gDNA, and CDS were obtained with the TBtools sequence extraction tool (Fasta Extract) [28].
2.3. Chromosomal Locations of MaATG8s
Chromosome location mapping was conducted with the Gene Location Visualize from GTF/GFF function of TBtools based on the MaATG8 gene IDs from the M. acuminata gff3 files. M. acuminata genome sequences were analyzed in pairwise comparisons using One Step MCScanX in TBtools. MaATG8 gene family collinearity was visualized in other species with Multiple Synteny Plot in TBtools. The chromosomal coordinates of MaATG8 genes were extracted from the annotated M. acuminata genome files and visualized using TBtools.
2.4. RNA Extraction and Quantitative Reverse Transcription (qRT)-PCR
RNA from banana roots was extracted with an RNA extraction kit (Accurate Biotech, Guluo, Hunan, China) following the manufacturer’s instructions. Briefly, the banana roots were ground into powder in liquid nitrogen at low temperatures. The RNA extraction kit was used to extract the RNA from roots. The kit contained RNase-free Recombinant DNase I to eliminate genomic DNA. Reverse transcription was performed with the Evo M-MLV One Step RT-PCR Kit (Accurate Biotech, Hunan, China). qRT-PCR was performed on a StepOne real-time PCR system (Applied Biosystems, Waltham, MA, USA) with ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China) following the manufacturer’s instructions. Gene relative expression levels were determined using the 2−∆∆Ct method with the endogenous reference gene MaTUB (β-tubulin). There were three biological replicates of each sample type, and qRT-PCR was performed in technical triplicate. All primer pairs are shown in Table S2.
2.5. Banana Transcriptomic Analysis
The roots of BX and ZJ6 plants were inoculated with Foc TR4 strain II5 and collected after 18, 32, and 56 h. There were three biological replicates per cultivar for each time point. The Illumina HiSeq×10 platform was used to generate 150 bp paired-end reads with Gene Denovo Biotechnology Co. (Guangzhou, China). Filtered reads were aligned to the M. acuminata genome using HISAT2 v2.0.5 [29]. Differential gene expression was calculated using the ‘DESeq2’ R package. Genes were considered significantly differentially expressed at|log2 (fold change)| > 1 (p < 0.05), indicating genes were upregulated or downregulated.
2.6. Plasmid Construction
MaATG8 genes were cloned from cDNA generated from Cavendish banana roots following the manufacturer’s instructions (2 × Phanta Max Master Mix, Vazyme Biotech, Nanjing, China). The amplified fragments were ligated into the BamHI-digested pCAMBIA1300-GFP (Green Fluorescent Protein, GFP) empty vector (MiaoLingBio, Wuhan, China), or SmaI-digested pGBKT7 or pGADT7 empty vector (Clontech, Mountain View, CA, USA) using the In-Fusion Cloning Kit (Vazyme Biotech, Nanjing, China). The primers used for plasmid construction are listed in Table S2. Individual colonies containing each construct were verified via PCR and sequencing (Sangon, Shanghai, China).
2.7. Subcellular Localization
The MaATG8F CDS was cloned into the pCAMBIA1300-35S:GFP empty vector, and the resulting constructs were transformed into Agrobacterium tumefaciens strain GV3101 (WeidiBio, Shanghai, China). Agrobacterium-mediated transient expression in N. benthamiana leaves was performed as previously described with some modifications [30]. Briefly, transformed cells were cultured for 8 h in liquid Luria–Bertani (LB) medium at 28 °C. Bacterial cells were harvested via centrifugation, then resuspended in infiltration medium containing 10 mM 2-(4-Morpholino) ethanesulfonic acid, 10 mM MgCl2, and 200 μM acetosyringone (pH = 5.6) (Sangon Biotech, Shanghai, China). The concentration of the cell suspension was adjusted to an OD600 of 1.0, and then the suspension was incubated for 2–3 h at room temperature prior to infiltration into the leaves of four-week-old N. benthamiana plants. The leaves were observed under a confocal microscope (LSM 710, Carl Zeiss, Oberkochen, Germany) at 72 h postinfiltration.
2.8. Inoculation Assays
To determine banana plant phenotypes after Foc TR4 infection, banana cultivars of two different resistance levels were selected: BX and ZJ6. ZJ6 is a resistant cultivar whereas BX is highly susceptible to Foc TR4. The inoculation assays were performed as previously described with some modifications [31]. Briefly, these two banana cultivars were grown in a greenhouse to the five- to six-leaf stage for use in inoculation assays. Banana cultivars were dipped with Foc TR4 suspension at a concentration of 1×107 conidia/L for half an hour. The plants were then replanted in soil. A total of 30 banana plantlets were used for each treatment. Each experiment was repeated three times independently. Banana corms were observed and photographed at 35 d postinoculation and graded for disease appearance as follows: 0 (no symptoms), 1 (some brown spots in the inner rhizome), 2 (less than 25% of the inner rhizome browning), 3 (up to 75% of the inner rhizome browning), and 4 (entire inner rhizome and pseudostem dark brown and dead). The disease index was calculated as follows:
100 × [∑ (total number of plants × disease grade)/(total number of plants × 4)]
2.9. Yeast Two-Hybrid (Y2H) Assays
The Y2H system was used to identify protein–protein interactions in vitro. The MaATG4B CDS was cloned inframe into the bait vector pGBKT7, and the CDSs of MaATG8 genes were cloned inframe into the prey vector pGADT7 using the In-Fusion Cloning Kit (Vazyme Biotech, Nanjing, China). The primers used for plasmid construction are listed in Table S2. The Y2H Gold yeast strain (WeidiBio, Shanghai, China) was cotransformed with the resulting bait and prey vectors to identify positive interactions following the manufacturer’s protocol (Clontech, Mountain View, CA, USA). The cotransformants of pGBKT7-53 + pGADT7-T and pGBKT7-lam + pGADT7-T are presented as positive control and negative control, respectively. SD/-leucine-tryptophan-adenine-histidine medium (SD/-LWAH) with the addition of X-α-gal was used to screen the positive interactors at 30 °C in the dark for about 3 days, and SD/-leucine-tryptophan medium (SD/-LWAH) was used to screen the positive transformants. X-α-gal was used to confirm the positive interactions by turning the colonies blue. The analysis of this experiment was to observe if the yeast colonies grew on SD/-LWAH and SD/-LW media, and if colonies turned blue on the SD/-LWAH medium with added x-α-gal. If the colonies grew on SD/-LW and appeared blue on SD/-LWAH after about 3 days, the results would indicate that they were interacting. The plates were then photographed using a Nikon camera.
2.10. Gene Silencing
dsRNAs of ~300 bp in size that were complementary to MaATG8F and MaATG4B were generated with the T7 RNAi Transcription Kit following the manufacturer’s protocol (Vazyme Biotech, Nanjing, China). The PCR technique was used to add the T7 promoter sequence to both ends of the RNA interference (RNAi) target fragments. The primers used for PCR are listed in Table S2. Sequences including the T7 promoters were purified and used as the templates for dsRNA amplification. Infection assays were then performed to establish the effects of knocking out target genes performed as in a previous study with some modifications [32]. Specifically, banana leaves of the same age were detached and pressed with the tip of a pipette head to make even wounds, then treated with 10 μL dsRNA at a concentration of 500 ng/μL with 0.02% Silwet L-77 (Solarbio Science & Technology, Beijing, China). The sites in the same leaves treated with 10 μL water with 0.02% Silwet L-77 were used as a control. The Silwet L-77 was a surfactant used for facilitating the adsorption and spread of dsRNA or water on the banana leaves. The dsRNA solution and water were allowed to dry for about 1 h, then mycelial plugs of 5 mm in diameter were taken from plates containing 6-day-old Foc TR4 strain II5. Plugs were taken only from the growing edges and were placed onto the portion of banana leaves treated with dsRNA. The mycelium was facing down, touching the banana leaves. A total of 10 banana leaves were used for each treatment. Each experiment was repeated three times independently. All leaves were then moved to plastic trays, each of which was lined with two layers of moistened paper towel. To maintain sufficiently high humidity, each tray was covered with plastic film. Trays containing inoculated leaves were incubated at 28 °C in the dark for 10 days. Leaves were then photographed, and the fungal lesion size was quantified in ImageJ V1.8.0 software (https://imagej.nih.gov/ij/, access on 20 November 2023). Leaves were also harvested for qRT-PCR to verify gene silencing.
2.11. Trypan Blue Staining
Trypan blue staining was performed as previously described [33] with some modifications. Briefly, banana leaves were harvested and soaked in trypan blue solution (0.02% trypan blue in 1:1:1:1:8 phenol: glycerol: lactic acid: water: ethanol (v/v/v/v/v)) at room temperature overnight. Leaf samples were destained in 75% alcohol several times until the destaining solution remained clear. The samples were then photographed using a Nikon camera.
3. Results
3.1. Genomewide Identification of MaATG8 Genes
Analysis of the M. acuminata genome revealed 10 putative MaATG8 genes (Table S1). The longest MaATG8 protein (MaATG8D) was 141 amino acids (aa) in length, and the shortest (MaATG8E) was 80 aa. A neighbor-joining phylogenetic tree constructed from ATG8 genes in banana, A. thaliana, rice, citrus, and ginger showed five major phylogenetic clades, which were conserved in banana (10 genes), A. thaliana (8 genes), rice (6 genes), citrus (11 genes), and ginger (20 genes) (Table S1, Figure 1A). MaATG8E alone formed a separate branch (Figure 1A). Overall, this phylogenetic analysis showed that ATG8 homologs were conserved among several plant species of varying evolutionary distances from one another. Furthermore, aa sequence alignment showed that the MaATG8 proteins were highly evolutionarily conserved (Figure 1B).
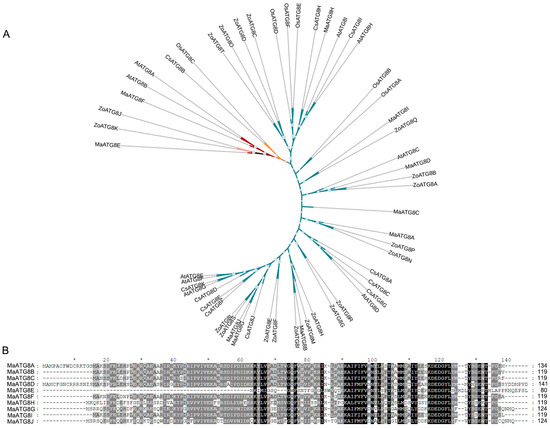
Figure 1.
Genomewide identification of ATG8 genes. (A) Phylogenetic analysis of MaATG8 proteins in banana, Arabidopsis thaliana, citrus, rice, and ginger. The subgroups are indicated by different frame colors. (B) Amino acid sequences alignment of 10 MaATG8 proteins in banana.
3.2. MaATG8 Gene Structures and Conserved Motif Analysis
Gene structure analysis showed notable differences in the numbers and lengths of exons in MaATG8 genes. There were nine motifs identified across the MaATG8 genes (Figure 2A). MaATG8C, MaATG8A, MaATG8F, MaATG8J, MaATG8E, and MaATG8G contained just two motifs each (motifs 1 and 2). MaATG8H had three motifs (2, 6, and 8); MaATG8B contained four motifs (1, 2, 3, and 5); and MaATG8I had five motifs (1, 2, 3, 5, and 9). MaATG8D contained the largest number of motifs at seven (motifs 2, 6, and 9 and two each of motifs 4 and 7) (Figure 2A). MaATG8D also contained two exons and no untranslated region (UTR), whereas the other nine MaATG8 genes had five exons each (Figure 2B) and one (MaATG8B and MaATG8I), two (MaATG8G, MaATG8J, and MaATG8H), or three (MaATG8E, MaATG8C, MaATG8A, and MaATG8F) UTRs (Figure 2B). These results indicate varying degrees of evolutionary conservation and divergence between the MaATG8 genes.
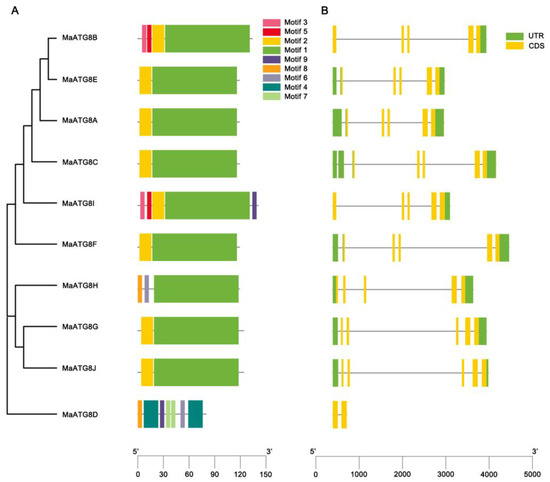
Figure 2.
Genomewide identification of banana ATG8 genes. (A) Phylogenetic analysis and motif analysis of MaATG8 proteins in banana, A. thaliana, citrus, rice, and ginger. The subgroups are indicated by different frame colors. (B) Gene structures of MaATG8 genes in banana. Yellow boxes indicate exons (coding sequence), green boxes indicate untranslated regions and gray lines indicate introns.
3.3. Predicted Cis-Acting Elements in MaATG8 Promoters
To understand the putative functional properties of MaATG8 gene family members, the PlantCARE database was used to predict cis-acting elements in the promoter regions (classified as 2000 bp upstream of the transcription start site for each gene). This analysis revealed many cis-acting elements, including low-temperature responsiveness, defense and stress responsiveness, and hormone responsiveness elements in addition to MYB binding sites. Six MaATG8s had abscisic acid (ABA) response elements and six had auxin responsiveness elements (Figure 3). For example, MaATG8A contained multiple ABRE (ABA-responsive) elements. Five MaATG8s were predicted to respond to methyl jasmonate (MeJA) (Figure 3); MaATG8D, MaATG8E, and MaATG8J each contained multiple binding sites for MeJA responsiveness. Four MaATG8s were predicted to be salicylic acid (SA)-responsive. The MaATG8s also included elements for stress responses (Figure 3). These results suggest that ATG8 genes play important roles in banana stress and hormone responses.
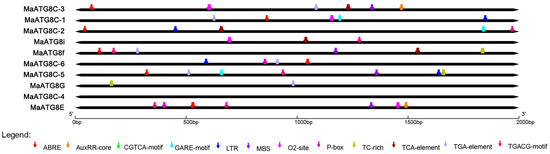
Figure 3.
The analysis of cis-acting elements in the MaATG8 gene promoters.
3.4. Chromosomal Distribution of MaATG8s
Analysis of the 10 MaATG8 locations showed that they were distributed across chromosome (Chr) 2, Chr 4, Chr 5, Chr 8, Chr 10, and Chr 11. The other banana chromosomes did not contain any ATG8 genes (Figure 4). Chr4 contained more MaATG genes (four) than any other chromosome: MaATG8B, MaATG8C, MaATG8D, and MaATG8E. Chr5 contained two MaATG8 genes (MaATG8H and MaATG8F); the remaining MaATG8s were present on Chr2, Chr8, Chr10, and Chr11, which contained just one MaATG8 each (MaATG8A, MaATG8G, MaATG8I, and MaATG8J, respectively). These results indicate that MaATG8s are distributed unevenly throughout the genome.
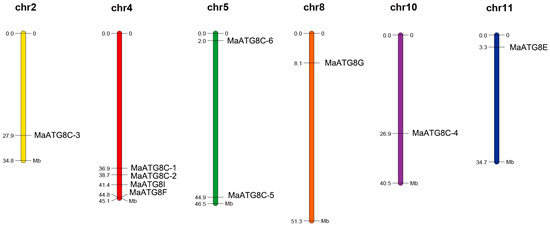
Figure 4.
Chromosomal distribution of banana MaATG8 genes. The chromosome number is shown above each chromosome. The left number represents the length of chromosomes.
3.5. Involvement of MaATG8 Family Members in the Foc TR4 Infection Response
The resistant M. acuminata cultivar ZJ6 and the susceptible cultivar BX were inoculated with Foc TR4 strain II5 at the five- to six-leaf stage. At 35 d after inoculation, the corms of BX plants showed significant browning symptoms; the disease incidence exceeded 95%, and the disease index was higher than 78% (Figure 5A,B). Among ZJ6 plants, only 43.3% showed mild disease symptoms (Figure 5A,B), and the disease index in ZJ6 plants reached only 18.3%, significantly lower than that of BX (Figure 5C). This demonstrated ZJ6 resistance to Foc TR4. To investigate MaATG8 gene expression profiles during the early infection process (at 18, 32, and 56 h postinoculation), we performed transcriptomic analyses of the susceptible and resistance cultivars. Nine of the MaATG8 genes were induced in both the resistant and susceptible cultivars; only MaATG8I was not induced (Table S3; Figure 5D). Furthermore, the remaining nine genes were all highly expressed in the resistant cultivar, but only MaATG8C (Macma4_04_g32300), MaATG8G (Macma4_08_g10850), MaATG8A (Macma4_02_g16130), MaATG8D (Macma4_04_g36560), and MaATG8H (Macma4_05_g30830) were continuously induced during the infection process (Table S3; Figure 5D). MaATG8F (Macma4_05_g02860), MaATG8J (Macma4_11_g04160), and MaATG8E (Macma4_04_g42290) were most strongly induced in the resistant cultivar (Table S3; Figure 5D). These results showed that MaATG8 genes were responsive to Foc TR4 infection and were more highly expressed in the roots of the resistant cultivar (ZJ6) than in the susceptible cultivar (BX), clearly demonstrating participation of MaATG8 genes in the banana immune response.
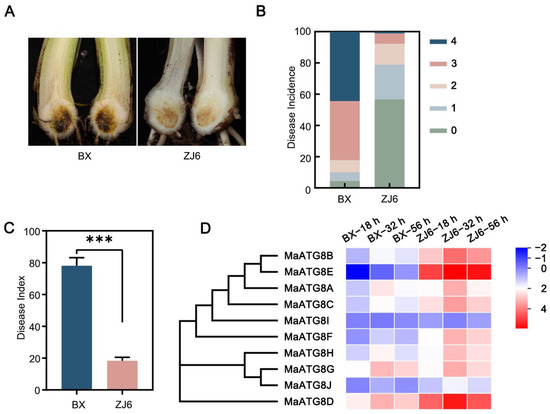
Figure 5.
MaATG8 genes were induced during Foc TR4 infection. (A) Disease symptoms, (B) disease incidence, and (C) disease index in resistant and susceptible plants inoculated with II5 strains for 5 weeks. ZJ6 is resistant cultivar while BX is susceptible cultivar. Data are presented as means ± SDs (n = 3). “***” symbol atop the columns indicates a significant difference at p < 0.001 (two-tailed Student’s t-test). (D) Heatmap for the MaATG8 genes in resistant and susceptible plants inoculated with II5 strains for different time points (18 h, 32 h, 56 h).
3.6. MaATG8F Silencing Reduced Foc TR4 Resistance
To investigate the biological function of MaATG8F in the Foc TR4 infection response, wounded banana leaves were treated with MaATG8F-dsRNA or a water control, then plugs of Foc TR4 II5 were placed on the same areas. As expected, MaATG8F expression was strongly downregulated after treatment with MaATG8F-dsRNA compared with that in the water-treated control leaves, demonstrating successful MaATG8F silencing (Figure 6A). Furthermore, leaves treated with MaATG8F-dsRNA showed more extensive necrosis than water-treated control leaves, as confirmed with both a visual comparison of the leaves (Figure 6B,C) and trypan blue staining (Figure 6D). To determine MaATG8F subcellular localization, a MaATG8F–green fluorescent protein (GFP) construct was generated with the pCAMBIA1300-35S:GFP vector, which was transiently expressed in N. benthamiana leaves. Microscopic observations of N. benthamiana leaves collected at 3 d post-agro-infiltration revealed nuclear and cytoplasmic localization of GFP:MaATG8, identical to the results of leaves infiltrated with the GFP empty vector (Figure 6E). Overall, these results indicated that MaATG8F positively regulated banana disease resistance to Foc TR4.
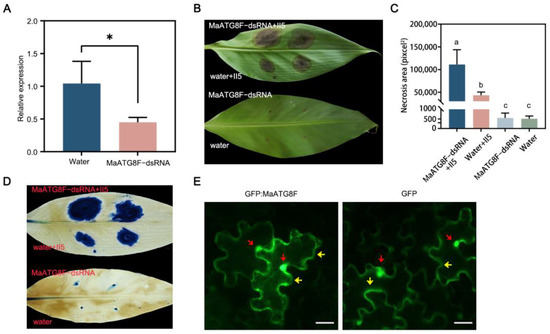
Figure 6.
MaATG8F positively regulated banana disease resistance against Foc TR4. (A) The expression level of MaATG8 in dsRNA- and water-treated banana leaves by qRT-PCR analysis. Data are presented as means ± SDs (n = 3). “*” symbol atop the columns indicates a significant difference at p < 0.05 (two-tailed Student’s t-test). (B) The disease symptoms and (C) necrosis area of dsRNA- and water-treated banana leaves. The pictures were taken 10 days postinoculation. The necrosis area was calculated with ImageJ V1.8.0 software. Data are presented as means ± SDs (n = 3). Different letters atop the columns indicate a significant difference at p < 0.01 (one-way analysis of variance). (D) Trypan blue staining of banana leaves. (E) Subcellular localization of MaATG8F in N. benthmiana leaves. Photographs were taken at 3 days postinfiltration. The empty vector (GFP) was used as control. Red arrowheads indicate nucleus, and yellow arrowheads indicate cytoplasm. Scale bar = 10 μm.
3.7. MaATG4B Interacted with MaATG8F
To investigate the molecular mechanism underlying MaATG8-mediated activation of the host immune response, yeast two-hybrid (Y2H) assays were performed to identify potential host targets of MaATG4, which are autophagy-related members of the ATG family. Interactions of ATG4s and ATG8s were previously identified in Arabidopsis [34]. MaATG4A and MaATG4B were found to be induced during Foc TR4 infection, whereas MaATG4C was not (Figure 7A). Notably, MaATG4B was only induced in the resistant cultivar ZJ6, whereas MaATG4A was induced in both ZJ6 and BX (Figure 7A). Thus, MaATG4B was selected for confirmation of physical interactions with MaATG8 homologs. The full-length coding sequences (CDSs) of MaATG4B and MaATG8 genes were cloned into the bait and prey vectors, respectively. Colony growth of cotransformants on selection medium containing X-α-gal confirmed pairwise interactions between MaATG4B and all 10 MaATG8 proteins in yeast. Only MaATG8F interacted with MaATG4B (Figure 7B). These results indicate that the interactions of MaATG8F and MaATG4B could also happen in banana plants.
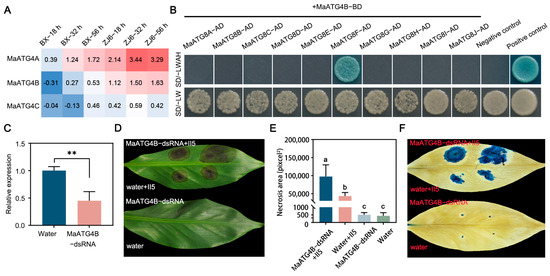
Figure 7.
MaATG4B positively regulates the banana resistance against Foc TR4. (A) Heatmap for the MaATG4 genes in resistant and susceptible plants inoculated with II5 strains at different time points (18 h, 32 h, 56 h). (B) MaATG4B interacts with MaATG8F. The cotransformants of p53+pGADT7-T and lam+pGADT7-T are presented as positive control and negative control, respectively. SD/-LWAH is presented as SD/-leucine-tryptophan-adenine-histidine, SD/-LW is presented as SD/-leucine-tryptophan. (C) The expression level of MaATG4B in dsRNA- and water-treated banana leaves from qRT-PCR. Data are presented as means ± SDs (n = 3). “**” symbol atop the columns indicates a significant difference at p < 0.01 (two-tailed Student’s t-test). (D) The disease symptoms and (E) necrosis area of dsRNA- and water-treated banana leaves. The pictures were taken 10 days postinoculation. The necrosis area was calculated with ImageJ V1.8.0 software. Data are presented as means ± SDs (n = 3). Different letters atop the columns indicate a significant difference at p < 0.01 (one-way analysis of variance). (F) Trypan blue staining of banana leaves.
To investigate the biological function of MaATG4B in response to Foc TR4 infection, banana leaves were treated with MaATG4B-dsRNA or a water control. qRT-PCR analysis showed significant MaATG4B downregulation in dsRNA-treated leaves compared with that in water-treated control leaves (Figure 7C), indicating that dsRNA treatment silenced MaATG4B as expected. To confirm whether silencing MaATG4B influenced Foc TR4 resistance, dsRNA-treated leaves were inoculated with Foc TR4 plugs. The dsRNA-treated leaves showed larger necrotic areas than the controls, as confirmed with both a visual comparison of the leaves (Figure 7D,E) and trypan blue staining (Figure 7F). Taken together, these results indicate that MaATG4B functions as a positive regulator of banana defense responses and interacts with the autophagy-related protein MaATG8F.
4. Discussion
Plant growth is a continuous process that is regulated by several genetic and environmental factors. Stressors such as salt, high temperature, drought, and pathogen infection in particular have substantial influences on plant growth and yield throughout the world. Plants have evolved various mechanisms to protect themselves from environmental stressors, enabling survival. Autophagy is one of the most important strategies that allows plants to survive under adverse conditions [18,35,36]. The molecular mechanisms by which members of the autophagy-related ATG8 protein family function in banana have not previously been characterized. In the present study, 10 MaATG8 genes were identified throughout the banana genome. Phylogenetic analysis of ATG8 genes in A. thaliana, rice, citrus, ginger, and banana demonstrated relatively high conservation between species, indicating that ATG8 proteins may perform similar functions in different species.
MaATG8 was here found to be induced by Foc TR4 infection and was more highly expressed in a resistant than a susceptible banana cultivar. This led to the hypothesis that MaATG8 expression levels in resistant cultivars may be associated with their mechanisms of disease resistance. Silencing MaATG8 in banana leaves enhanced host susceptibility to Foc TR4 infection, which might be one of explanations for the hypothesis. An increasing number of studies have shown that single nucleotide polymorphisms (SNPs) play important roles in various plant processes [37,38,39]. A cis-acting element analysis here revealed that the MaATG8 promoter sequences contained a large number of resistance-related cis-elements. This suggested that the MaATG8 promoter elements of resistant and susceptible cultivars contained SNPs, leading to differences in the binding abilities of associated transcription factors, resulting in cultivar-specific differences in MaATG8 gene expression. This is another possible explanation for differential MaATG8 expression between resistant and susceptible cultivars. Due to the difficulty of banana plant transformation and the length of time required, MaATG8 transgenic plants have not yet been obtained. To verify the biological functions of MaATG8s, banana suspension cells should be transformed to generate transgenic plants, including those overexpressing MaATG8s or expressing CRISPR/Cas9-edited versions of MaATG8s. Further studies should also address the mechanisms by which MaATG8s induce autophagy to degrade effectors secreted by Foc TR4. These mechanisms may protect banana plants from pathogen infection.
Autophagy-related proteins are known to play important roles in plant life processes, including pathogen resistance. For example, MdATG8i can mediate disease resistance and drought tolerance in apples [21,40]. Many mechanisms of interaction between ATG4 and ATG8 have been revealed previously. For example, the cysteine protease ATG4 facilitates normal ATG8 functioning by first hydrolyzing the C-terminal arginine residue, exposing a glycine residue for PE binding, then by cleaving ATG8 from PE to enable autophagosome formation [14,19]. The results of the present study verified interactions between MaATG4 and MaATG8 in vitro, suggesting that MaATG4–MaATG8 interactions also regulated autophagy in banana. Interestingly, Y2H assays showed that MaATG4 could not interact with every one of the MaATG8 family members, indicating that some of these proteins may have evolved new functions in banana. Further studies should be conducted to determine if the other two MaATG4 homologs in M. acuminata can interact with all MaATG8 family members.
ATG6 is another protein with an important role in ATG8-mediated autophagosome formation. In rice, the C-terminus of a Rhabdovirus glycoprotein interacts with SnRK1B, promoting ATG6b phosphorylation. Rice ATG6b can also interact with the N-terminus of the viral glycoprotein; this connects the glycoprotein with ATG8 on the autophagosome membrane, promoting removal of the glycoprotein into the autophagosome for degradation [22]. A prior study indicated that ATG6, ATG8, and ATG4 all interact to form a protein complex. However, whether this is true in banana, and whether such a complex participates in Foc TR4 resistance, remains unknown. Previous research demonstrated that interactions between ATG8 and ATG8-family interacting motif (AIM)-containing proteins participate in cell-selective autophagy and regulate plant disease resistance [41,42]. Our research group determined with Y2H assays that an AIM-containing protein interacts with MaATG8 (unpublished data), but the relationship between this interaction and disease resistance requires further study.
In summary, a total of 10 MaATG8 genes were here identified in the banana genome. Phylogenetic relationships, gene structures, chromosomal locations, and expression pattern analyses were conducted for all 10 MaATG8 genes. Nearly all of the MaATG8 genes were upregulated in disease-resistant cultivars after Foc TR4 infection. Based on the results of MaATG8 expression analyses, the role of MaATG8 was explored further. MaATG8F silencing indicated that this gene positively regulated banana resistance to Foc TR4. Y2H results showed that MaATG4B could interact with MaATG8F in vitro but not with the other MaATG8 homologs. This study provides novel resistant gene resources for subsequent functional research of autophagy-related proteins in banana.
5. Conclusions
In the present study, 10 MaATG8 genes were identified in the banana genome, some of which were determined to be involved in the host immune response against Foc TR4. Nine of the ten MaATG8 genes were significantly induced during the infection process in the resistant and susceptible cultivars, with the exception of MaATG8I. The results of several analyses indicated that MaATG8F partially regulated banana disease resistance to Foc TR4. MaATG4A and MaATG4B were strongly upregulated in response to Foc TR4 infection, with MaATG4B only induced in the disease-resistant banana cultivar. MaATG4B was found to interact with MaATG8F (but not with other MaATG8s) and to partially regulate banana disease resistance to Foc TR4. Taken together, this comprehensive analysis of the MaATG8 family and interaction between MaATG8F and MaATG4B in a wild banana species provides valuable new information to facilitate mining of related resistance genes for future genetic improvement of banana cultivars.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10020091/s1, Table S1: ATG8 homologs identified in different plant species; Table S2: Primers used in this study; Table S3: DEGs of MaATG8 genes.
Author Contributions
H.H. carried out the experiments and data analysis; Y.T. and Y.H. provided RNA-seq data analysis and performed bioinformatics; Y.L. (Yushan Liu) performed the subcellular localization; W.Y. and Y.L. (Yuqing Li) performed synthesis of dsRNA; M.Z. and D.X. performed the inoculation assays; G.Y. and C.L. conceived the project; C.L. and S.L. carried out experiment design and manuscript design; H.H. wrote the manuscript. G.Y., C.L. and S.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Guangdong Science and Technology Project (2019B1515120088), the Science and Technology Department of Guangdong Province (2022A1515110433), the Guangdong Science and Technology Project (110263295131), the Laboratory of Lingnan Modern Agriculture Project (NT2021004), the Research Fund of Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture (2021TDQD003), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams, Department of Agriculture and Rural Affairs of Guangdong Province (2023KJ109), and the Earmarked Fund for CARS (CARS-31).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Files.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. Food and Agricultural Organization (FAO). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 31 March 2023).
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium Wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.K.; Harris, S.D.; Marten, M.R. Autophagy in filamentous fungi. Fungal Genet. Biol. 2009, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Schmelzle, T.; Hall, M.N. TOR, a central controller of cell growth. Cell 2000, 103, 253–262. [Google Scholar] [CrossRef]
- Ismayil, A.; Yang, M.; Liu, Y. Role of autophagy during plant-virus interactions. Semin. Cell Dev. Biol. 2020, 101, 36–40. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. BZR1 mediates brassinosteroid-induced autophagy and nitrogen starvation in tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The master of bulk and selective recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Ohsumi, Y. Unveiling the molecular mechanisms of plant autophagy-from autophagosomes to vacuoles in plants. Plant Cell Physiol. 2018, 59, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Harding, T.M.; Morano, K.A.; Scott, S.V.; Klionsky, D.J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1995, 131, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Cregg, J.M.; Dunn, W.A., Jr.; Emr, S.D.; Sakai, Y.; Sandoval, I.V.; Sibirny, A.; Subramani, S.; Thumm, M.; Veenhuis, M.; et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 2003, 5, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, D.N.; Liu, H.L.; Luo, J.; Wang, P.; Wang, M.L.; Guo, F.; Wang, Y.; Zhao, H.; Ni, D.J. Genome-wide identification of CsATGs in tea plant and the involvement of CsATG8e in nitrogen utilization. Int. J. Mol. Sci. 2020, 21, 7043. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kataura, T.; Sedlackova, L.; Otten, E.G.; Kumari, R.; Shapira, D.; Scialo, F.; Stefanatos, R.; Ishikawa, K.I.; Kelly, G.; Seranova, E.; et al. Autophagy promotes cell survival by maintaining NAD levels. Dev. Cell 2022, 57, 2584–2598.e11. [Google Scholar] [CrossRef]
- Kirisako, T.; Ichimura, Y.; Okada, H.; Kabeya, Y.; Mizushima, N.; Yoshimori, T.; Ohsumi, M.; Takao, T.; Noda, T.; Ohsumi, Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000, 151, 263–276. [Google Scholar] [CrossRef]
- Doelling, J.H.; Walker, J.M.; Friedman, E.M.; Thompson, A.R.; Vierstra, R.D. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 33105–33114. [Google Scholar] [CrossRef]
- Che, R.; Liu, C.; Wang, Q.; Tu, W.; Wang, P.; Li, C.; Gong, X.; Mao, K.; Feng, H.; Huang, L.; et al. The Valsa Mali effector Vm1G-1794 protects the aggregated MdEF-Tu from autophagic degradation to promote infection in apple. Autophagy 2023, 19, 1745–1763. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Chen, S.; Liu, S.; Li, Z.; Wang, Z.; Chen, B.; Zhang, C.; Zhang, Y.; Wu, J.; et al. Rhabdovirus encoded glycoprotein induces and harnesses host antiviral autophagy for maintaining its compatible infection. Autophagy 2023, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Czymmek, K.; Tallóczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005, 121, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gong, Y.; Shi, H.; Ma, X.; Zhu, Y.; Yang, F.; Wang, D.; Fu, Y.; Lin, Y.; Yang, N.; et al. ‘Candidatus Liberibacter asiaticus’ secretory protein SDE3 inhibits host autophagy to promote Huanglongbing disease in citrus. Autophagy 2023, 19, 2558–2574. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Xie, X.; Yue, N.; Li, J.; Wang, X.B.; Han, C.; Yu, J.; Liu, Y.; Li, D. Barley stripe mosaic virus γb protein subverts autophagy to promote viral infection by disrupting the ATG7-ATG8 interaction. Plant Cell 2018, 30, 1582–1595. [Google Scholar] [CrossRef]
- Pei, D.; Zhang, W.; Sun, H.; Wei, X.; Yue, J.; Wang, H. Identification of autophagy-related genes ATG4 and ATG8 from wheat (Triticum aestivum L.) and profiling of their expression patterns responding to biotic and abiotic stresses. Plant Cell Rep. 2014, 33, 1697–1710. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, W.; Hu, W.; Liu, G.; Wu, C.; Liu, W.; Zeng, H.; He, C.; Shi, H. Genome-wide analysis of autophagy-related genes in banana highlights MaATG8s in cell death and autophagy in immune response to Fusarium wilt. Plant Cell Rep. 2017, 36, 1237–1250. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Hwang, I.S.; Choi, D.S.; Kim, N.H.; Kim, D.S.; Hwang, B.K. Pathogenesis-related protein 4b interacts with leucine-rich repeat protein 1 to suppress PR4b-triggered cell death and defense response in pepper. Plant J. 2014, 77, 521–533. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Zhang, Y.; Yi, G.; Xie, J.; Viljoen, A.; Wang, W.; Mostert, D.; Fu, G.; Peng, C.; et al. FocECM33, a GPI-anchored protein, regulates vegetative growth and virulence in Fusarium oxysporum f. sp. cubense tropical race 4. Fungal Biol. 2022, 126, 213–223. [Google Scholar] [CrossRef]
- Wu, J.; Yin, S.; Lin, L.; Liu, D.; Ren, S.; Zhang, W.; Meng, W.; Chen, P.; Sun, Q.; Fang, Y.; et al. Host-induced gene silencing of multiple pathogenic factors of Sclerotinia sclerotiorum confers resistance to Sclerotinia rot in Brassica napus. Crop J. 2022, 10, 661–671. [Google Scholar] [CrossRef]
- Fernández-Bautista, N.; Domínguez-Núñez, J.A.; Moreno, M.M.C.; Berrocal-Lobo, M. Plant tissue trypan blue staining during phytopathogen infection. Bio-Protocol 2016, 6, e2078. [Google Scholar] [CrossRef]
- Park, E.; Woo, J.; Dinesh-Kumar, S.P. Arabidopsis ATG4 cysteine proteases specificity toward ATG8 substrates. Autophagy 2014, 10, 926–927. [Google Scholar] [CrossRef]
- Tang, J.; Bassham, D.C. Autophagy during drought: Function, regulation, and potential application. Plant J. 2022, 109, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef]
- Xing, A.; Gao, Y.; Ye, L.; Zhang, W.; Cai, L.; Ching, A.; Llaca, V.; Johnson, B.; Liu, L.; Yang, X.; et al. A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J. Exp. Bot. 2015, 66, 3791–3802. [Google Scholar] [CrossRef]
- Li, N.; Lin, B.; Wang, H.; Li, X.; Yang, F.; Ding, X.; Yan, J.; Chu, Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 2019, 51, 1540–1548. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef]
- Jia, X.; Gong, X.; Jia, X.; Li, X.; Wang, Y.; Wang, P.; Huo, L.; Sun, X.; Che, R.; Li, T.; et al. Overexpression of MdATG8i Enhances drought tolerance by alleviating oxidative damage and promoting water uptake in transgenic apple. Int. J. Mol. Sci. 2021, 22, 5517. [Google Scholar] [CrossRef]
- Kalvari, I.; Tsompanis, S.; Mulakkal, N.C.; Osgood, R.; Johansen, T.; Nezis, I.P.; Promponas, V.J. iLIR: A web resource for prediction of Atg8-family interacting proteins. Autophagy 2014, 10, 913–925. [Google Scholar] [CrossRef]
- Nolan, T.M.; Brennan, B.; Yang, M.; Chen, J.; Zhang, M.; Li, Z.; Wang, X.; Bassham, D.C.; Walley, J.; Yin, Y. Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Dev. Cell 2017, 41, 33–46.e7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).