Nitric Oxide in Fungi: Production and Function
Abstract
1. Introduction
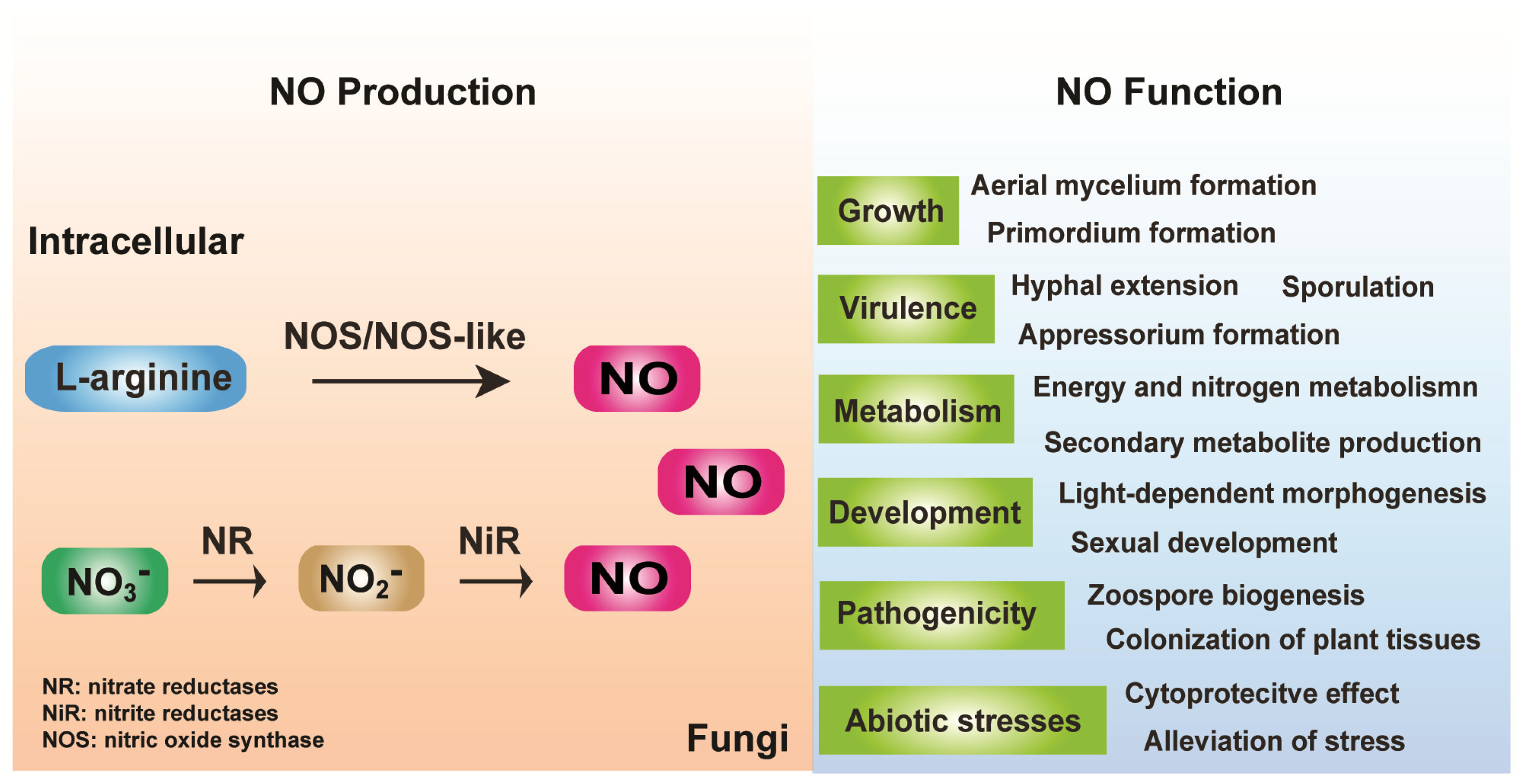
2. Fungal Endogenous NO Generation and Removal
2.1. Arginine-Dependent NO Formation
2.2. Nitrite (NO2−)-Dependent NO Formation
2.3. Other NO Formation Pathways and Regulation of NO Homeostasis in Fungi
3. Function of Endogenous NO and NO Signaling in Fungi
3.1. Growth and Development Regulation
3.2. Response to Stressors
3.3. Metabolism Regulation
3.4. Virulence and Pathogenicity
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamattina, L.; Garcia-Mata, C.; Graziano, M.; Pagnussat, G. Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, D.; Marcos, J.F.; Marcos, A.T.; Strauss, J. Nitric oxide in fungi: Is there NO light at the end of the tunnel? Curr. Genet. 2016, 62, 513–518. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.W. Nitric Oxide Acts as a Key Signaling Molecule in Plant Development under Stressful Conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, W.; Li, L.; Fan, X. Endothelial nitric oxide synthase: A potential therapeutic target for cerebrovascular diseases. Mol Brain. 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef]
- Palavan-Unsal, N.; Arisan, D. Nitric oxide signalling in plants. Bot. Rev. 2009, 75, 203–229. [Google Scholar] [CrossRef]
- Hancock, J.T. Nitric oxide signaling in plants. Plants 2020, 9, 1550. [Google Scholar] [CrossRef]
- Arc, E.; Galland, M.; Godin, B.; Cueff, G.; Rajjou, L. Nitric oxide implication in the control of seed dormancy and germination. Front. Plant Sci. 2013, 4, 346. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef]
- Gayatri, G.; Agurla, S.; Raghavendra, A.S. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 2013, 4, 425. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Okamoto, M.; Crawford, N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Foresi, N.; Correa-Aragunde, N.; Parisi, G.; Calo, G.; Salerno, G.; Lamattina, L. Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 2010, 22, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Chamizo-Ampudia, A.; Sanz-Luque, E.; Ocaña-Calahorro, F.; Llamas, A.; Fernandez, E.; Galvan, A. How Chlamydomonas handles nitrate and the nitric oxide cycle. J. Exp. Bot. 2017, 68, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Crane, B.R.; Sudhamsu, J.; Patel, B.A. Bacterial nitric oxide synthases. Annu. Rev. Biochem. 2010, 79, 445–470. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, L.L.; Wang, W.W.; Gao, H.C. Nitric Oxide, Nitric Oxide Formers and Their Physiological Impacts in Bacteria. Int. J. Mol. Sci. 2022, 23, 10778. [Google Scholar] [CrossRef]
- Coleman, R.E.; Lancaster, K.M. Heme P460: A (Cross) Link to Nitric Oxide. Acc. Chem. Res. 2020, 53, 2925–2935. [Google Scholar] [CrossRef]
- Poole, R.K. Flavohaemoglobin: The pre-eminent nitric oxide-detoxifying machine of microorganisms. F1000Research 2020, 9, F1000 Faculty Rev-7. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.R.; Arvai, A.S.; Ghosh, D.K.; Wu, C.; Getzoff, E.D.; Stuehr, D.J.; Tainer, J.A. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science 1998, 279, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Aulak, K.S.; Stuehr, D.J. Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis. J. Biol. Chem. 2002, 277, 16167–16171. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.A.; Wach, M.J.; Krasnoff, S.B.; Widom, J.; Cameron, K.D.; Bukhalid, R.A.; Gibson, D.M.; Crane, B.R.; Loria, R. Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature 2004, 429, 79–82. [Google Scholar] [CrossRef]
- Li, H.; Poulos, T.L. Structure-function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Ito, S.; Yajima, S.; Sasaki, Y. Nitric oxide signaling for aerial mycelium formation in Streptomyces coelicolor A3 (2). Appl. Environ. Microbiol. 2022, 88, e0122222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Lim, J.; Xu, J.Y.; Yu, J.H.; Zheng, W.F. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Bachurina, G.P. Nitric oxide in fungal metabolism (Review). Appl. Biochem. Microbiol. 2021, 57, 694–705. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R. 5-Fungal biodiversity patterns. In Biodiversity of Fungi; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: Cambridge, UK, 2004; pp. 59–75. [Google Scholar]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.L.; Moali, C.; Tenu, J.P. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell. Mol. Life Sci. 1999, 55, 1015–1028. [Google Scholar] [CrossRef]
- Kanadia, R.N.; Kuo, W.N.; Mcnabb, M.; Botchway, A. Constitutive nitric oxide synthase in Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. 1998, 45, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Song, N.K.; Jeong, C.S.; Choi, H.S. Identification of nitric oxide synthase in Flammulina velutipes. Mycologia 2000, 92, 1027–1032. [Google Scholar] [CrossRef]
- Maier, J.; Hecker, R.; Rockel, P.; Ninnemann, H. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol. 2001, 126, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Buttner, S.; Ohlmeier, S.; Silva, A.; Mesquita, A.; Sampaio-Marques, B.; Osório, N.S.; Kollau, A.; Mayer, B.; Leão, C.; et al. NO-mediated apoptosis in yeast. J. Cell Sci. 2007, 120, 3279–3288. [Google Scholar] [CrossRef]
- Vieira, A.L.; Linares, E.; Augusto, O.; Gomes, S.L. Evidence of a Ca2+-NO-cGMP signaling pathway controlling zoospore biogenesis in the aquatic fungus Blastocladiella emersonii. Fungal Genet. Biol. 2009, 46, 575–584. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.P.; Jiang, D.H.; Xie, J.T.; Cheng, J.S.; Li, G.Q.; Hamid, M.I.; Yi, X.H. Cyclic GMP as a second messenger in the nitric oxide-mediated conidiation of the mycoparasite Coniothyrium minitans. Appl. Environ. Microbiol. 2010, 76, 2830–2836. [Google Scholar] [CrossRef]
- Zhao, Y.; Xi, Q.; Xu, Q.; He, M.; Ding, J.; Dai, Y.; Keller, N.P.; Zheng, W. Correlation of nitric oxide produced by an inducible nitric oxide synthase-like protein with enhanced expression of the phenylpropanoid pathway in Inonotus obliquus cocultured with Phellinus morii. Appl. Microbiol. Biotechnol. 2015, 99, 4361–4372. [Google Scholar] [CrossRef]
- Huang, H.; Huang, M.; Lv, W.; Hu, Y.; Wang, R.; Zheng, X.; Ma, Y.; Chen, C.; Tang, H. Inhibition of Trichophyton rubrum by 420-nm intense pulsed light: In vitro activity and the role of nitric oxide in fungal death. Front. Pharmacol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Onufriev, M.V.; Peregud, D.I.; Bachurina, G.P.; Kritsky, M.S. Nitric-oxide synthase activity in the photomorphogenesis of Neurospora crassa. Appl. Biochem. Microbiol. 2020, 56, 446–452. [Google Scholar] [CrossRef]
- Franco-Cano, A.; Marcos, A.T.; Strauss, J.; Canovas, D. Evidence for an arginine-dependent route for the synthesis of NO in the model filamentous fungus Aspergillus nidulans. Environ. Microbiol. 2021, 23, 6924–6939. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Yang, H.; Liu, Z.; Zhang, Z.; Yan, R.; Zhu, D. L-Arginine enhanced perylenequinone production in the endophytic fungus Shiraia sp. Slf14(w) via NO signaling pathway. Appl. Microbiol. Biotechnol. 2022, 106, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bi, S.; Meng, J.; Liu, T.; Li, P.; Yu, C.; Peng, X. Arbuscular mycorrhizal fungi enhanced rice proline metabolism under low temperature with nitric oxide involvement. Front. Plant Sci. 2022, 13, 962460. [Google Scholar] [CrossRef]
- Ninnemann, H.; Maier, J. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem. Photobiol. 1996, 64, 393–398. [Google Scholar] [CrossRef]
- Wang, J.; Higgins, V.J. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet. Biol. 2005, 42, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Fu, Y.P.; Jiang, D.H.; Li, G.Q.; Yi, X.H.; Peng, Y.L. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet. Biol. 2007, 44, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Prats, E.; Carver, T.L.; Mur, L.A. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Res. Microbiol. 2008, 159, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Miao, K.; Zhang, Y.; Pan, S.; Zhang, M.; Jiang, H. Nitric oxide mediates the fungal-elicitor-enhanced biosynthesis of antioxidant polyphenols in submerged cultures of Inonotus obliquus. Microbiology 2009, 155, 3440–3448. [Google Scholar] [CrossRef]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.S.; Biswas, P.; Ghosh, S.K.; Ghosh, S. Nitric oxide production by necrotrophic pathogen Macrophomina phaseolina and the host plant in charcoal rot disease of jute: Complexity of the interplay between necrotroph-host plant interactions. PLoS ONE 2014, 9, e107348. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.T.; Ramos, M.S.; Marcos, J.F.; Carmona, L.; Strauss, J.; Cánovas, D. Nitric oxide synthesis by nitrate reductase is regulated during development in Aspergillus. Mol. Microbiol. 2016, 99, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Fushinobu, S.; Zhou, S.; Wakagi, T.; Shoun, H. Eukaryotic nirK genes encoding copper-containing nitrite reductase: Originating from the protomitochondrion? Appl. Environ. Microbiol. 2009, 75, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gardiner, D.M.; Xiao, D.; Kazan, K. Regulators of nitric oxide signaling triggered by host perception in a plant pathogen. Proc. Natl. Acad. Sci. USA 2020, 117, 11147–11157. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shoun, H. The Copper-containing Dissimilatory Nitrite Reductase Involved in the Denitrifying System of the Fungus Fusarium oxysporum. J. Biol. Chem. 1995, 270, 4146–4151. [Google Scholar] [CrossRef]
- Shi, L.; Yue, S.; Gao, T.; Zhu, J.; Ren, A.; Yu, H.; Wang, H.; Zhao, M. Nitrate reductase-dependent nitric oxide plays a key role on MeJA-induced ganoderic acid biosynthesis in Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2020, 104, 10737–10753. [Google Scholar] [CrossRef]
- Al-Hosni, K.; Shahzad, R.; Khan, A.L.; Imran, Q.M.; Al Harrasi, A.; Al Rawahi, A.; Asaf, S.; Kang, S.M.; Yun, B.W.; Lee, I.J. Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J. Plant Interact. 2018, 13, 112–118. [Google Scholar] [CrossRef]
- Castello, P.R.; Woo, D.K.; Ball, K.; Wojcik, J.; Liu, L.; Poyton, R.O. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 8203–8208. [Google Scholar] [CrossRef]
- Xu, C.; Lin, W.; Chen, Y.; Gao, B.; Zhang, Z.; Zhu, D. Heat stress enhanced perylenequinones biosynthesis of Shiraia sp. Slf14(w) through nitric oxide formation. Appl. Microbiol. Biotechnol. 2023, 107, 3745–3761. [Google Scholar] [CrossRef]
- Astuti, R.I.; Nasuno, R.; Takagi, H. Nitric oxide signaling in yeast. Appl. Microbiol. Biotechnol. 2016, 100, 9483–9497. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Chamizo-Ampudia, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Llamas, A. From the eukaryotic molybdenum cofactor biosynthesis to the moonlighting enzyme mARC. Molecules 2018, 23, 3287. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zheng, L.; Zhang, L.; Huang, X.; Ren, Q. mARC dependent NO synthesis activates CanA-Relish-AMPs signal pathway in Eriocheir sinensis during nitrite stress. Fish Shellfish Immunol. 2023, 141, 109076. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Kim, S.W.; Fushinobu, S.; Zhou, S.M.; Wakagi, T.; Shoun, H. The possible involvement of copper-containing nitrite reductase (NirK) and flavohemoglobin in denitrification by the fungus Cylindrocarpon tonkinense. Biosci. Biotechnol. Biochem. 2010, 74, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.-W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. B 2012, 367, 1186–1194. [Google Scholar] [CrossRef]
- Aldossari, N.; Ishii, S. Fungal denitrification revisited—Recent advancements and future opportunities. Soil Biol. Biochem. 2021, 157, 108250. [Google Scholar] [CrossRef]
- Morozkina, E.V.; Kurakov, A.V. Dissimilatory nitrate reduction in fungi under conditions of hypoxia and anoxia: A review. Appl. Biochem. Microbiol. 2007, 43, 607–613. [Google Scholar] [CrossRef]
- Schinko, T.; Berger, H.; Lee, W.; Gallmetzer, A.; Pirker, K.; Pachlinger, R.; Buchner, I.; Reichenauer, T.; Guldener, U.; Strauss, J. Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol. Microbiol. 2010, 78, 720–738. [Google Scholar] [CrossRef]
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol. 2011, 155, 1023–1036. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Llamas, A.; Galvan, A.; Fernandez, E. Role of Nitrate Reductase in NO Production in Photosynthetic Eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Kulbir; Das, S.; Devi, T.; Goswami, M.; Yenuganti, M.; Bhardwaj, P.; Ghosh, S.; Sahoo, S.C.; Kumar, P. Oxygen atom transfer promoted nitrate to nitric oxide transformation: A step-wise reduction of nitrate → nitrite → nitric oxide. Chem. Sci. 2021, 12, 10605–10612. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, B.; Zhang, X.; Bu, Y.; Shen, Z.; Zou, J.; Chen, Y. Cloning of Nitrate Reductase and Nitrite Reductase Genes and Their Functional Analysis in Regulating Cr (VI) Reduction in Ectomycorrhizal Fungus Pisolithus sp. 1. Front. Microbiol. 2022, 13, 926748. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Takaya, N. Denitrification by the fungus Fusarium oxysporum involves NADH-nitrate reductase. Biosci. Biotechnol. Biochem. 2008, 72, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Samalova, M.; Johnson, J.; Illes, M.; Kelly, S.; Fricker, M.; Gurr, S. Nitric oxide generated by the rice blast fungus Magnaporthe oryzae drives plant infection. New Phytol. 2013, 197, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Planchet, E.; Kaiser, W.M. Nitric oxide production in plants: Facts and fictions. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Nonenzymatic nitric oxide production in humans. Nitric Oxide 1998, 2, 1–7. [Google Scholar] [CrossRef]
- Jedelska, T.; Luhova, L.; Petrivalsky, M. Nitric oxide signalling in plant interactions with pathogenic fungi and oomycetes. J. Exp. Bot. 2021, 72, 848–863. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. Nitric oxide: An effective weapon of the plant or the pathogen? Mol. Plant Pathol. 2014, 15, 406–416. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. Nitric oxide in the offensive strategy of fungal and oomycete plant pathogens. Front. Plant Sci. 2016, 7, 252. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Fernández-Espinosa, M.G.; Lorenzo, O. Nitric oxide molecular targets: Reprogramming plant development upon stress. J. Exp. Bot. 2019, 70, 4441–4460. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Calatrava, V.; Llamas, A.; Fernandez, E.; Sanz-Luque, E.; Galvan, A. Nitrous Oxide Emissions from Nitrite Are Highly Dependent on Nitrate Reductase in the Microalga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2022, 23, 9412. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Ocaña-Calahorro, F.; de Montaigu, A.; Chamizo-Ampudia, A.; Llamas, Á.; Galván, A.; Fernández, E. THB1, a truncated hemoglobin, modulates nitric oxide levels and nitrate reductase activity. Plant J. 2015, 81, 467–479. [Google Scholar] [CrossRef]
- Marcos, A.T.; Ramos, M.S.; Schinko, T.; Strauss, J.; Cánovas, D. Nitric oxide homeostasis is required for light-dependent regulation of conidiation in Aspergillus. Fungal Genet. Biol. 2020, 137, 103337. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, M.; Hausladen, A.; Heitman, J.; Stamler, J.S. Protection from nitrosative stress by yeast flavohemoglobin. Proc. Natl. Acad. Sci. USA 2000, 97, 4672–4676. [Google Scholar] [CrossRef]
- Ullmann, B.D.; Myers, H.; Chiranand, W.; Lazzell, A.L.; Zhao, Q.; Vega, L.A.; Lopez-Ribot, J.L.; Gardner, P.R.; Gustin, M.C. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot. Cell 2004, 3, 715–723. [Google Scholar] [CrossRef]
- Turrion-Gomez, J.L.; Eslava, A.P.; Benito, E.P. The flavohemoglobin BCFHG1 is the main NO detoxification system and confers protection against nitrosative conditions but is not a virulence factor in the fungal necrotroph Botrytis cinerea. Fungal Genet. Biol. 2010, 47, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Narukami, T.; Masuo, S.; Shimizu, M.; Fujita, T.; Doi, Y.; Kamimura, Y.; Takaya, N. NO-inducible nitrosothionein mediates NO removal in tandem with thioredoxin. Nat. Chem. Biol. 2013, 9, 657–663. [Google Scholar] [CrossRef]
- Koch, B.; Barugahare, A.A.; Lo, T.L.; Huang, C.; Schittenhelm, R.B.; Powell, D.R.; Beilharz, T.H.; Traven, A. A metabolic checkpoint for the yeast-to-hyphae developmental switch regulated by endogenous nitric oxide signaling. Cell Rep. 2018, 25, 2244–2258.e7. [Google Scholar] [CrossRef]
- Hou, L.; Huang, C.; Wu, X.; Zhang, J.; Zhao, M. Nitric Oxide Negatively Regulates the Rapid Formation of Pleurotus ostreatus Primordia by Inhibiting the Mitochondrial aco Gene. J. Fungi 2022, 8, 1055. [Google Scholar] [CrossRef]
- Pengkit, A.; Jeon, S.S.; Son, S.J.; Shin, J.H.; Baik, K.Y.; Choi, E.H.; Park, G. Identification and functional analysis of endogenous nitric oxide in a filamentous fungus. Sci. Rep. 2016, 6, 30037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, N.N.; Veerana, M.; Ketya, W.; Sun, H.N.; Park, G. RNA-seq-based transcriptome analysis of nitric oxide scavenging response in Neurospora crassa. J. Fungi 2023, 9, 985. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Liu, Y.Q.; Liao, B.Y.; Zong, Y.W.; Shi, Y.Y.; Liao, M.; Wang, J.N.; Zhou, X.D.; Cheng, L.; et al. Extracellular vesicles of Candida albicans regulate its own growth through the l-arginine/nitric oxide pathway. Appl. Microbiol. Biotechnol. 2023, 107, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Golderer, G.; Werner, E.R.; Leitner, S.; Gröbner, P.; Werner-Felmayer, G. Nitric oxide synthase is induced in sporulation of Physarum polycephalum. Genes Dev. 2001, 15, 1299–1309. [Google Scholar] [CrossRef]
- Yin, S.; Gao, Z.; Wang, C.; Huang, L.; Kang, Z.; Zhang, H. Nitric oxide and reactive oxygen species coordinately regulate the germination of Puccinia striiformis f. sp. tritici urediniospores. Front. Microbiol. 2016, 7, 178. [Google Scholar] [CrossRef]
- Oiki, S.; Nasuno, R.; Urayama, S.-i.; Takagi, H.; Hagiwara, D. Intracellular production of reactive oxygen species and a DAF-FM-related compound in Aspergillus fumigatus in response to antifungal agent exposure. Sci. Rep. 2022, 12, 13516. [Google Scholar] [CrossRef]
- Xia, J.L.; Wu, C.G.; Ren, A.; Hu, Y.R.; Wang, S.L.; Han, X.F.; Shi, L.; Zhu, J.; Zhao, M.W. Putrescine regulates nitric oxide accumulation in Ganoderma lucidum partly by influencing cellular glutamine levels under heat stress. Microbiol. Res. 2020, 239, 11. [Google Scholar] [CrossRef] [PubMed]
- Loshchinina, E.A.; Nikitina, V.E. Role of the NO synthase system in response to abiotic stress factors for basidiomycetes Lentinula edodes and Grifola frondosa. Mikrobiologiia 2016, 85, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.W.; Huang, C.Y.; Chen, Q.; Zou, Y.J.; Zhao, M.R.; Zhang, J.X. Nitric oxide is involved in the regulation of trehalose accumulation under heat stress in Pleurotus eryngii var. tuoliensis. Biotechnol. Lett. 2012, 34, 1915–1919. [Google Scholar] [CrossRef]
- Domitrovic, T.; Palhano, F.L.; Barja-Fidalgo, C.; DeFreitas, M.; Orlando, M.T.; Fernandes, P.M. Role of nitric oxide in the response of Saccharomyces cerevisiae cells to heat shock and high hydrostatic pressure. FEMS Yeast Res 2003, 3, 341–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef]
- Baidya, S.; Cary, J.W.; Grayburn, W.S.; Calvo, A.M. Role of nitric oxide and flavohemoglobin homolog genes in Aspergillus nidulans sexual development and mycotoxin production. Appl. Environ. Microbiol. 2011, 77, 5524–5528. [Google Scholar] [CrossRef]
- Yu, N.N.; Ketya, W.; Park, G. Intracellular nitric oxide and cAMP are involved in cellulolytic enzyme production in Neurospora crassa. Int. J. Mol. Sci. 2023, 24, 4503. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Wang, Y.; Ma, Y.J.; Wang, J.W.; Zheng, L.P. Nitric oxide and hydrogen peroxide signaling in extractive Shiraia fermentation by Triton X-100 for hypocrellin A production. Int. J. Mol. Sci. 2020, 21, 882. [Google Scholar] [CrossRef]
- Ma, Y.J.; Li, X.P.; Wang, Y.; Wang, J.W. Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb. Cell Factories 2021, 20, 92. [Google Scholar] [CrossRef]
- Turrion-Gomez, J.L.; Benito, E.P. Flux of nitric oxide between the necrotrophic pathogen Botrytis cinerea and the host plant. Mol. Plant Pathol. 2011, 12, 606–616. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Staats, M.; Van Kan, J.A.L. Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica. Mol. Plant Pathol. 2004, 5, 559–574. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.Y.; Chai, R.Y.; Qiu, H.P.; Jiang, H.; Mao, X.Q.; Wang, Y.L.; Liu, F.Q.; Sun, G.C. An S-(hydroxymethyl) glutathione dehydrogenase is involved in conidiation and full virulence in the rice blast fungus. PLoS ONE 2015, 10, e0120627. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Wang, Q.F.; Lu, D.H.; Zhang, H.; Wang, J.; Fu, R.T. Transcriptional profiling provides new insights into the role of nitric oxide in enhancing Ganoderma oregonense resistance to heat stress. Sci. Rep. 2017, 7, 15694. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.T.; Shinyashiki, M.; Switzer, C.H.; Valentine, J.S.; Gralla, E.B.; Thiele, D.J.; Fukuto, J.M. Effects of nitric oxide on the copper-responsive transcription factor Ace1 in Saccharomyces cerevisiae: Cytotoxic and cytoprotective actions of nitric oxide. Arch. Biochem. Biophys. 2000, 377, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, W.; Xiong, C.; Zhao, J. Transcriptome analysis reveals the role of nitric oxide in Pleurotus eryngii responses to Cd2+ stress. Chemosphere 2018, 201, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Demain, A.L. Bioactive products from fungi. Food Bioactives 2017, 2017, 59–87. [Google Scholar] [CrossRef]
- Zheng, W.F.; Zhang, M.M.; Zhao, Y.X.; Wang, Y.; Miao, K.J.; Wei, Z.W. Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Bioresour. Technol. 2009, 100, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, H.; Liang, S.; Ning, G.; Xu, N.; Lu, J.; Liu, X.; Lin, F. MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae. Microbiol. Res. 2015, 180, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Marroquin-Guzman, M.; Hartline, D.; Wright, J.D.; Elowsky, C.; Bourret, T.J.; Wilson, R.A. The Magnaporthe oryzae nitrooxidative stress response suppresses rice innate immunity during blast disease. Nat. Microbiol. 2017, 2, 17054. [Google Scholar] [CrossRef]
- Liu, R.; Shi, L.; Zhu, T.; Yang, T.; Ren, A.; Zhu, J.; Zhao, M.-W. Cross talk between nitric oxide and calcium-calmodulin regulates ganoderic acid biosynthesis in Ganoderma lucidum under heat stress. Appl. Environ. Microbiol. 2018, 84, e00043-18. [Google Scholar] [CrossRef]
- Zeng, F.; Gong, X.; Hamid, M.I.; Fu, Y.; Jiatao, X.; Cheng, J.; Li, G.; Jiang, D. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet. Biol. 2012, 49, 347–357. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the induction of nitric oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhao, M.; Huang, C.; He, Q.; Zhang, L.; Zhang, J. Alternative oxidase gene induced by nitric oxide is involved in the regulation of ROS and enhances the resistance of Pleurotus ostreatus to heat stress. Microb. Cell Factories 2021, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
| Fungus | Mechanism for NO Synthesis | Experiments for Testing Mechanisms | Reference |
|---|---|---|---|
| Aspergillus nidulans | NOS dependent | NOS-like enzyme activity was measured. | [45] |
| NO2− dependent | NR enzyme activity was measured. | [55] | |
| Blastocladiella emersonii | NOS dependent | NOS-like enzyme activity was measured. | [40] |
| Blumeria graminis | NOS dependent | Enzyme activity was inhibited by NOS inhibitors. | [51] |
| Colletotrichum coccodes | NOS dependent | Enzyme activity was inhibited by NOS inhibitors. | [49] |
| Coniothyrium minitans | NOS dependent | NOS-like enzyme activity was measured. | [41] |
| Enzyme activity was inhibited by NOS inhibitors. | [50] | ||
| Cylindrocarpon tonkinense | NO2− dependent | Nitrite reductase was expressed and purified. Enzyme activity (NO2− reduction to NO) was measured. | [56] |
| Flammulina velutipes | NOS dependent | NOS protein was purified using column chromatography, and activity of purified NOS enzyme was measured. | [37] |
| Fusarium graminearum | NO2− dependent | Identification of protein that may possibly induce NR enzyme expression. | [57] |
| Fusarium oxysporum | NO2− dependent | Nitrite reductase was expressed and purified. Enzyme activity (NO2− reduction to NO) was measured. | [58] |
| Ganoderma lucidum | NO2− dependent | NR gene was silenced, and activity of NR was inhibited. | [59] |
| Inonotus obliquus | NOS dependent | Enzyme activity was inhibited by NOS inhibitors. | [52] |
| Inonotus obliquus co-cultured with Phellinus morii | NOS dependent | Enzyme activity was inhibited by NOS inhibitors in I. obliquus. Genes homologous to constitutive and inducible mammalian NOS were identified, and inducible NOS was expressed in I. obliquus during co-culture. Cloned inducible NOS showed enzyme activity. | [42] |
| Macrophomina phaseolina | NOS dependent | Enzyme activity was inhibited by NOS inhibitors, and gene homologous to mammalian NOS was identified. | [54] |
| Neurospora crassa | NOS dependent | Enzyme activity was inhibited by NOS inhibitors. | [48] |
| NOS dependent | NOS-like enzyme activity was measured. | [44] | |
| Phycomyces blakesleeanus | NOS dependent | NOS-like enzyme activity was measured and inhibited by NOS inhibitors. | [38] |
| Pleurotus eryngii var. tuoliensis | NOS dependent | Enzyme activity was inhibited by NOS inhibitors. | [53] |
| Preussia sp. BSL-10 | NOS dependent NO2− dependent | Genes encoding NOS-like protein, nitrate reductase, and nitrite reductase were expressed. | [60] |
| Saccharomyces cerevisiae | NOS dependent | NOS-like enzyme activity was measured. | [39] |
| NOS dependent | Constitutive NOS-like protein was detected by Western blot. Activity of NOS was measured and inhibited by NOS inhibitors. | [36] | |
| NO2− dependent | Nitrite reduction to NO by mitochondrial cytochrome c oxidase under hypoxia condition. | [61] | |
| Shiraia sp. Slf14 | NOS dependent NO2− dependent | Genes homologous to constitutive and inducible mammalian NOS were identified. Cloned inducible NOS showed higher enzyme activity and gene expression under heat stress. Expression of inducible NOS and NR was elevated under heat stress. | [62] |
| Transcription level and activity of NOS and NR were elevated. | [46] | ||
| Trichophyton rubrum | NOS dependent | NOS-like enzyme activity was measured. | [43] |
| Category | Fungus | Function | Reference |
|---|---|---|---|
| Growth and development | Aspergillus nidulans | Reduce conidiation and induce the formation of cleistothecia | [55] |
| Light regulation of conidiation | [88] | ||
| Blastocladiella emersonii | Controlling zoospore biogenesis | [40] | |
| Candida albicans | Growth promotion and pathogenesis by extracellular vesicles | [97] | |
| Colletotrichum coccodes | Regulation of spore germination | [49] | |
| Coniothyrium minitans | Nitric-oxide-mediated conidiation | [41,50] | |
| Neurospora crassa | Light-induced conidiation and carotenogenesis | [44,48] | |
| Regulate mycelial development and conidia formation | [95] | ||
| Impacting the growth and development of hyphae (vegetative growth) | [96] | ||
| Phycomyces blakesleeanus | Light-induced development of sporangiophores | [38] | |
| Physarum polycephalum | Sporulation | [98] | |
| Pleurotus ostreatus | Primordia formation | [94] | |
| Puccinia striiformis f.sp. tritici | Induce spore germination | [99] | |
| Response to stresses | Aspergillus fumigatus | Effects of antifungal agent (farnesol) on germination | [100] |
| Ganoderma lucidum | Heat-stress-induced ganoderic acid levels | [101] | |
| Lentinula edodes and Grifola frondosa | Tolerance to superoptimal pH and in nitrogen limitation | [102] | |
| Pleurotus eryngii var. tuoliensis | Heat-stress-induced oxidative damage | [53] | |
| Heat-stress-induced trehalose accumulation | [103] | ||
| Rhizophagus irregularis | Enhanced host plant tolerance to low temperature stress by regulating proline accumulation in plant | [47] | |
| Saccharomyces cerevisiae | Cytoprotective effect from heat shock or high hydrostatic pressure | [104] | |
| Hypoxia signaling | [61,105] | ||
| H2O2-induced apoptosis | [39] | ||
| Shiraia sp. Slf14(w) | Heat-stress-enhanced perylenequinone biosynthesis | [62] | |
| Trichophyton rubrum | Reduction in fungal viability by 420 nm intense pulsed light | [43] | |
| Metabolism | Aspergillus nidulans | Mycotoxin production | [106] |
| Ganoderma lucidum | Methyl-jasmonate-induced ganoderic acid biosynthesis | [59] | |
| Inonotus obliquus | Biosynthesis of antioxidant polyphenols, accumulation of antioxidant phenolic constituents | [52] | |
| Inonotus obliquus and Phellinus morii | Increase in level of styrylpyrone polyphenols in fungal interspecific interaction | [42] | |
| Neurospora crassa | Cellulolytic enzyme production | [107] | |
| Carbohydrate and amino acid metabolism | [96] | ||
| Preussia sp. BSL-10 | Improve rice plant growth and related gene expression | [60] | |
| Shiraia sp. S9 | Hypocrellin A production | [108,109] | |
| Shiraia sp. Slf14(w) | Production of secondary metabolite perylenequinone | [46,62] | |
| Virulence and pathogenicity | Aspergillus nidulans | Mycotoxin production | [106] |
| Blumeria graminis | Influences formation of the appressorium infection structure | [51] | |
| Botrytis cinerea | Saprophytic growth and plant infection | [110] | |
| Botrytis elliptica | Induction of programmed cell death in lily | [111] | |
| Fusarium graminearum | Host recognition and virulence | [57] | |
| Magnaporthe oryzae | Drives plant infection (delays germling development and reduces disease lesion numbers) | [77] | |
| Conidial germination and appressorium formation (infectious morphogenesis) | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.-N.; Park, G. Nitric Oxide in Fungi: Production and Function. J. Fungi 2024, 10, 155. https://doi.org/10.3390/jof10020155
Yu N-N, Park G. Nitric Oxide in Fungi: Production and Function. Journal of Fungi. 2024; 10(2):155. https://doi.org/10.3390/jof10020155
Chicago/Turabian StyleYu, Nan-Nan, and Gyungsoon Park. 2024. "Nitric Oxide in Fungi: Production and Function" Journal of Fungi 10, no. 2: 155. https://doi.org/10.3390/jof10020155
APA StyleYu, N.-N., & Park, G. (2024). Nitric Oxide in Fungi: Production and Function. Journal of Fungi, 10(2), 155. https://doi.org/10.3390/jof10020155







