Diversity and Distribution of Fungi in the Marine Sediments of Zhanjiang Bay, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Sediment Sample Collection
2.2. Environmental Factor Analyses
2.3. DNA Extraction, PCR Amplification, ITS Sequencing, Data Processing, and Bioinformatic Analyses
2.4. Data Analyses
2.5. Accession Numbers for Nucleotide Sequences
3. Results
3.1. Distribution Characteristics of Environmental Factors in Zhanjiang Bay
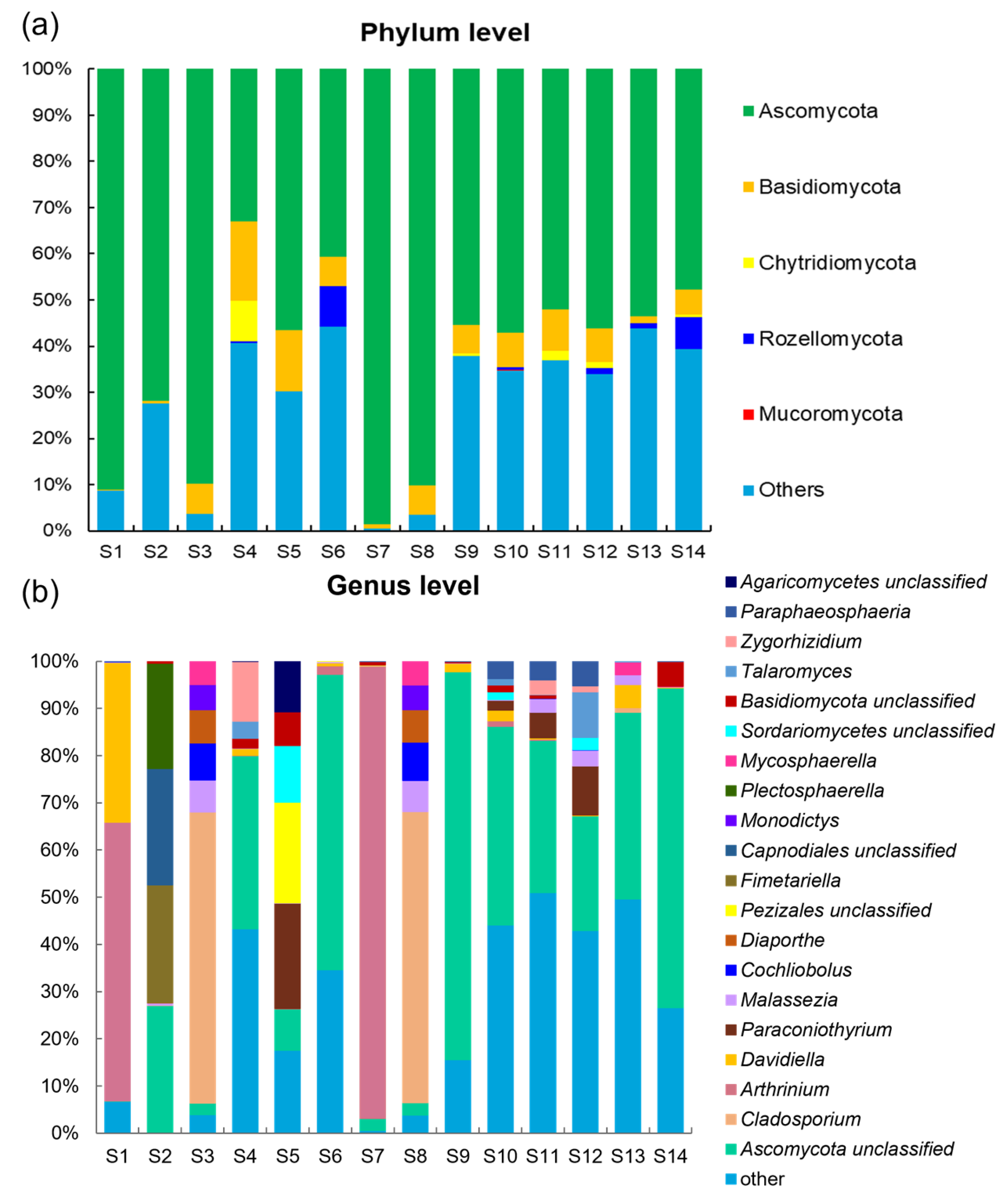
3.2. Composition and Distribution of Fungi in the Sediments of Zhanjiang Bay
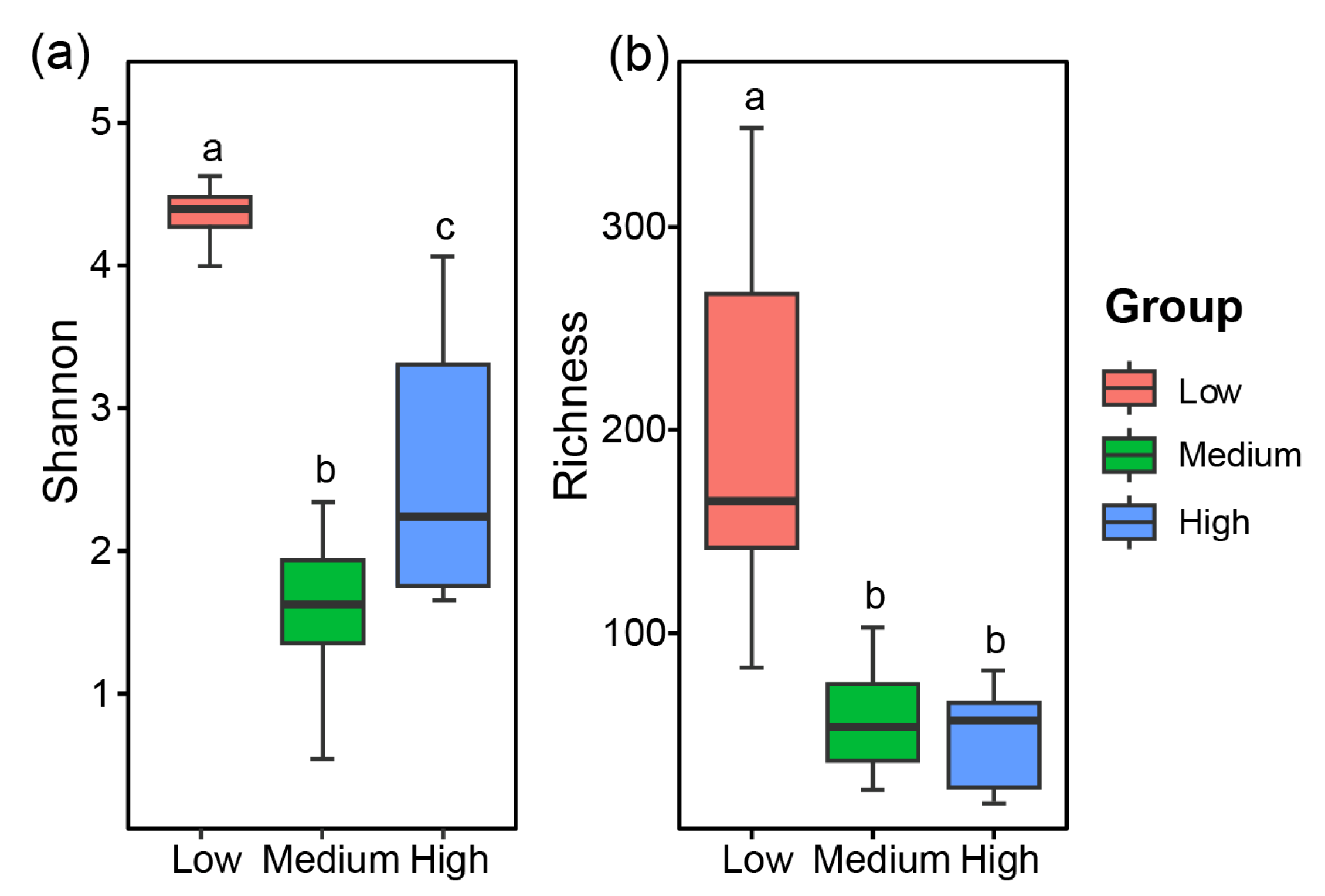
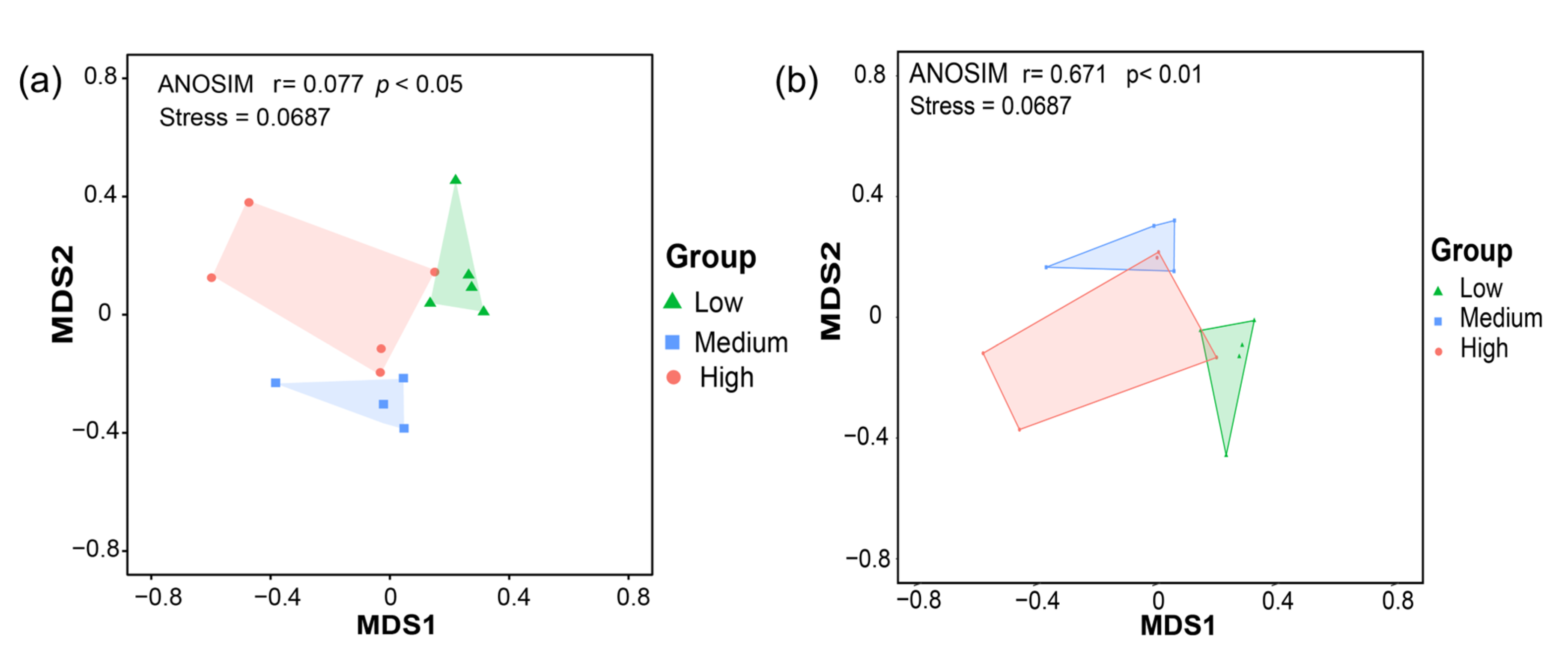
3.3. Diversity of Fungal Communities in the Sediments of Zhanjiang Bay, China
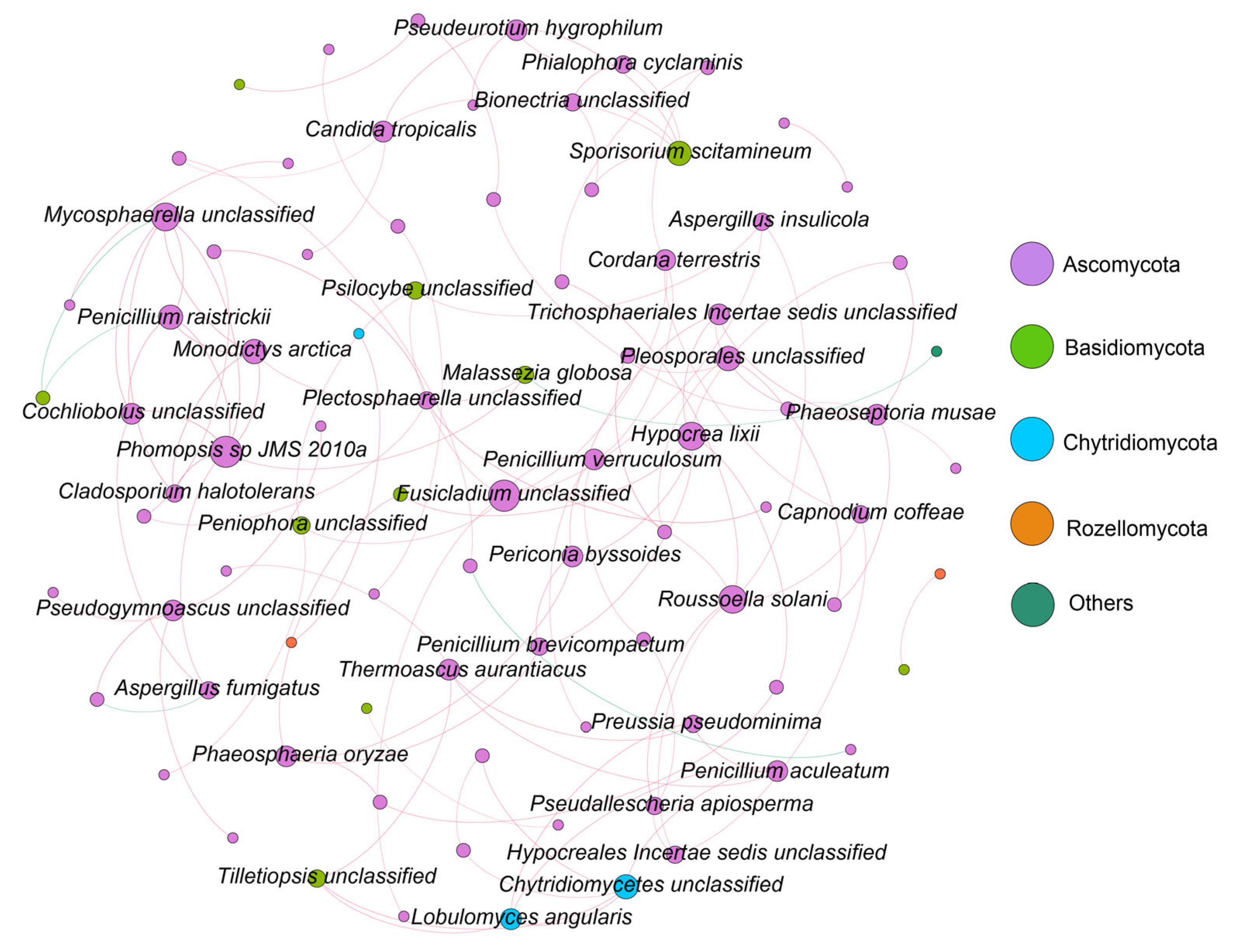
3.4. Molecular Ecological Network Analysis of Fungal Communities
3.5. Trophic Types and Functional Groups of Fungi in Zhanjiang Bay Sediments
3.6. Correlation Analysis Between Fungi and Environmental Factors
4. Discussion
4.1. Fungal Community Composition and Diversity in Zhanjiang Bay Sediments
4.2. Fungal Community Molecular Network Analysis in Zhanjiang Bay Sediments Based on ITS Metabarcoding
4.3. Potential Functional Traits of Fungal Communities in the Sediments of Zhanjiang Bay
4.4. Environmental Drivers of Fungal Community Structure in the Sediments of Zhanjiang Bay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasdemir, D. Marine fungi in the spotlight: Opportunities and challenges for marine fungal natural product discovery and biotechnology. Fungal Biol. Biotechnol. 2017, 4, 5. [Google Scholar] [CrossRef]
- Bass, D.; Howe, A.; Brown, N.; Barton, H.; Demidova, M.; Michelle, H. Yeast forms dominate fungal diversity in the deep oceans. Proc. R. Soc. B Biol. Sci. 2007, 274, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Carlile, M.J.; Watkinson, S.C.; Gooday, G.W. The fungi, 2nd ed.; Gulf Professional Publishing: San Diego, CA, USA, 2001; pp. 1–600. [Google Scholar]
- Dimitrios, F.; Binder, M.; Riley, R.; Barry, K. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012, 336, 1715–1719. [Google Scholar]
- Tian, J.; Zhu, D.; Wang, J.; Wu, B.; Hussain, M.; Liu, X. Environmental factors driving fungal distribution in freshwater lake sediments across the Headwater Region of the Yellow River, China. Sci. Rep. 2018, 8, 3768. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Dickie, I.A.; Paula Wilkie, J.; Paulus, B.C.; Park, D.; Roberts, A.; Buchanan, P.K.; Allen, R.B. Assembly history dictates ecosystem functioning: Evidence from wood decomposer communities. Ecol. Lett. 2010, 13, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Multiple competitive mechanisms underlie the effects of a strong invader on early-to late-seral tree seedlings. J. Ecol. 2012, 100, 1204–1215. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justic, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Heisle, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Gobler, C.; Dortch, Q.; Heil, C.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Duan, Y.; Xie, N.; Song, Z. A high-resolution time series reveals distinct seasonal patterns of planktonic fungi at a temperate coastal ocean site (Beaufort, North Carolina, USA). Appl. Environ. Microbiol. 2018, 84, e00967-18. [Google Scholar] [CrossRef]
- Cudowski, A.; Pietryczuk, A. Biodiversity of mycoplankton in the profile of eutrophic lakes with varying water quality. Fungal Ecol. 2020, 48, 100978. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2010, 9, 18–26. [Google Scholar]
- Banos, S.; Gysi, D.M.; Richter-Heitmann, T.; Glckner, F.O.; Reich, M. Seasonal dynamics of pelagic mycoplanktonic communities: Interplay of taxon abundance, temporal occurrence, and biotic interactions. Front. Microbiol. 2020, 11, 1305. [Google Scholar]
- Ramón, A.-R.; Daniel, C.-M.; Rudolph, A.; Reinoso, R.; Torres, C.; Silva, M.; Becerra, J. Variation of sterols and fatty acids as an adaptive response to changes in temperature, salinity and pH of a marine fungus Epicoccum nigrum isolated from the Patagonian Fjords. Rev. Biol. Mar. Oceanogr. 2014, 49, 293–305. [Google Scholar]
- Tisthammer, K.H.; Cobian, G.M.; Amend, A.S. Global biogeography of marine fungi is shaped by the environment. Fungal Ecol. 2015, 19, 39–46. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Y.; Liao, X.; Yang, X.; Chen, Z.; Mo, L.; Zhong, S.; Zhang, X. Fungal diversity and its relationship with environmental factors in coastal sediments from Guangdong, China. J. Fungi 2023, 9, 101. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Rieck, A.; Wurzbacher, C.; Jürgens, K.; Grossart, H.P. A salinity threshold separating fungal communities in the Baltic Sea. Front. Microbiol. 2019, 10, 680. [Google Scholar]
- Burgaud, G.; Woehlke, S.; Redou, V.; Orsi, W.; Edgcomb, V.P. Deciphering the presence and activity of fungal communities in marine sediments using a model estuarine system. Aquat. Microb. Ecol. 2013, 70, 45–62. [Google Scholar]
- Gilbert, J.A.; Field, D.; Swift, P.; Newbold, L.; Joint, I. The seasonal structure of microbial communities in the Western English Channel. Environ. Microbiol. 2010, 11, 3132–3139. [Google Scholar]
- Sen, K.; Bai, M.; Sen, B.; Wang, G. Disentangling the structure and function of mycoplankton communities in the context of marine environmental heterogeneity. Sci. Total Environ. 2021, 766, 142635. [Google Scholar]
- Zhao, H.; Brearley, F.Q.; Huang, L.; Tang, J.; Qiang, S. Abundant and rare taxa of planktonic fungal community exhibit distinct assembly patterns along coastal eutrophication gradient. Microb. Ecol. Int. J. 2023, 85, 495–507. [Google Scholar]
- Zhang, J.; Zhou, F.; Chen, C.; Sun, X.; Shi, Y.; Zhao, H.; Chen, F.; Hong, Y. Spatial distribution and correlation characteristics of heavy metals in the seawater, suspended particulate matter and sediments in Zhanjiang Bay, China. PLoS ONE 2018, 13, e0201414. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, F.; Chen, F.; Zhu, Q.; Meng, Y. Spatial and seasonal variations of chlorophyll a in Zhanjiang Bay, China, and controlling factors. Front. Mar. Sci. 2024, 11, 1329864. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Fan, C.; Zhu, B.; Fu, M.; Zhang, P. Effects of terrestrial input on heavy metals in Zhanjiang Bay, a typical subtropical bay in the South China Sea. Mar. Pollut. Bull. 2024, 199, 116015. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Z.; Yan, W. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediments from Zhanjiang Bay and Leizhou Bay, South China. Mar. Pollut. Bull. 2012, 64, 1962–1969. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, F.; Chen, F.; Meng, Y.; Zhu, Q. Spatiotemporal distribution characteristics of nutrients in the drowned tidal inlet under the influence of tides: A case study of zhanjiang bay, China. Int. J. Environ. Res. Public Health 2021, 18, 2089. [Google Scholar] [CrossRef]
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The potential role of marine fungi in plastic degradation—A review. Front. Mar. Sci. 2021, 8, 738877. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’Anno, A. Bacteria, fungi and microalgae for the bioremediation of marine sediments contaminated by petroleum hydrocarbons in the omics era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef]
- Lotlikar, N.P.; Damare, S.R.; Meena, R.M.; Linsy, P.; Mascarenhas, B. Potential of marine-derived fungi to remove hexavalent chromium pollutant from culture broth. Indian J. Microbiol. 2018, 58, 182–192. [Google Scholar]
- Dell’Anno, F.; Rastelli, E.; Buschi, E.; Barone, G.; Beolchini, F.; Dell’Anno, A. Fungi can be more effective than bacteria for the bioremediation of marine sediments highly contaminated with heavy metals. Microorganisms 2022, 10, 993. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Zienkiewicz, K.; Vande Pol, N.; Ostrom, N.E.; Benning, C.; Bonito, G.M. Algal-fungal symbiosis leads to photosynthetic mycelium. Elife 2019, 8, e47815. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Wang, Y.; Lin, Z.; Zhu, Q.; Li, D.; Li, D. Characteristics and influencing factors of rhizosphere fungi of six mangrove plants of the genus Sonneratia. Acta Ecologica Sinica. 2024, 44, 2517–2530. [Google Scholar]
- GB 12763-2007; Standardization Administration of China. Specification for Oceanographic Survey. Ocean Press: Beijing, China, 2007.
- Ihrmark, K.; Bödeker, I.T.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- White, T. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjøller, R.; Kõljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J. Fungal community analysis by high-throughput sequencing of amplified markers—A user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef]
- Möhlenhoff, P.; Müller, L.; Gorbushina, A.A.; Karin, P. Molecular approach to the characterisation of fungal communities: Methods for DNA extraction, PCR amplification and DGGE analysis of painted art objects. FEMS Microbiol. Lett. 2001, 195, 169–173. [Google Scholar] [CrossRef]
- Pang, K.L.; Mitchell, J.I. Molecular approaches for assessing fungal diversity in marine substrata. Bot. Mar. 2005, 50, 283–347. [Google Scholar] [CrossRef][Green Version]
- Leal, M.C.; Ferrier-Pagès, C. Molecular trophic markers in marine food webs and their potential use for coral ecology. Mar. Genom. 2016, 29, 1–7. [Google Scholar] [CrossRef]
- Wirta, H.K.; Vesterinen, E.J.; Hambck, P.A.; Weingartner, E.; Rasmussen, C.; Reneerkens, J.; Schmidt, N.M.; Gilg, O.; Roslin, T. Exposing the structure of an Arctic food web. Ecol. Evol. 2015, 5, 3842–3856. [Google Scholar] [CrossRef]
- Zhang, P.; Peng, C.H.; Zhang, J.B.; Zhang, J.X.; Chen, J.Y.; Zhao, H. Long-Term harmful algal blooms and nutrients patterns affected by climate change and anthropogenic pressures in the Zhanjiang Bay, China. Front. Mar. Sci. 2022, 9, 849819. [Google Scholar] [CrossRef]
- Taylor, J.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118–2128. [Google Scholar] [CrossRef]
- Wang, Y.; Sen, B.; He, Y.; Xie, N.; Wang, G. Spatiotemporal distribution and assemblages of planktonic fungi in the coastal waters of the Bohai Sea. Front. Microbiol. 2018, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Huang, J.; Luo, K.; Lin, X.; Su, M.; Lu, J. Bacterial, archaeal, and fungal community structure and interrelationships of deep-sea shrimp intestine and the surrounding sediment. Environ. Res. 2021, 205, 112461. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Ramos, J.; Plemenita, A. Halotolerant and halophilic fungi. Mycol. Res. 2009, 113, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef]
- Crampon, M.; Soulier, C.; Sidoli, P.; Hellal, J.; Joulian, C.; Charron, M.; Guillemoto, Q.; Picot-Colbeaux, G.; Pettenati, M. Dynamics of soil microbial communities during diazepam and oxazepam biodegradation in soil flooded by water from a WWTP. Front. Microbiol. 2021, 12, 742000. [Google Scholar] [CrossRef]
- Barone, G.; Rastelli, E.; Corinaldesi, C.; Tangherlini, M.; Danovaro, R.; Dell’Anno, A. Benthic deep-sea fungi in submarine canyons of the Mediterranean Sea. Prog. Oceanogr. 2018, 168, 57–64. [Google Scholar] [CrossRef]
- Varrella, S.; Barone, G.; Corinaldesi, C.; Giorgetti, A.; Nomaki, H.; Nunoura, T.; Rastelli, E.; Tangherlini, M.; Danovaro, R.; Dell’Anno, A. Fungal abundance and diversity in the Mariana Trench, the deepest ecosystem on earth. J. Fungi 2024, 10, 73. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Zhang, X.; Zhang, J.; Gong, J. Marine fungal communities in water and surface sediment of a sea cucumber farming system: Habitat-differentiated distribution and nutrients driving succession. Fungal Ecol. 2015, 14, 87–98. [Google Scholar] [CrossRef]
- García-García, N.; Tamames, J.; Linz, A.M.; Pedrós-Alió, C.; Puente-Sánchez, F. Microdiversity ensures the maintenance of functional microbial communities under changing environmental conditions. Int. Soc. Microb. Ecol. J. 2019, 13, 2969–2983. [Google Scholar] [CrossRef]
- Wainwright, B.J.; Zahn, G.L.; Arlyza, I.S.; Amend, A.S. Seagrass-associated fungal communities follow Wallace’s line, but host genotype does not structure fungal community. J. Biogeogr. 2018, 45, 762–770. [Google Scholar] [CrossRef]
- Zheng, Y.; Maitra, P.; Gan, H.-Y.; Chen, L.; Li, S.; Tu, T. Soil fungal diversity and community assembly: Affected by island size or type? FEMS Microbiol. Ecol. 2021, 97, fiab062. [Google Scholar] [CrossRef]
- Jeffries, T.C.; Curlevski, N.J.; Brown, M.V.; Harrison, D.P. Partitioning of fungal assemblages across different marine habitats. Environ. Microbiol. Rep. 2016, 8, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sen, K.; He, Y.; Xie, Y.; Wang, G. Impact of environmental gradients on the abundance and diversity of planktonic fungi across coastal habitats of contrasting trophic status. Sci. Total Environ. 2019, 683, 822–833. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Issa, A.A.; Gomaa, M. Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 2014, 740, 37–49. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yan Yu, L. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (High Arctic). Sci. Rep. 2015, 5, 14524. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling interactions in the microbiome: A network perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, Y.; Chen, F.; Li, X.; Wang, H. Molecular ecological network complexity drives stand resilience of soil bacteria to mining disturbances among typical damaged ecosystems in China. Microorganisms 2020, 8, 433. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Zhou, J. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, J.; Sun, B. Structure of functional ecological networks of soil microbial communities for nitrogen transformations and their response to cropping in major soils in eastern China. Chin. Sci. Bull. 2014, 59, 387–396. (In Chinese) [Google Scholar]
- Wu, X.; Wang, R.; Hu, H.; Xiu, W. Response of bacterial and fungal communities to chemical fertilizer reduction combined with organic fertilizer and straw in Fluvo-aquic soil. Environ. Sci. 2020, 41, 4670–4681. (In Chinese) [Google Scholar]
- Hyde, K.D.; Jones, E.B.G.; Pointing, S.B.; Poonyth, A.D.; Vrijmoed, L.L.P. Role of fungi in marine ecosystems. Biodivers. Conserv. 1998, 7, 1147–1161. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C. Fungi in the Marine Environment: Open Questions and Unsolved Problems. Am. Soc. Microbiol. 2019, 10, e01189-18. [Google Scholar] [CrossRef]
- Ettinger, C.L.; Vann, L.E.; Eisen, J.A. Global diversity and biogeography of the Zostera marina mycobiome. Appl. Environ. Microbiol. 2021, 87, e02795-20. [Google Scholar] [CrossRef]
- Summerbell, R.; Lévesque, C.; Seifert, K.; Bovers, M.; Fell, J.; Diaz, M.; Boekhout, T.; De Hoog, G.; Stalpers, J.; Crous, P. Microcoding: The second step in DNA barcoding. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1897–1903. [Google Scholar] [CrossRef]
- Raghukumar, S. Fungi in Coastal and Oceanic Marine Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; Volume 378. [Google Scholar]
- Sen, K.; Sen, B.; Wang, G. Diversity, abundance, and ecological roles of planktonic fungi in marine environments. J. Fungi 2022, 8, 491. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, Y.; Xiong, S.; Yu, H.; Xi, B. Untangling the response of fungal community structure, composition and function in soil aggregate fractions to food waste compost addition. Sci. Total Environ. 2021, 769, 145248. [Google Scholar] [CrossRef]
- Jia, J.; Wang, L.; Tang, Y.; Zhang, W. Variability in and factors influencing soil microbial respiration in the Jiuduansha Wetland under different successional stages. Acta Ecol. Sin. 2010, 30, 4529–4538. [Google Scholar]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2010, 10, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.W.; Sharpley, A.; Walker, D. Sources of nutrient pollution to coastal waters in the United States: Implications for achieving coastal water quality goals. Estuaries 2002, 25, 656–676. [Google Scholar] [CrossRef]
- Meybeck, M. Global analysis of river systems: From Earth system controls to Anthropocene syndromes. Philos. Trans. R. Soc. B-Biol. Sci. 2003, 358, 1935–1955. [Google Scholar] [CrossRef] [PubMed]
- Shish, F.-K.; Ducklow, H.W. Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol. Oceanogr. 1994, 39, 1243–1258. [Google Scholar] [CrossRef]
- Jiang, C.; Fu, D.; Li, Q.; Liu, D. Thermohaline structure and ecological characteristics of the Zhanjiang Bay and its estuary in autumn. Acta Oceanol. Sin. 2016, 38, 20–31. [Google Scholar]
- Huang, R.; Xie, L.; Li, M.; Wang, L. Observational analysis on 3-D distribution and seasonal variation of thermohaline characteristics in Zhanjiang Bay. Acta Oceanol. Sin. 2021, 43, 46–60. [Google Scholar]
- Kogej, T.; Ramos, J.; Plemenitaš, A.; Gunde-Cimerman, N. The halophilic fungus Hortaea werneckii and the halotolerant fungus Aureobasidium pullulans maintain low intracellular catio concentrations in hypersaline environments. Appl. Environ. Microbiol. 2005, 71, 6600–6605. [Google Scholar] [CrossRef]
- Mohamed, D.J.; Martiny, J.B. Patterns of fungal diversity and composition along a salinity gradient. Int. Soc. Microb. Ecol. J. 2010, 5, 379–388. [Google Scholar] [CrossRef]
- Ahumada-Rudolph, R.; Novoa, V.; Becerra, J. Morphological response to salinity, temperature, and pH changes by marine fungus Epicoccum nigrum. Environ. Monit. Assess. 2018, 191, 35–51. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, H.; Yang, S.; Qin, X.; Liao, N.; Li, X.; Wei, Q.; Li, W.; Jiang, G.; Li, N.; et al. Mycoplanktonic community structure and their roles in monitoring environmental changes in a subtropical estuary in the Beibu Gulf. J. Mar. Sci. Eng. 2022, 10, 1940. [Google Scholar] [CrossRef]
- Sun, J.Y.; Song, Y.; Ma, Z.P.; Zhang, H.J.; Yang, Z.D.; Cai, Z.H.; Zhou, J. Fungal community dynamics during a marine dinoflagellate (Noctiluca scintillans) bloom. Mar. Environ. Res. 2017, 131, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, X.; Bai, Y.; Lin, Z.; Qiu, M.; Nie, X.; Wang, S.; Zhang, F.; Zhuang, Z.; Yuan, J.; et al. Effects of nitrogen metabolism on growth and aflatoxin biosynthesis in Aspergillus flavus. J. Hazard. Mater. 2016, 324, 691–700. [Google Scholar] [CrossRef] [PubMed]




| Station | Depth (m) | T (°C) | pH | Salt | Chl-a (µg/L) | DIN (mg/L) | SiO3-Si (mg/L) | PO4-P (mg/L) | COD (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 15.4 | 24.63 | 7.65 | 30.63 | 0.49 | 0.240 | 1.408 | 0.008 | 0.88 |
| S2 | 27.3 | 24.87 | 7.72 | 31.67 | 0.49 | 0.231 | 1.387 | 0.003 | 1.36 |
| S3 | 36.4 | 24.74 | 7.71 | 32.07 | 0.49 | 0.234 | 1.538 | 0.002 | 1.12 |
| S4 | 9.6 | 25.13 | 7.69 | 32.01 | 1.47 | 0.238 | 1.565 | 0.030 | 1.04 |
| S5 | 7.9 | 24.84 | 7.70 | 31.32 | 1.47 | 0.209 | 1.470 | 0.004 | 0.96 |
| S6 | 4.9 | 24.64 | 7.68 | 28.75 | 0.98 | 0.281 | 1.626 | 0.045 | 1.28 |
| S7 | 6.8 | 24.65 | 7.67 | 30.01 | 1.47 | 0.262 | 1.385 | 0.014 | 0.96 |
| S8 | 10.6 | 24.08 | 7.67 | 30.12 | 1.47 | 0.278 | 1.385 | 0.042 | 1.20 |
| S9 | 6.6 | 24.66 | 7.65 | 29.92 | 1.97 | 0.274 | 1.646 | 0.027 | 1.20 |
| S10 | 18.5 | 24.15 | 7.63 | 29.18 | 1.97 | 0.348 | 2.123 | 0.066 | 1.20 |
| S11 | 14.3 | 24.29 | 7.61 | 29.08 | 3.44 | 0.302 | 1.894 | 0.089 | 1.28 |
| S12 | 7.7 | 24.74 | 7.70 | 27.56 | 1.47 | 0.381 | 2.790 | 0.094 | 1.44 |
| S13 | 7.2 | 25.07 | 7.64 | 24.44 | 0.49 | 0.340 | 2.451 | 0.071 | 1.60 |
| S14 | 2.1 | 24.64 | 7.50 | 18.48 | 2.95 | 0.770 | 5.428 | 0.117 | 2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Liu, B.; Li, J.; Deng, Y.; Zhang, Y.; Zhang, N.; Li, F.; Li, C.; Huang, X.; Hu, Z. Diversity and Distribution of Fungi in the Marine Sediments of Zhanjiang Bay, China. J. Fungi 2024, 10, 867. https://doi.org/10.3390/jof10120867
Gao M, Liu B, Li J, Deng Y, Zhang Y, Zhang N, Li F, Li C, Huang X, Hu Z. Diversity and Distribution of Fungi in the Marine Sediments of Zhanjiang Bay, China. Journal of Fungi. 2024; 10(12):867. https://doi.org/10.3390/jof10120867
Chicago/Turabian StyleGao, Menghan, Bihong Liu, Jianming Li, Yunyan Deng, Yulei Zhang, Ning Zhang, Feng Li, Changling Li, Xianghu Huang, and Zhangxi Hu. 2024. "Diversity and Distribution of Fungi in the Marine Sediments of Zhanjiang Bay, China" Journal of Fungi 10, no. 12: 867. https://doi.org/10.3390/jof10120867
APA StyleGao, M., Liu, B., Li, J., Deng, Y., Zhang, Y., Zhang, N., Li, F., Li, C., Huang, X., & Hu, Z. (2024). Diversity and Distribution of Fungi in the Marine Sediments of Zhanjiang Bay, China. Journal of Fungi, 10(12), 867. https://doi.org/10.3390/jof10120867












