Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Temperature, and Relative Humidity
2.2. Experimental Design and Water Treatment
2.3. Crop Management
2.4. Measurements and Data Collection
Yield and Its Components
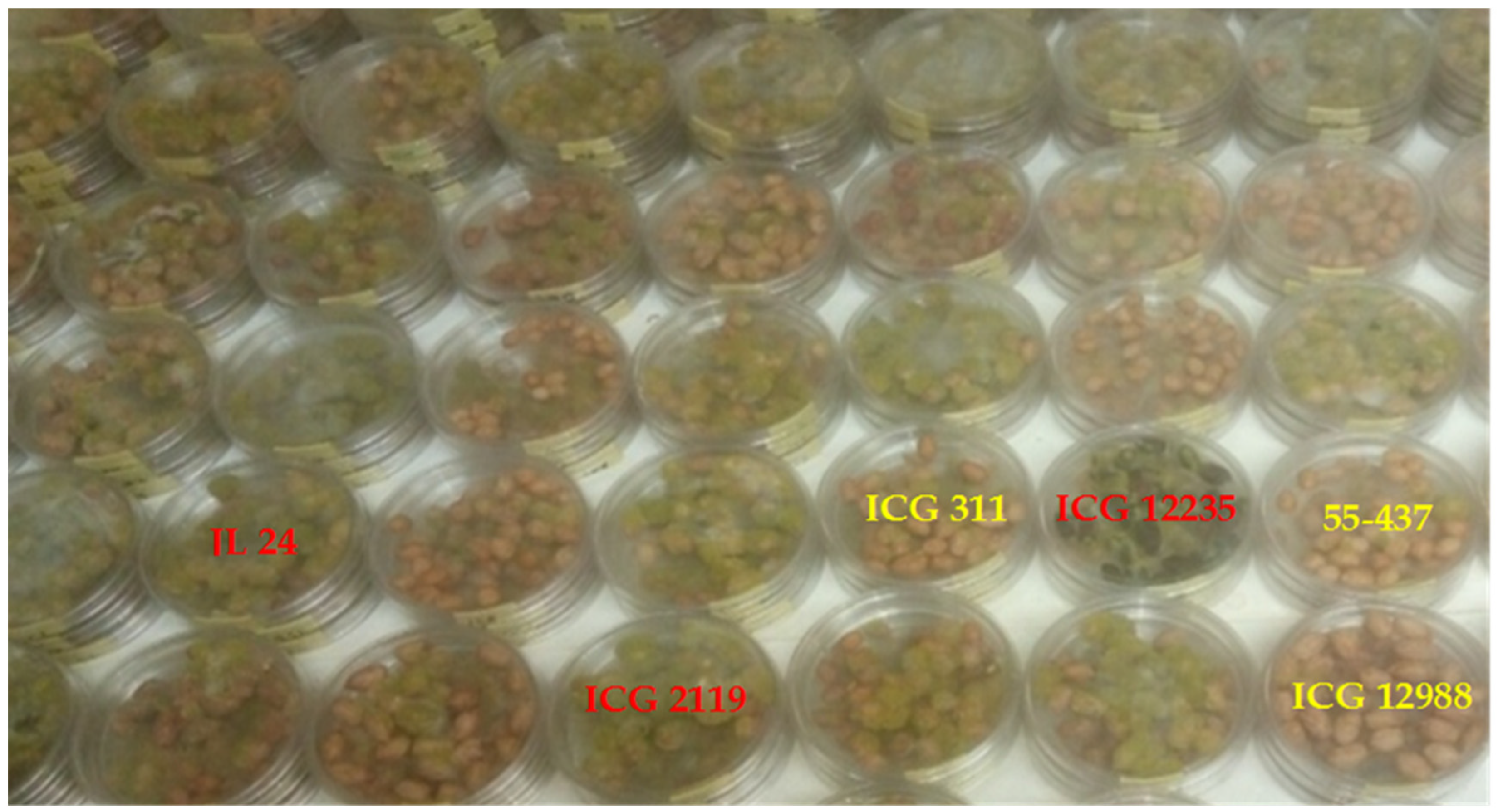
2.5. In-Vitro Seed Colonization Assay
2.6. Seed Coat Total Polyphenol Extraction and Quantification
2.7. Genotypes Aflatoxin Content Quantification
2.8. Analysis
3. Results
3.1. Yields and Yield Components
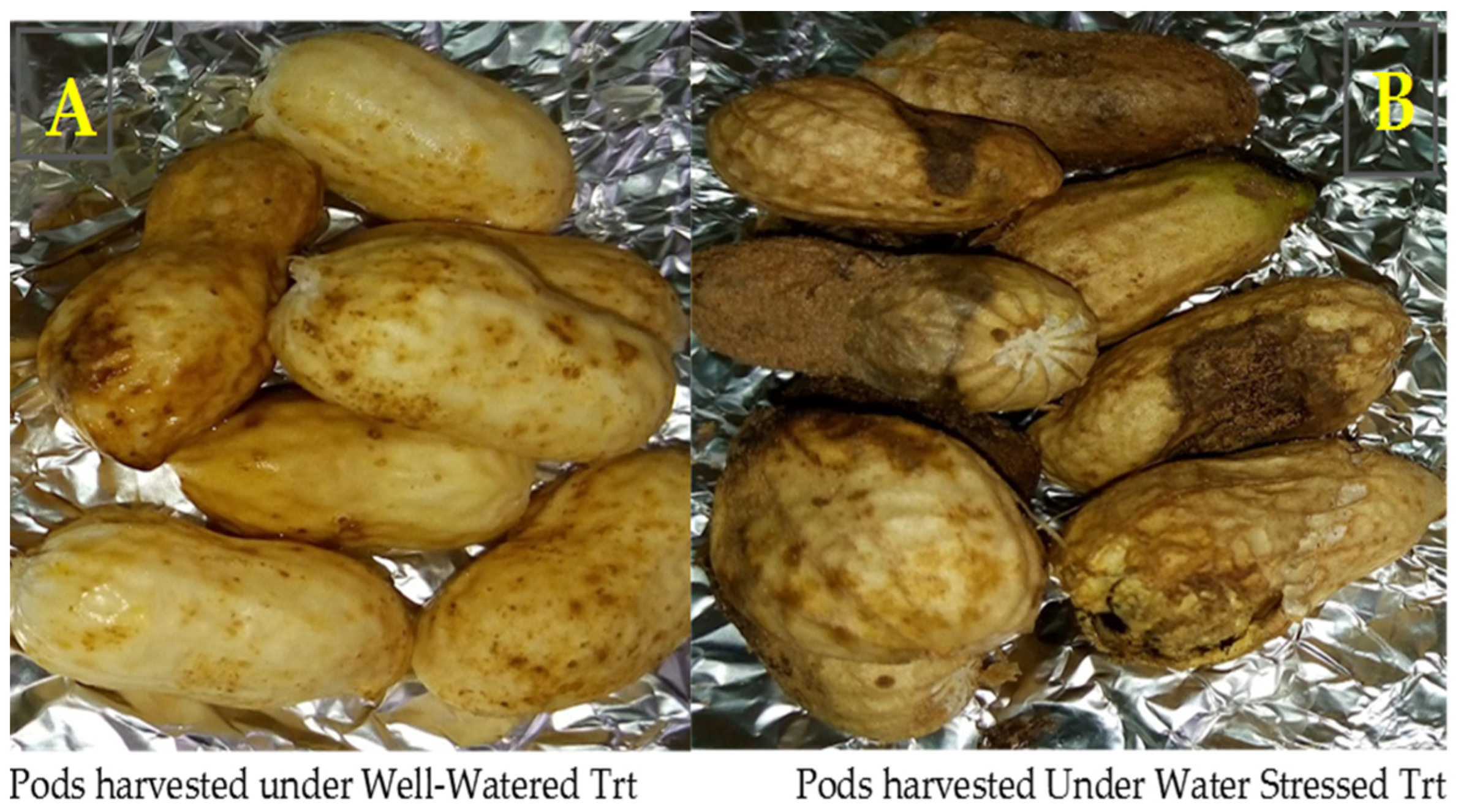
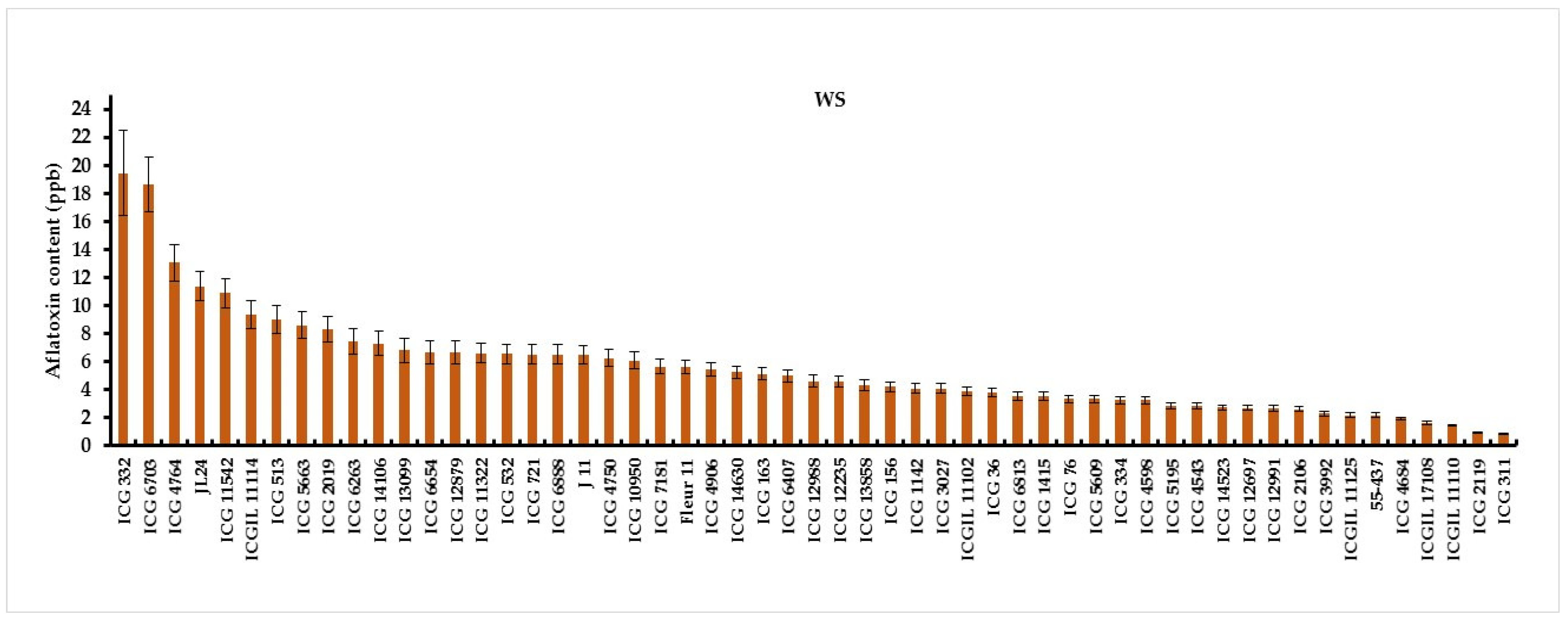
3.2. Intermittent Water Deficit (WS) Effect on Pre-Harvest Aflatoxin B1 Contamination (AC)
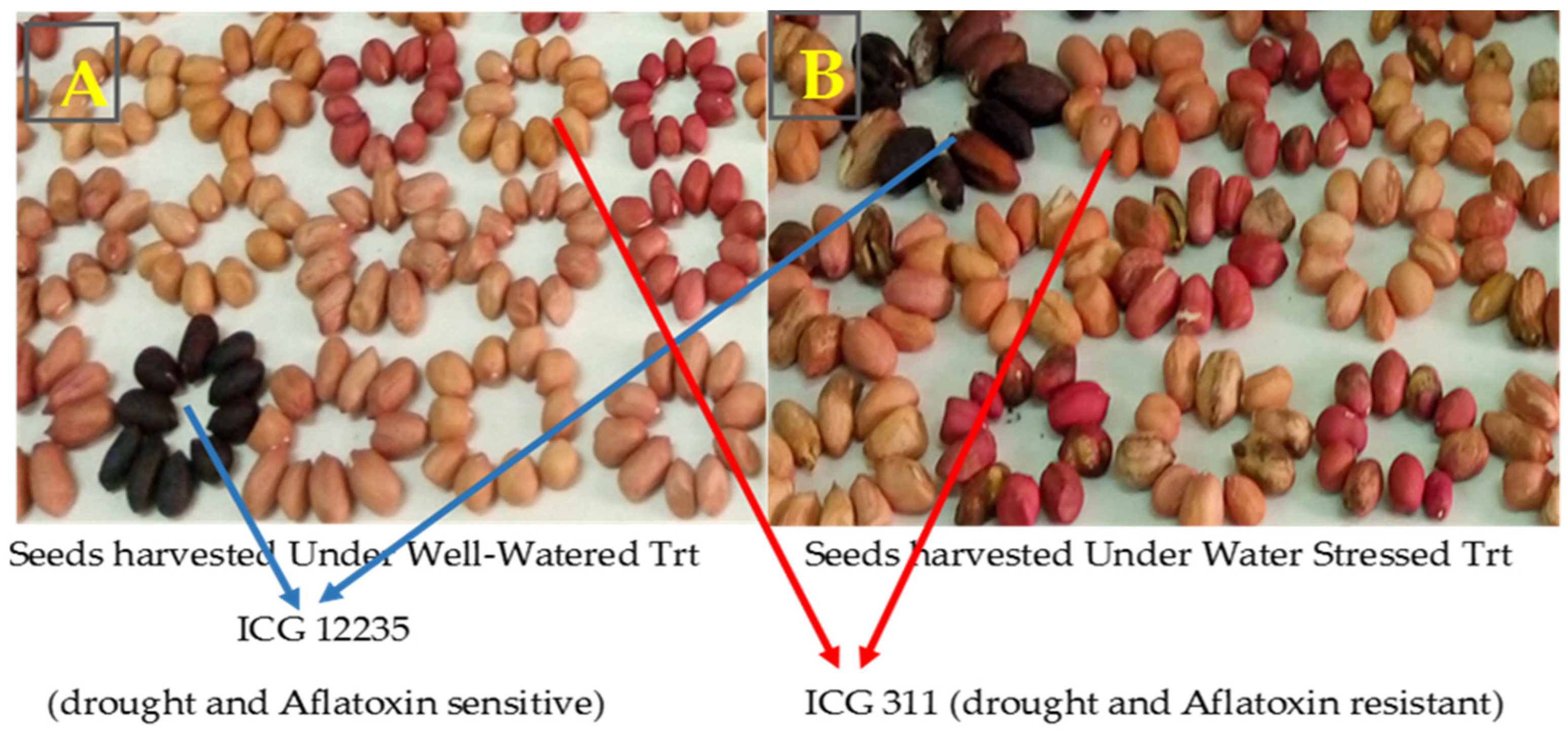
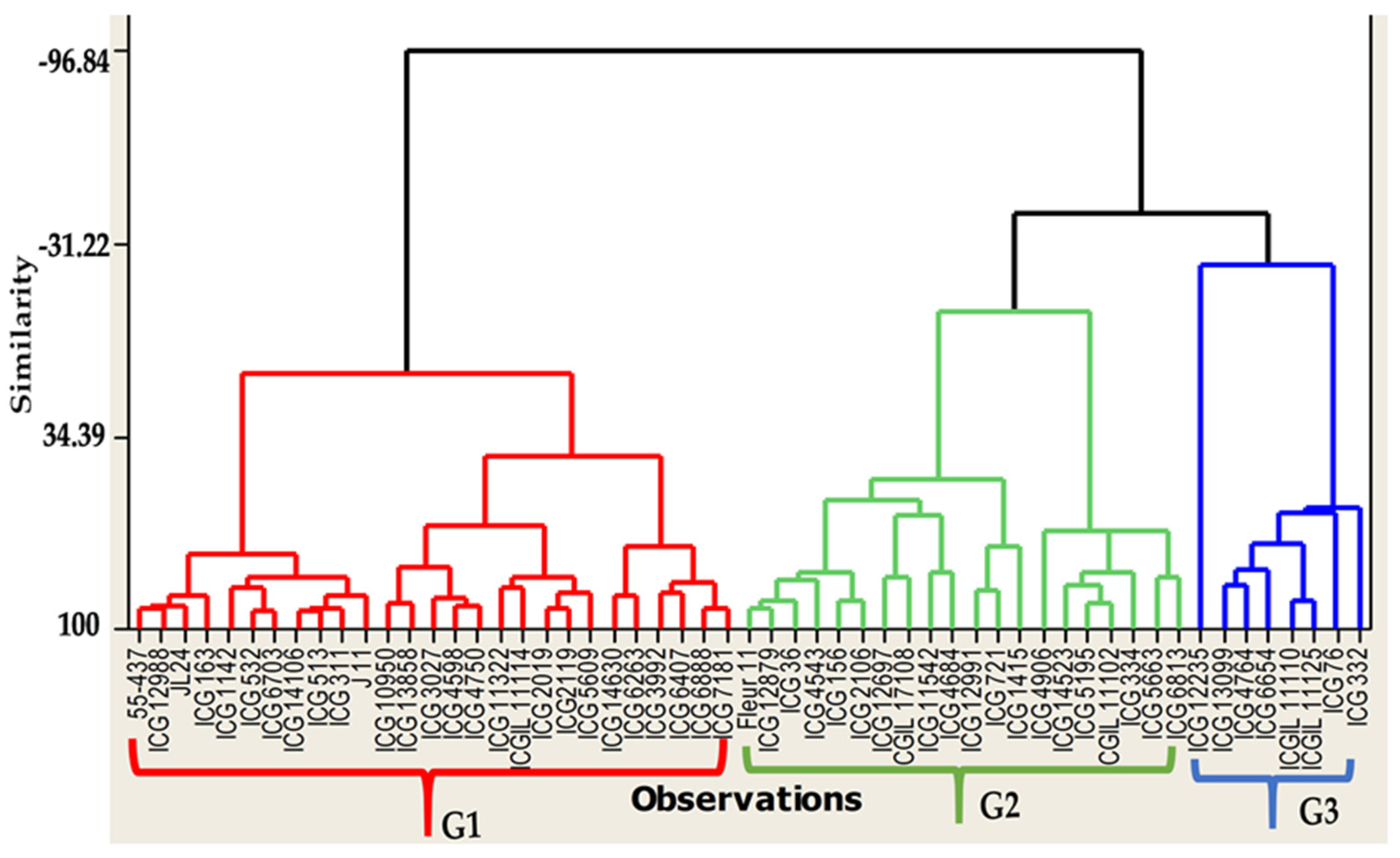
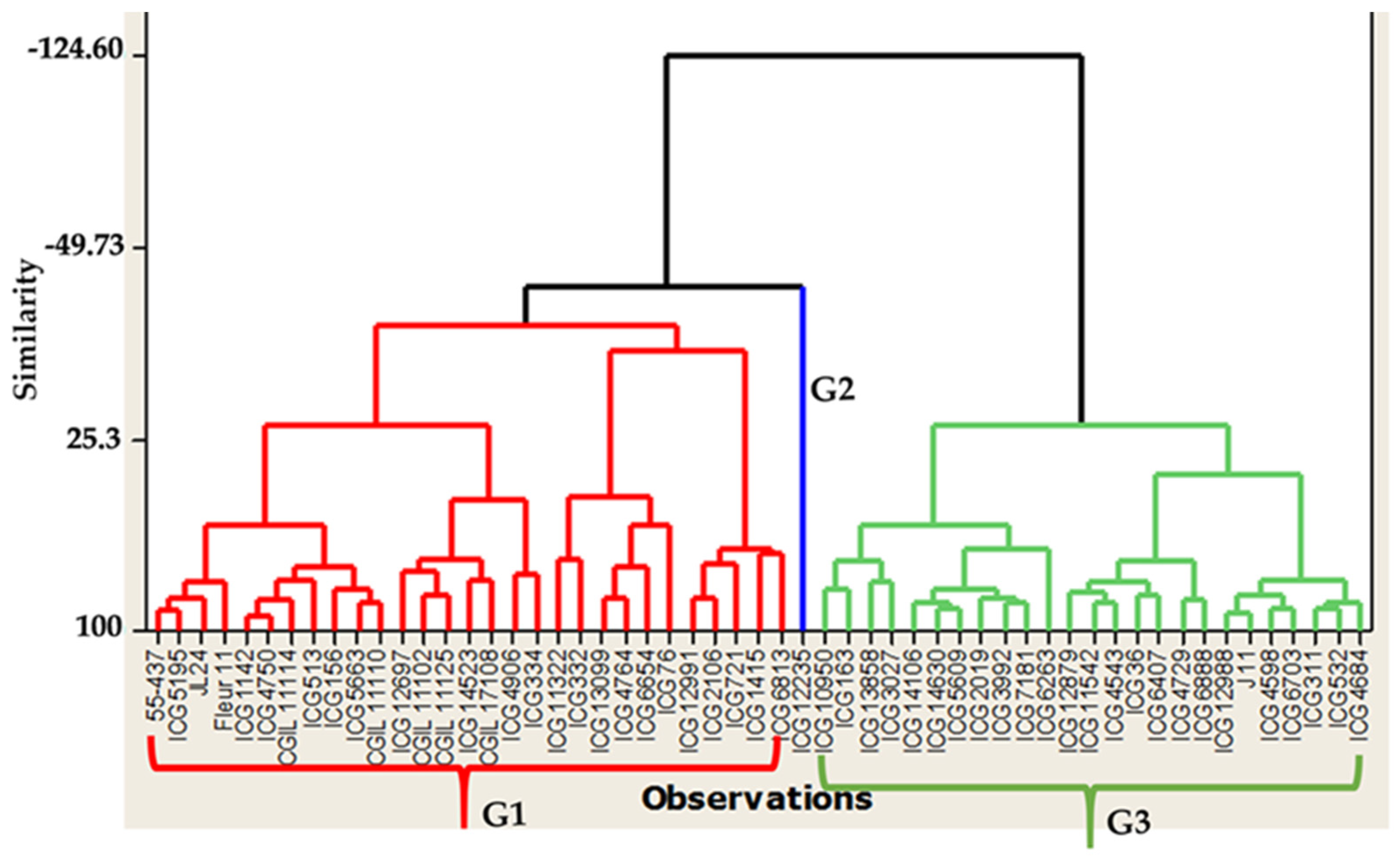
3.3. Seeds Colonization and Total Polyphenol Content of Seed Coat
3.4. Relationship between Aflatoxin Contaminations with Total Polyphenol, Pod Yield, Seed Yield, and Haulm Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underwood, C.; Taylor, H.; Hoveland, C. Soil Physical Factors Affecting Peanut Pod Development 1. Agron. J. 1971, 63, 953–954. [Google Scholar] [CrossRef]
- Horn, B. Colonization of wounded peanut seeds by soil fungi: Selectivity for species from Aspergillus section Flavi. Mycologia 2005, 97, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; Samsudin, N.I.P.; Azri, F.A. Aspergillus section Flavi and aflatoxins: Occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front. Microbiol. 2019, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Massomo, S.M. Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Sci. Afr. 2020, 10, e00606. [Google Scholar] [CrossRef]
- Arunyanark, A.; Pimratch, S.; Jogloy, S.; Wongkaew, S.; Vorasoot, N.; Akkasaeng, C.; Kesmala, T.; Patanothai, A.; Holbrook, C.C. Association between aflatoxin contamination and N2 fixationin peanut under drought conditions. Int. J. Plant Prod. 2012, 6, 161–172. [Google Scholar]
- Song, R.; Yao, L.; Sun, C.; Yu, D.; Lin, H.; Li, G.; Lian, Z.; Zhuang, S.; Zhang, D. Electrospun Membranes Anchored with g-C3N4/MoS2 for Highly Efficient Photocatalytic Degradation of Aflatoxin B1 under Visible Light. Toxins 2023, 15, 133. [Google Scholar] [CrossRef]
- Alshannaq, A.F.; Gibbons, J.G.; Lee, M.-K.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018, 8, 16871. [Google Scholar] [CrossRef]
- Dieme, R.M.A.; Faye, I.; Zoclanclounon, Y.A.B.; Fonceka, D.; Ndoye, O.; Diedhiou, P.M. Identification of Sources of Resistance for Peanut Aspergillus flavus Colonization and Aflatoxin Contamination. Int. J. Agron. 2018, 2018, 5468602. [Google Scholar] [CrossRef]
- Bediako, K.A.; Ofori, K.; Offei, S.K.; Dzidzienyo, D.; Asibuo, J.Y.; Amoah, R.A. Aflatoxin contamination of groundnut (Arachis hypogaea L.): Predisposing factors and management interventions. Food Control. 2019, 98, 61–67. [Google Scholar] [CrossRef]
- Ndunguru, B.; Ntare, B.; Williams, J.; Greenberg, D. Assessment of groundnut cultivars for end-of-season drought tolerance in a Sahelian environment. J. Agric. Sci. 1995, 125, 79–85. [Google Scholar] [CrossRef]
- Falade, T.D.O.; Neya, A.; Bonkoungou, S.; Dagno, K.; Basso, A.; Senghor, A.L.; Atehnkeng, J.; Ortega-Beltran, A.; Bandyopadhyay, R. Aflatoxin contamination of maize, groundnut, and sorghum grown in Burkina Faso, Mali, and Niger and aflatoxin exposure assessment. Toxins 2022, 14, 700. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.; Sy, A.T.; Blaise, K.; Sondé, A.L.M.K. Assessment of sixteen varieties of groundnut in two agro ecological zones in Burkina Faso for yield and tolerance to aflatoxin. Afr. J. Agric. Res. 2021, 17, 66–78. [Google Scholar] [CrossRef]
- Hamidou, F.; Halilou, O.; Vadez, V. Assessment of groundnut under combined heat and drought stress. J. Agron. Crop Sci. 2013, 199, 1–11. [Google Scholar] [CrossRef]
- Waliyar, F.; Traore, A.; Fatondji, D.; Ntare, B. Effect of irrigation interval, planting date, and cultivar on Aspergillus flavus and aflatoxin contamination of peanut in a sandy soil of Niger. Peanut Sci. 2003, 30, 79–84. [Google Scholar] [CrossRef]
- Craufurd, P.; Prasad, P.; Waliyar, F.; Taheri, A. Drought, pod yield, pre-harvest Aspergillus infection and aflatoxin contamination on peanut in Niger. Field Crops Res. 2006, 98, 20–29. [Google Scholar] [CrossRef]
- Ferrari, L.; Rizzi, N.; Grandi, E.; Clerici, E.; Tirloni, E.; Stella, S.; Bernardi, C.E.M.; Pinotti, L. Compliance between food and feed safety: Eight-year survey (2013–2021) of aflatoxin M1 in raw milk and aflatoxin B1 in feed in northern Italy. Toxins 2023, 15, 168. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Sanders, T.H.; Blankenship, P.D. Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stressed peanuts. Mycopathologia 1989, 105, 117–128. [Google Scholar] [CrossRef]
- Girdthai, T.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Wongkaew, S.; Holbrook, C.C.; Patanothai, A. Heritability of, and genotypic correlations between, aflatoxin traits and physiological traits for drought tolerance under end of season drought in peanut (Arachis hypogaea L.). Field Crops Res. 2010, 118, 169–176. [Google Scholar] [CrossRef]
- Waliyar, F.; Hassan, H.; Bonkoungou, S. Sources of resistance to Aspergillus flavus and aflatoxin contamination in groundnut genotypes in West Africa. Plant Dis. 1994, 78, 704–708. [Google Scholar] [CrossRef]
- Keller, N.P.; Butchko, R.A.; Sarr, B.; Phillips, T.D. A visual pattern of mycotoxin production in maize kernels by Aspergillus spp. Phytopathology 1994, 84, 483–488. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Nigam, S.; Mehan, V.; Lenne, J. Aflatoxin Contamination of Groundnut: Prospects for a Genetic Solution Through Conventional Breeding. In Proceedings of the First Asia Working Group Meeting, Hanoi, Vietnam, 27–29 May 1996. [Google Scholar]
- Mendu, L.; Cobos, C.J.; Tengey, T.K.; Commey, L.; Balasubramanian, V.K.; Williams, L.D.; Dhillon, K.K.; Sharma, D.; Pandey, M.K.; Falalou, H.; et al. Seed coat mediated resistance against Aspergillus flavus infection in peanut. Plant Gene 2022, 32, 100381. [Google Scholar] [CrossRef]
- Souza, F.H.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Braz. J. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Pandey, M.K.; Kumar, R.; Pandey, A.K.; Soni, P.; Gangurde, S.S.; Sudini, H.K.; Fountain, J.C.; Liao, B.; Desmae, H.; Okori, P.; et al. Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post-harvest management practices. Toxins 2019, 11, 315. [Google Scholar] [CrossRef]
- Mukanga, M.; Matumba, L.; Makwenda, B.; Alfred, S.; Sakala, W.; Kanenga, K.; Chancellor, T.; Mugabe, J.; Bennett, B. Participatory evaluation of groundnut planting methods for pre-harvest aflatoxin management in Eastern Province of Zambia. Cah. Agric. 2019, 28, 1. [Google Scholar] [CrossRef]
- Arunyanark, A.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Kesmala, T.; Patanothai, A. Stability of Relationship Between Chlorophyll Density and Soil Plant Analysis Development Chlorophyll Meter Readings in Peanut Across Different Drought Stress Conditions. Asian J. Plant Sci. 2009, 8, 102–110. [Google Scholar] [CrossRef]
- Olwari, F.; Bisikwa, J.; Kaaya, A.N.; Okello, D.K. Tolerance Levels of Peanut Varieties against Aspergillus flavus Infection. J. Plant Pathol. Microbiol. 2013, 4, 195. [Google Scholar]
- Commey, L.; Tengey, T.K.; Cobos, C.J.; Dampanaboina, L.; Dhillon, K.K.; Pandey, M.K.; Sudini, H.K.; Falalou, H.; Varshney, R.K.; Burow, M.D.; et al. Peanut Seed Coat Acts as a Physical and Biochemical Barrier against Aspergillus flavus Infection. J. Fungi 2021, 7, 1000. [Google Scholar] [CrossRef]
- Mixon, A. Reducing Aspergillus species infection of peanut seed using resistant genotypes. J. Environ. Qual. 1986, 15, 101–103. [Google Scholar] [CrossRef]
- Waliyar, F.; Kumar, K.V.K.; Diallo, M.; Traore, A.; Mangala, U.N.; Upadhyaya, H.D.; Sudini, H. Resistance to pre-harvest aflatoxin contamination in ICRISAT’s groundnut mini core collection. Eur. J. Plant Pathol. 2016, 145, 901–913. [Google Scholar] [CrossRef]
- Waliyar, F.; Bockelee-Morvan, A. Resistance of Groundnut Varieties to Aspergillus flavus in Senegal. In Proceedings of the International Workshop on Aflatoxin Contamination of Groundnut, Patancheru, India, 6–9 October 1987. [Google Scholar]
- Thakur, R.; Rao, V.; Reddy, S.; Ferguson, M. Evaluation of wild Arachis germplasm accessions for in vitro seed colonization and anatoxin production by Aspergillus flavus. Int. Arachis Newsl. 2000, 20, 44–46. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Reddy, S.; Kiran Mayi, D.; Uma Reddy, M.; Thirumala-Devi, K.; Reddy, D. Aflatoxins B1 in different grades of chillies (Capsicum annum L.) in India as determined by indirect competitive-ELISA. Food Addit. Contam. 2001, 18, 553–558. [Google Scholar] [CrossRef]
- Kalariya, K.A.; Singh, A.L.; Goswami, N.; Mehta, D.; Mahatma, M.K.; Ajay, B.C.; Chakraborty, K.; Zala, P.V.; Chaudhary, V.; Patel, C.B. Photosynthetic characteristics of peanut genotypes under excess and deficit irrigation during summer. Physiol. Mol. Biol. Plants 2015, 21, 317–327. [Google Scholar] [CrossRef][Green Version]
- Boontang, S.; Girdthai, T.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Patanothai, A.; Tantisuwic, N. Responses of released cultivars of peanut to terminal drought for traits related to drought tolerance. Asian J. Plant Sci. 2010, 9, 423–431. [Google Scholar] [CrossRef]
- Songsri, P.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Patanothai, A.; Holbrook, C. Root distribution of drought-resistant peanut genotypes in response to drought. J. Agron. Crop Sci. 2008, 194, 92–103. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Babitha, M.; Reddy, P.; Naidu, P. Relationship of drought tolerance traits with aflatoxin contamination in groundnut. Indian J. Plant Physiol. 2007, 12, 261. [Google Scholar]
- Holbrook, C.; Kvien, C.; Rucker, K.; Wilson, D.; Hook, J.; Matheron, M. Preharvest aflatoxin contamination in drought-tolerant and drought-intolerant peanut genotypes. Peanut Sci. 2000, 27, 45–48. [Google Scholar] [CrossRef]
- Arunyanark, A.; Jogloy, S.; Wongkaew, S.; Akkasaeng, C.; Vorasoot, N.; Kesmala, T.; Patanothai, A. Heritability of aflatoxin resistance traits and correlation with drought tolerance traits in peanut. Field Crops Res. 2010, 117, 258–264. [Google Scholar] [CrossRef]
- Okello, D.K.; Kaaya, A.N.; Bisikwa, J.; Were, M.; Oloka, H.K. Management of Aflatoxins in Groundnuts: A Manual for Farmers, Processors, Traders and Consumers in Uganda; National Agricultural Research Organization: Entebbe, Uganda, 2010. [Google Scholar]
- Hamidou, F.; Rathore, A.; Waliyar, F.; Vadez, V. Although drought intensity increases aflatoxin contamination, drought tolerance does not lead to less aflatoxin contamination. Field Crops Res. 2014, 156, 103–110. [Google Scholar] [CrossRef]
- Wilson, J.S.; Otsuki, T. Global Trade and Food Safety: Winners and Losers in a Fragmented System; World Bank Publications: Chicago, IL, USA, 2001. [Google Scholar]
- Holbrook, C.C.; Stalker, H.T.; Janick, J. Peanut breeding and genetic resources. Plant Breed. Rev. 2002, 22, 297–356. [Google Scholar]
- LaPrade, J.; Bartz, J.; Norden, A.; Demuynk, T. Correlation of peanut seed-coat surface wax accumulations with tolerance to colonization by Aspergillus flavus. J. Am. Peanut. Res. Educ. Soc. 1973, 5, 89–94. [Google Scholar]
- Liang, X.; Zhou, G.; Pan, R. Study on the relationship of wax and cutin layers in peanut seeds and resistance to invasion and aflatoxin production by Aspergillus flavus. J. Trop. Subtrop. Bot 2003, 11, 11–14. [Google Scholar]
- Lindsey, D.; Turner, R.B. Inhibition of growth of Aspergillus flavus and Trichoderma viride by peanut embryos. Mycopathologia 1975, 55, 149–152. [Google Scholar] [CrossRef]
- Mehan, V. Screening Groundnuts for Resistance to Seed Invasion by Aspergillus flavus and to Aflatoxin Production. Proceedings of International Workshop on Aflatoxin Contamination of Groundnut, Patancheru, India, 6–9 October 1987. [Google Scholar]
- Kasno, A.; Trustinah, T.; Purnomo, J.; Sumartini, S. Seed Coat Resistance of Groudnut to Aspergillus flavus and Their Stability Performance in The Field. AGRIVITA J. Agric. Sci. 2011, 33, 53–62. [Google Scholar]


 ) = percentage of total polyphenol; PY (
) = percentage of total polyphenol; PY ( ) = pod yield, SY (
) = pod yield, SY ( ) = seed yield, and HY (
) = seed yield, and HY ( ) = haulm yield. (
) = haulm yield. ( ) = linear %TPP curve, (
) = linear %TPP curve, ( ) = linear pod yield curve, (
) = linear pod yield curve, ( ) = linear seed yield curve, and (
) = linear seed yield curve, and ( ) = linear haulm yield curve.
) = linear haulm yield curve.
 ) = percentage of total polyphenol; PY (
) = percentage of total polyphenol; PY ( ) = pod yield, SY (
) = pod yield, SY ( ) = seed yield, and HY (
) = seed yield, and HY ( ) = haulm yield. (
) = haulm yield. ( ) = linear %TPP curve, (
) = linear %TPP curve, ( ) = linear pod yield curve, (
) = linear pod yield curve, ( ) = linear seed yield curve, and (
) = linear seed yield curve, and ( ) = linear haulm yield curve.
) = linear haulm yield curve.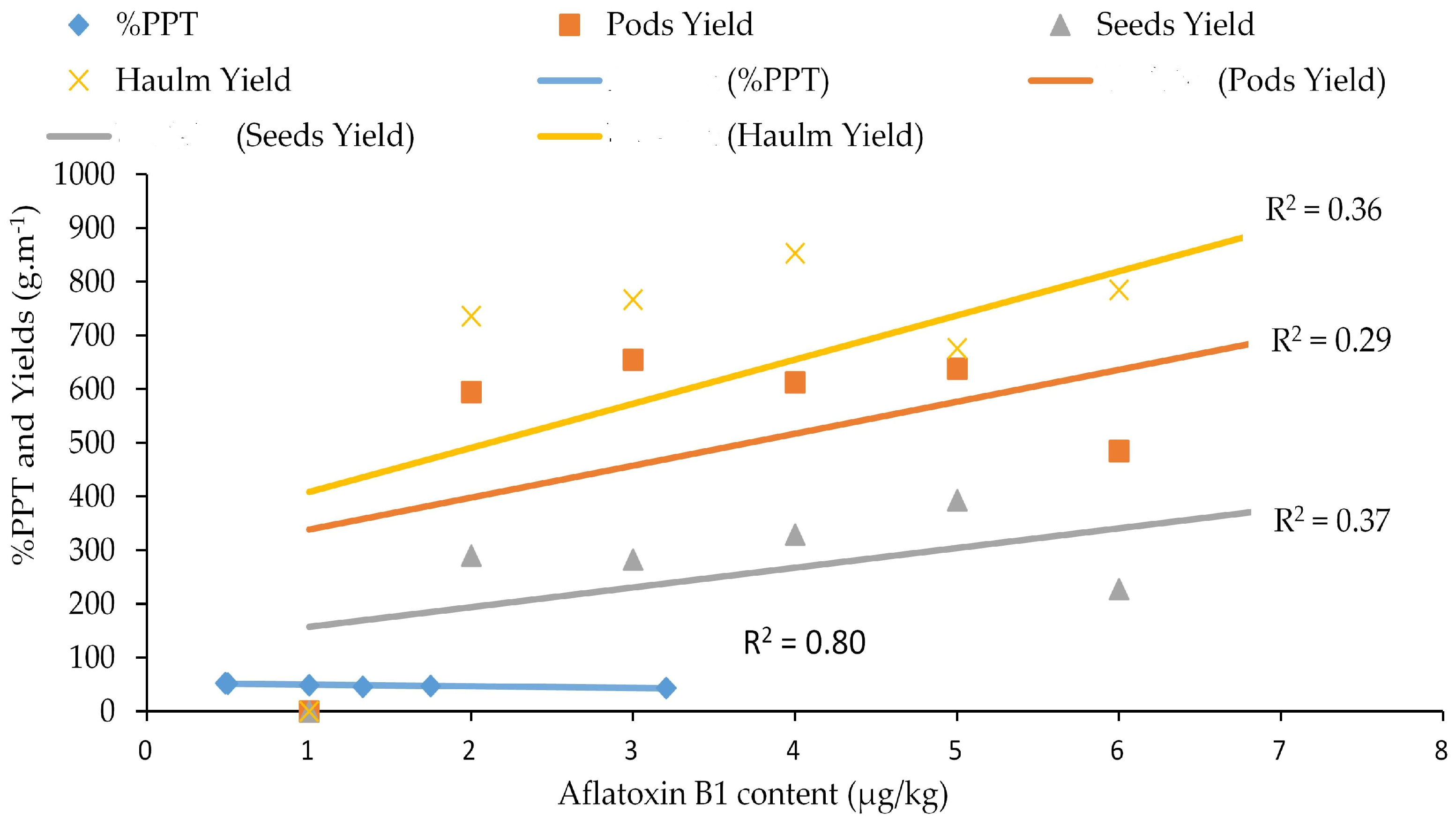
 ) = percentage of total polyphenol; PY (
) = percentage of total polyphenol; PY ( ) = pod yield, SY (
) = pod yield, SY ( ) = seed yield, and HY (
) = seed yield, and HY ( ) = haulm yield. (
) = haulm yield. ( ) = linear %TPP curve, (
) = linear %TPP curve, ( ) = linear pod yield curve, (
) = linear pod yield curve, ( ) = linear seed yield curve, and (
) = linear seed yield curve, and ( ) = linear haulm yield curve.
) = linear haulm yield curve.
 ) = percentage of total polyphenol; PY (
) = percentage of total polyphenol; PY ( ) = pod yield, SY (
) = pod yield, SY ( ) = seed yield, and HY (
) = seed yield, and HY ( ) = haulm yield. (
) = haulm yield. ( ) = linear %TPP curve, (
) = linear %TPP curve, ( ) = linear pod yield curve, (
) = linear pod yield curve, ( ) = linear seed yield curve, and (
) = linear seed yield curve, and ( ) = linear haulm yield curve.
) = linear haulm yield curve.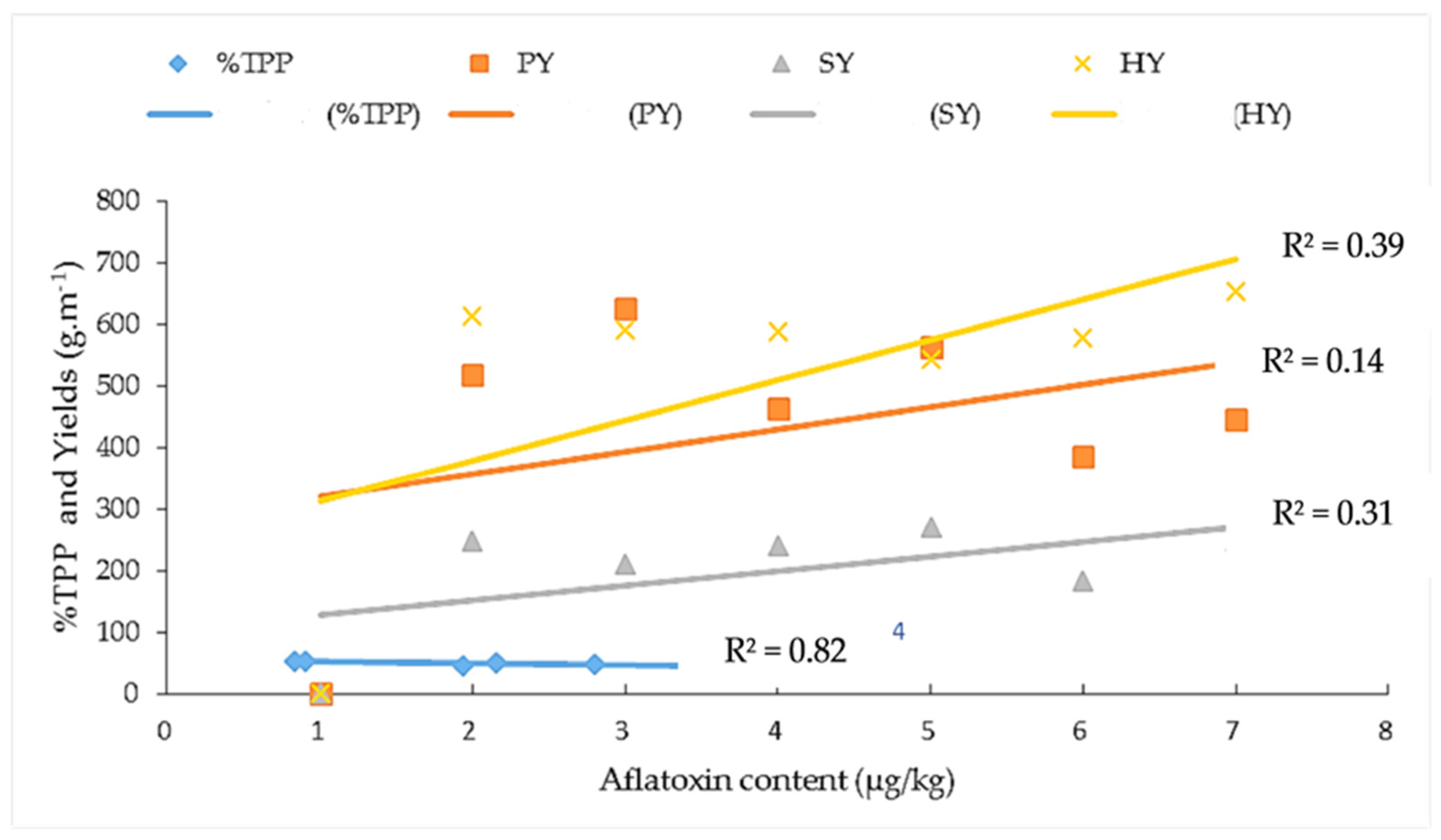
| Sources of Variance | PNP | IMPN | SNP | HY (gm−2) | PY (gm−2) | SY (gm−2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | WS | WW | WS | WW | WS | WW | WS | WW | WS | WW | WS | |
| F Vr.Value | 31.62 | 31.75 | 12 | 3.25 | 14.23 | 12 | 8.25 | 4.24 | 14 | 12 | 7.24 | 9.16 |
| G (F prob) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Wtrt (F prob) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| G × (Wtrt) | <0.001 | <0.001 | <0.001 | 0.015 | 0.027 | 0.020 | ||||||
| Traits | Water Treatments | WS Negative Effect (%) | |
|---|---|---|---|
| WW | WS | ||
| Pod number per plant | 102 ± 31 | 80 ± 25 | 21.92 |
| Immature pods per plant (increasing) | 9 ± 5 | 21 ± 10 | 57.14 |
| Seed number per plant | 123 ± 31 | 89 ± 27 | 27.36 |
| Pod yield (gm−2) | 678.54 ± 203.51 | 546.15 ± 163.5 | 19.43 |
| Seed yield (gm−2) | 316.67 ± 90.26 | 230.37 ± 69.3 | 27.24 |
| Haulm yield (gm−2) | 828.53 ± 85.30 | 645.57 ± 150.13 | 22.07 |
| Aflatoxin B1 | ||
|---|---|---|
| Genotypes | WW | WS |
| Means | 1.81 | 5.51 |
| LSD | 1.14 | 1.49 |
| Vr.Value | 9.55 | 56 |
| F (Prob) | <0.001 | <0.001 |
| G × W(Trt) | <0.001 | |
| Genotypes | %TPP | %AFC |
|---|---|---|
| Means | 50.50 | 85 |
| LSD | 2.54 | 16.63 |
| Vr. Value | 19.38 | 4.12 |
| F (Prob) | <0.001 | <0.001 |
| Genotypes | %AFC | %TPP | Genotypes | %AFC | %TPP | Genotypes | %AFC | %TPP | |
|---|---|---|---|---|---|---|---|---|---|
| CI ≤ 25% | ICG 311 | 19 | 53.08 | 55-437 | 21 | 49 | ICG 12988 | 18 | 52.1 |
| CI ˃ 25% ≤ 50% | ICG 11322 | 50 | 50.43 | ICG 14523 | 43 | 51.61 | ICG 1415 | 43 | 51.61 |
| ICG 11542 | 28 | 43.90 | ICG 163 | 45 | 47.38 | ICG 3027 | 83 | 57.50 | |
| ICG 4543 | 28 | 47.55 | ICG 76 | 42 | 52.57 | J 11 | 40 | 55.16 | |
| CI ˃ 50% | Fleur 11 | 86 | 47.90 | ICG 10950 | 91 | 56.05 | ICG 1142 | 100 | 50.32 |
| ICG 12235 | 85 | 56.67 | ICG 12697 | 91 | 51.47 | ICG 12879 | 100 | 45.50 | |
| ICG 12991 | 81 | 48.75 | ICG 13099 | 66 | 50.35 | ICG 4906 | 83 | 46.86 | |
| ICG 13858 | 91 | 53.27 | ICG 14106 | 100 | 48.76 | ICG 1415 | 100 | 43.68 | |
| ICG 14630 | 81 | 56.95 | ICG 14630 | 81 | 56.95 | ICG 156 | 90 | 51.86 | |
| ICG 2019 | 100 | 52.34 | ICG 2106 | 83 | 48.63 | ICG 332 | 100 | 53.22 | |
| ICG 334 | 66 | 50.76 | ICG 36 | 66 | 49.14 | ICG 3992 | 83 | 53.17 | |
| ICG 4598 | 100 | 48.16 | ICG 4684 | 75 | 45.97 | ICG 2119 | 100 | 52.41 | |
| ICG 4750 | 65 | 47.38 | ICG 4764 | 70 | 50.23 | ICG 513 | 100 | 53.69 | |
| ICG 5195 | 100 | 52.36 | ICG 532 | 100 | 42.74 | ICG 5609 | 83 | 43.74 | |
| ICG 5663 | 81 | 49.36 | ICG 6263 | 91 | 54.01 | ICG 6407 | 61 | 52.14 | |
| ICG 6654 | 91 | 48.31 | ICG 6703 | 91 | 46.32 | ICG 6813 | 46 | 46.53 | |
| ICG 6888 | 83 | 46.09 | ICG 7181 | 100 | 45.92 | ICG 721 | 91 | 50.20 | |
| ICGIL 11102 | 100 | 53.09 | ICGIL 11110 | 75 | 51.29 | ICGIL 11114 | 75 | 54.34 | |
| ICGIL 11125 | 61 | 56.30 | ICGIL 17108 | 91 | 62.96 | JL24 | 100 | 47.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminou, M.M.; Falalou, H.; Abdou, H.; Mendu, V. Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought. J. Fungi 2024, 10, 850. https://doi.org/10.3390/jof10120850
Aminou MM, Falalou H, Abdou H, Mendu V. Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought. Journal of Fungi. 2024; 10(12):850. https://doi.org/10.3390/jof10120850
Chicago/Turabian StyleAminou, Maman Moutari, Hamidou Falalou, Harou Abdou, and Venugopal Mendu. 2024. "Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought" Journal of Fungi 10, no. 12: 850. https://doi.org/10.3390/jof10120850
APA StyleAminou, M. M., Falalou, H., Abdou, H., & Mendu, V. (2024). Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought. Journal of Fungi, 10(12), 850. https://doi.org/10.3390/jof10120850






