Abstract
Due to the high morbidity and mortality rates of invasive aspergillosis (IA) and the importance of early IA detection for successful treatment and subsequent outcome, this study aimed to determine a time course of detectable antigen in a mouse model of IA and correlate it with tissue invasion by using two novel monoclonal antibodies, 1D2 and 4E4, that can be used to detect the Aspergillus-derived glycoproteins. Immunocompromised mice were randomly divided into five groups: uninfected control, and inoculation with conidia from Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus terreus. Conidia (2 × 106 cells/mL) were administered intravenously via tail vein injection. Three mice from each group were euthanised at each time point (6 h, 12 h, 18 h, 24 h, and 48 h) after inoculation. Urine and blood were collected for analysis using a double-sandwich ELISA using 1D2 and 4E4. Liver, spleen, and kidney tissues were harvested for tissue staining. The levels of liver injury in the IA mice progressively increased with time after inoculation with Aspergillus conidia. Following inoculation with A. fumigatus, swollen conidia were identified in the spleen, as well as antigens in blood after 18 h. Hyphae were detected in the spleen, liver, and kidney after 48 h. For A. flavus, the antibodies detected hyphae in the liver and spleen as well as circulating antigens in blood samples 48 h after inoculation. Tissue injury was observed in the mice inoculated with A. terreus and A. niger, but there was no evidence of fungal invasion or antigens in the blood. Antigens were not detectable in mouse urine but could be detected in glomeruli of the kidney by immunofluorescence. In conclusion, the mAb-based antigen detection double-sandwich ELISA results were consistent with the IHC results in this study. Novel monoclonal antibodies 1D2 and 4E4 can serve as tools for the early identification of IA in mice infected by A. fumigatus and A. flavus. This study also suggests the potential usefulness of this approach in human disease.
1. Introduction
Invasive aspergillosis (IA) is one of the most severe disorders of invasive fungal disease [1]; it is most commonly (approximately 90% of cases) caused by Aspergillus fumigatus, followed by Aspergillus flavus, Aspergillus niger, and Aspergillus terreus [2]. The morbidity and mortality rate of IA remains high in those who receive immunosuppressive drugs [3], neutropenic patients [4], and patients suffering from severe influenza or coronavirus (COVID-19) [4,5,6] despite treatment with antifungal agents. To date, early-stage IA diagnosis remains a major challenge owing to sub optimal sensitivity and specificity of current diagnostic methods [2,4,7]. Delayed diagnosis results in delayed therapy, leading to an increase in morbidity and mortality for patients [2,4,7]. Given the specific binding between the monoclonal antibody (mAb) and the target antigen, mAb-based methods have paved a new way for assisting the rapid, early, and non-invasive diagnosis of IA [8,9,10]. Early studies using mAb 1D2 and 4E4 indicated the hyphal antigens were detected from all four Aspergillus species in filtrates of culture media after a two-week incubation [11].
IA animal models play a critical role in improving the translation of a new diagnostic or therapeutic approach prior to use in the clinical setting [9,12,13,14,15]. A successful animal model is central to understanding the capability of a novel method for diagnosing or treating IA [12,14,16]. We investigated two monoclonal antibodies (mAbs) 1D2 and 4E4 against glycoprotein antigens and showed they can detect circulating antigens in mice 48 h post-infection with A. fumigatus. Due to the importance of early IA detection for successful treatment and subsequent outcome, we aimed to determine a time course of detectable antigen in a mouse model of IA and correlate it with tissue invasion.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
A. fumigatus (AF 293) and A. flavus (NRRL 3357) were obtained from the American Type Culture Collection (ATCC). A. niger and A. terreus strains were clinical strains provided by Canterbury Health Laboratories, Christchurch, New Zealand. All fungal strains were cultivated on Sabouraud dextrose agar (SDA) plates or in Sabouraud dextrose (SD) liquid media at 37 °C.
2.2. Preparation of Aspergillus Conidial Suspension
Aspergillus conidial suspensions were prepared as previously described with minor changes [11]. Briefly, all Aspergillus species were sub-cultured on SDA plates and washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBST) to acquire conidia. The obtained conidial suspensions were filtered and washed three times. Finally, the conidial precipitates were resuspended with PBS and concentration adjusted to 2 × 106 conidia/mL.
2.3. The Production, Purification and Biotinylation of Monoclonal Antibodies
The generation and biotinylation of mAb 1D2 and 4E4 was described previously [11], and they were purified by a mouse IgM purification resin column (LT-145, LigaTrap Technologies, Raleigh, NC, USA) according to the manufacturer’s guidelines. Antibody concentrations were calculated according to the absorbance at 280 nm measured by a UV–visible spectrophotometer. A control mouse IgM antibody (6B10) was purified by the similar method.
2.4. Preparation and Separation of Aspergillus Hyphal Antigens
The Aspergillus hyphal antigens were prepared as reported previously [11]. In brief, cell wall-related proteins (CWPs) of Aspergillus were precipitated from the cell wall fragments (CWFs) by addition of cold ethanol. Molecular weight cut-off (MWCO) ultrafiltration tubes (3000 MWCO, 10,000 MWCO, 30,000 MWCO, 50,000 MWCO, and 100,000 MWCO) (GE Healthcare, Waukesha, WI, USA) were utilised to separate the CWFs and CWPs into different molecular weights. All filter tubes were pre-treated with sterile PBS to wash and wet the membrane. The samples of CWFs or CWPs were added to different MWCO sizes tubes and centrifuged at 14,000× g for 10 to 30 min depending on the MWCO of the membrane. The ultrafiltrate samples were collected in a microcentrifuge tube and stored at −20 °C for future usage.
2.5. Double-Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
The double-sandwich ELISA was performed similarly to that previously described with minor changes [11]. Briefly, mAb 1D2 (5 µg/mL) was used as the capture antibody. After washing and blocking, 100 μL of each sample or ultrafiltrate samples containing different sizes of CWPs was added and incubated for one hour. Afterward, 4E4-biotin (5 µg/mL) was added, followed by addition of streptavidin-HRP (Jackson ImmunoResearch, Philadelphia, PA, USA) at 1 µg/mL. The signals were detected by addition of tetramethybenzidine (TMB) substrate.
2.6. Immunohistochemical Staining and Immunofluorescent Staining
Immunohistochemical (IHC) staining of infected tissues from IA mice was performed as described previously [11]. Tissue fixation, embedding, deparaffinisation and rehydration, antigen retrieval, permeabilisation, blocking, and primary antibody incubation steps for immunofluorescent (IF) staining were similar with IHC but without inactivation of endogenous peroxidase. After three washes, the IF tissue samples were incubated with goat anti-mouse IgM-FITC (Invitrogen, Auckland, New Zealand) at 7.5 µg/mL for one hour at room temperature in the dark, while the IHC samples were incubated with goat anti-mouse IgM-HRP (Abcam, Cambridge, UK) at 0.5 µg/mL. Blocking buffer or isotype IgM 6B10 [17] were used in control groups, but samples were otherwise treated the same. The IF slides were mounted with ProLong™ Glass Antifade Mountant (Invitrogen, Auckland, New Zealand) and observed via an epifluorescence microscope (Carl Zeiss, Oberkochen, Germany). The IHC slides were mounted with Permount™ Mounting Medium (Thermo Fisher Scientific, Auckland, New Zealand) and observed using a light microscope (Olympus corporation, Tokyo, Japan).
2.7. The Mouse Model of Invasive Aspergillosis
Animal experiments were carried out in compliance with the New Zealand animal welfare regulation and approved by the Animal Ethics Committee of the University of Otago (AUP-21-145). Male Balb/c mice aged from 10 to 12 weeks were randomly divided into five groups, immunocompromised, A. fumigatus infected, A. flavus infected, A. niger infected, and A. terreus infected. All mice were intraperitoneally administrated with two doses of cyclophosphamide (Baxter Healthcare Limited, Auckland, New Zealand) at 150 mg/kg on day one and day four to induce immunosuppression. To establish the IA model, the immunocompromised mice were injected intravenously with 0.1 mL of A. fumigatus, A. flavus, A. niger, or A. terreus conidia suspension at 2 × 106 cells/mL on day five. The control group mice was inoculated with 0.1 mL of sterile saline. In each infection group, three mice were euthanised at varying time points (6 h, 12 h, 18 h, 24 h, and 48 h) after inoculation. Urine and blood (heparinised) were collected alongside liver, spleen, and kidney. Given that the neutropenic mice have high risks of bacterial infection, all mice received enrofloxacin (5 mg/kg) subcutaneously as a prophylaxis daily from the first dose of cyclophosphamide to the day they were euthanised.
2.8. Statistical Analysis
Statistical analysis was performed with SPSS software (version 21.0, IBM Analytics, New York, NY, USA) and GraphPad software (version 9, Prism, San Diego, CA, USA). All continuous variable results were expressed as [mean ± standard deviation (SD)]. Comparisons were evaluated by independent samples t-test or one-way ANOVA with post hoc Tukey’s test. Differences were considered significant when p value < 0.05.
3. Results
3.1. Tissue Samples from the Mouse Model
Gross examination of organs taken from mice after inoculation with A. fumigatus showed changes only in the liver specimens harvested 48 h post conidia inoculation. The liver showed evidence of haemorrhage on some surfaces and pallor in others, suggesting infarction and infection (Figure 1).
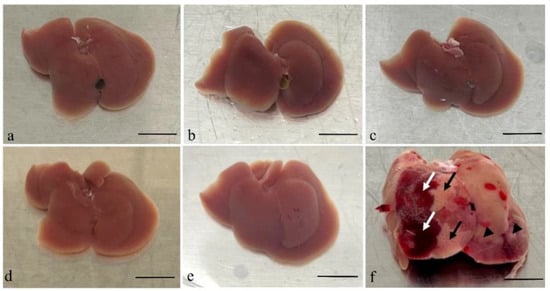
Figure 1.
Gross examination of the livers taken after the mice infected by A. fumigatus at different time points. Images of liver tissues from an immunocompromised mouse without Aspergillus inoculation (a) and tissues collected post-inoculation with Aspergillus are shown (b–f). Tissues harvested 6 h (b), 12 h (c), 18 h (d), and 24 h (e) post-inoculation showed no significant morphological changes. However, the liver specimen harvested 48 h (f) post-inoculation was significantly swollen and showed evidence of infection (black arrow head) and haemorrhage (white arrow) on the surface, as well as pallor suggestive of infarction (black arrow). Scale bars represent 1 cm.
3.2. Microscopic Examination of Tissues
3.2.1. Haematoxylin and Eosin (HE) Staining
Liver tissues from mice inoculated with A. fumigatus and A. flavus showed tissue injury that became progressively more severe 6, 12, 18, 24, and 48 h after inoculation when compared with the control group (Figure 2).
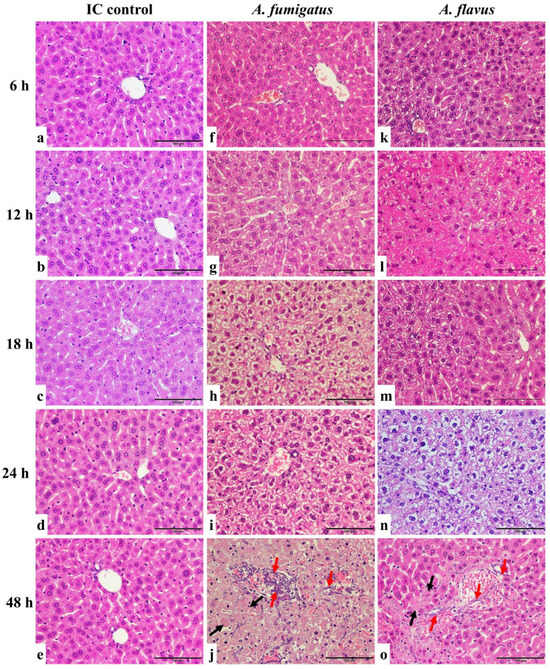
Figure 2.
HE staining of liver sections of A. fumigatus- and A. flavus-infected mice. The liver of immunocompromised (IC) control mice did not show significant tissue injury (a–e). The samples from A. fumigatus-inoculated (f–j)) and A. flavus-inoculated (k–o) mice showed no significant necrosis six hours post-inoculation (f,k). From 12 to 24 h, the liver progressively showed cytoplasmic vacuolation and necrosis of hepatocytes (g–i,l–n). After 48 h, the tissue showed significant injuries with confluent necrosis (black arrow) and Aspergillus hyphae infiltration (red arrow) (j,o). Scale bars represent 100 μm.
Mice in the control group were immunosuppressed but not inoculated with Aspergillus conidia. Liver tissues of immunosuppressed mice showed hepatocytes arranged in a radiation shape surrounding the round or oval central hepatic vein without red blood cells inside. The hepatocytes were normal in shape (cubic) with a clear cell nucleus in the centre. For mice infected by A. fumigatus and A. flavus, six hours post-conidial inoculation, there was no significant necrosis in the tissues. From 12 to 24 h, the liver progressively showed cytoplasmic vacuolation and necrosis of hepatocytes, indicating a progression of inflammation and injury in the liver caused by infection. After 48 h, the tissue showed significant injuries due to the fungal infection. The hepatic lobule structure was disordered, the radial structure disappeared, and there was confluent necrosis. The central hepatic vein was expanded, congested, and full of red blood cells. The hepatocytes were deformed and partly fused with a shrinking or disappearing nucleus. In addition, the Aspergillus hyphae infiltrated the blood vessels, but with no obvious neutrophil infiltration.
In parallel, liver tissues from mice inoculated with A. niger and A. terreus showed similar injurious features between 6 and 24 h after conidia inoculation as those inoculated with A. fumigatus- and A. flavus-infected mice. However, examination of the liver samples harvested 48 h post-inoculation showed progressive cytoplasmic vacuolation and necrosis of hepatocytes but no hyphal infiltration in the liver (Figure 2 and Supplementary Figure S1).
3.2.2. Immunohistochemistry (IHC) Staining
No fungal elements were detectable in the tissues from control mice or mice inoculated with A. fumigatus conidia in samples harvested at 6 and 12 h, as well as A. flavus between 6 and 24 h, post-inoculation. Both 1D2 and 4E4 recognised the cell wall of hyphae of A. fumigatus and A. flavus in formalin-fixed paraffin tissue sections from IA mice (Figure 3 and Figure 4), indicating that both monoclonal antibodies could bind to the cell wall antigens of A. fumigatus and A. flavus after they swelled or germinated in mouse tissue. Both antibodies identified the swollen A. fumigatus conidia in spleen after 18 h inoculation. Additionally, they recognised the hyphal wall of A. fumigatus and A. flavus in the liver, spleen, and kidney 48 h post-inoculation. However, there was no positive staining in the tissues from the IA mice infected with A. niger and A. terreus or the controls at any time post-inoculation. Also, no staining was observed in negative controls with blocking buffer or isotype IgM 6B10.
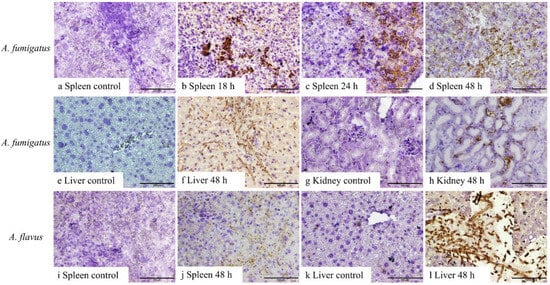
Figure 3.
IHC staining of 1D2 in tissue sections of liver, spleen, and kidney from A. fumigatus- and A. flavus-infected mice. A. fumigatus-infected tissues (a–h), compared to the negative IHC staining in spleen taken 6–12 h after inoculation (a), swollen conidial wall (b,c), and hyphal wall (d) (brown staining) seen after 18 h (b), 24 h (c), and 48 h (d) post-inoculation. In addition, compared to the tissues collected 6, 12, 18, and 24 h post-inoculation (e,g), 1D2 also identified hyphal wall in the liver (f) and kidneys (h) 48 h post-inoculation. Likewise, for A. flavus-infected animals (i–l), hyphal wall elements were detected in the spleen (j) and liver (l) 48 h post-inoculation when compared to samples collected at 6, 12, 18, and 24 h post-inoculation (i,k). Scale bars represent 100 μm.
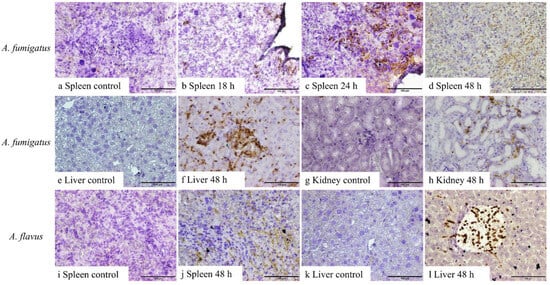
Figure 4.
IHC staining of 4E4 in tissue sections of the liver, spleen, and kidney from A. fumigatus- and A. flavus-infected mice. A. fumigatus-infected tissues (a–h), compared to the negative IHC staining in spleen taken 6–12 h after inoculation (a), swollen conidial wall (b,c), and hyphal wall (d) (brown staining) detected after 18 h (b), 24 h (c), and 48 h (d) inoculation. In addition, compared to the tissues collected 6, 12, 18, and 24 h post-inoculation (e,g), hyphal walls were identified in the liver (f) and kidneys (h) 48 h post-inoculation. Likewise, for A. flavus-infected samples (i–l), 4E4 can detect the hyphal wall in the spleen (j) and liver (l) 48 h post-inoculation when compared to samples collected at 6, 12, 18, and 24 h post-inoculation (i,k). Scale bars represent 100 μm.
3.2.3. Kidney Immunofluorescence
Immunofluorescence (IF) of kidney tissues from A. fumigatus-infected mice showed positive fluorescence staining in the glomerulus with both 1D2- and 4E4-tagged antigens (Figure 5). This suggested that some antigens were unable to penetrate the glomerular filtration barrier and subsequently remained trapped in the glomerulus.
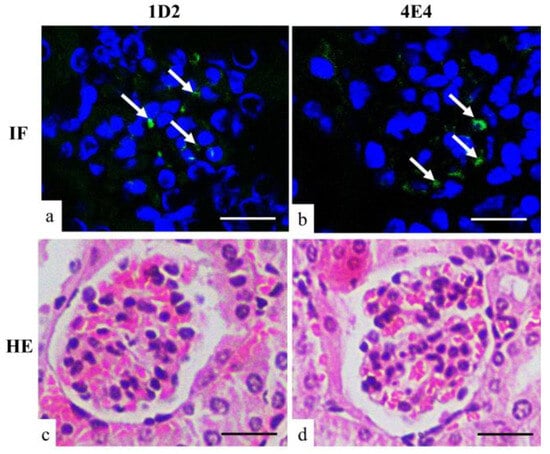
Figure 5.
Immunofluorescent (a,b) and HE (c,d) staining of mouse kidney. Both 1D2 (a) and 4E4 (b) showed positive fluorescent signals (arrow) in the glomerulus. Scale bars represent 20 μm.
3.3. Detection of Antigens in the Blood and Urine of Mice with Invasive Aspergillosis
The detection of hyphal cell wall antigens in serial dilutions of blood samples are shown in Figure 6. Antigens were detected in plasma samples from mice 18, 24, and 48 h post-inoculation with A. fumigatus conidia and 48 h post-inoculation with A. flavus conidia. Samples taken at times prior to this, including baseline, were negative. Interestingly, the titre of the 48 h plasma sample for A. flavus blood was the highest observed, despite a negative sample at 24 h. No Aspergillus antigen was detected in the plasma from those mice infected with A. niger and A. terreus. All urine samples were negative in both the inoculated and control mice.
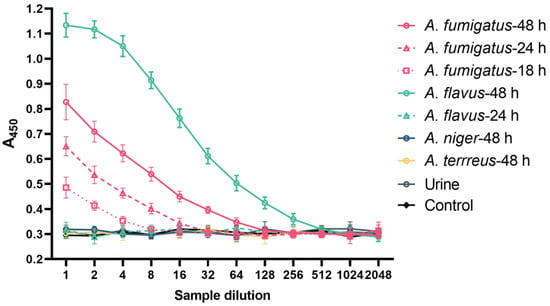
Figure 6.
The detection of antigens in the blood and urine of IA mice. The double-sandwich ELISA detects circulating antigens in dilutions of plasma from IA mice (n = 3 per time point) infected by A. fumigatus (after 18 h, 24 h and 48 h of inoculation) and A. flavus (after 48 h of inoculation). The assay was tested in triplicate. Data are presented as mean ± SD. A450: absorbance at 450 nm.
3.4. Correlation of Histologic and ELISA Findings
The results of histological findings and the ELISA assay are summarised in Table 1. These results showed that the antigens became detectable in blood in parallel with detectable A. fumigatus swollen spores in splenic tissue at 18 h. However, the blood antigens preceded detectable hyphae in liver and kidney tissues. In contrast, antigens became detectable in blood at 48 h after A. flavus inoculation, which followed the same timeline as the detection of hyphae in all the tissues.

Table 1.
Summary of plasma ELISA, tissue IHC, and HE staining results.
3.5. The Molecular Weight of Antigens Recognised by mAb 1D2 and 4E4
Cell wall proteins (CWPs) and cell wall fragments (CWFs) from various MWCO centrifugal filters showed differing reaction signals (Figure 7). Figure 7 shows that there was little reactivity in fractions with a molecular weight (MW) less than 30 kDa in CWP samples that were identified by 1D2 (Figure 7a) and 4E4 (Figure 7b). This demonstrated that most of the antigens had a MW more than 30 kDa. Of note, the antigen concentrations of both CWP and CWF samples gradually increased when they were collected from the 50 kDa and 100 kDa filter tubes. Together, these results indicated that the MW of antigens recognised by 1D2 and 4E4 varied from 30 kDa to 100 kDa, especially between 50 kDa and 100 kDa. Additionally, the concentration of antigens in CWP samples was significantly higher than those in CWF samples (p < 0.001), which meant there were more antigens in the CWP sample and the antigens were more likely to be a protein.
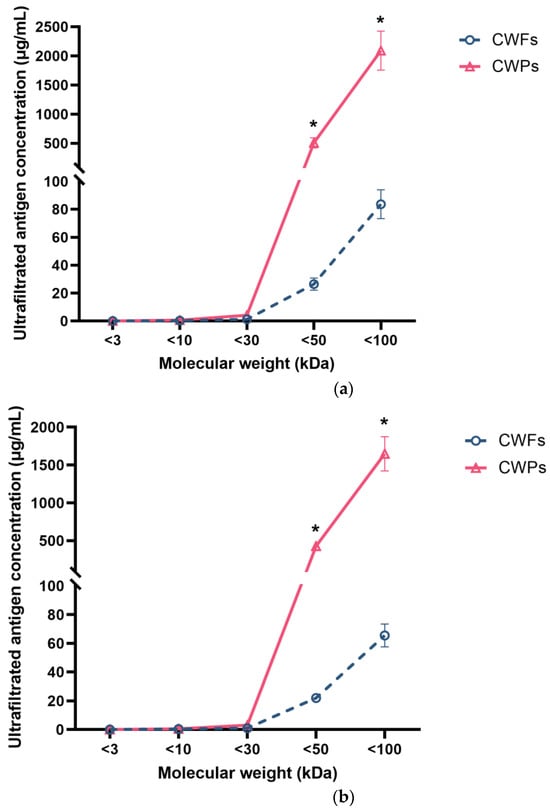
Figure 7.
The concentration of ultra-filtered antigens with various molecular weights detected by ELISA using monoclonal antibodies. MAb 1D2 (5 µg/mL) was coated overnight, followed by blocking with 5% BSA; 100 μL of ultrafiltrate samples of cell wall fragments (CWFs) or cell wall proteins (CWPs) were added to the wells. After this, the plate was incubated with 4E4-biotin (5 µg/mL). Little signal was detected in ultra-filtrate samples less than 30 kDa for both 1D2 (a) and 4E4 (b). The concentration of samples collected from the 50 kDa and 100 kDa filter tubes gradually increased. This assay was undertaken in triplicate. Data were presented as mean ± SD and analysed using a two-tailed Student’s t test. *: p < 0.001 verse CWPs.
4. Discussion
The current study confirms some of our previous results and adds to our knowledge on the potential value of the two monoclonal antibodies for the diagnosis of invasive aspergillosis by determining the time course for antigens to appear in the peripheral blood of immune-suppressed mice, the size of the cell wall proteins, and a possible reason why the antigen was not detectable in urine. Our results demonstrated that there was a progressive increase in severity of liver injury with time post-inoculation with Aspergillus conidia in the mice. The earliest evidence of fungal invasion was found in samples taken 18 h after A. fumigatus conidia inoculation, when swollen conidia were seen in splenic tissue samples and antigen was detected in the blood. A. fumigatus hyphae were present in tissue samples harvested at 48 h after conidia injection. In contrast, fungal elements were not detected in tissue samples until 48 h after A. flavus conidia inoculation, when they were identified in both the liver and spleen alongside antigen-positive blood samples. While there was tissue injury in mice inoculated with A. terreus and A. niger, there was no evidence of hyphal invasion or antigen present in blood. These results demonstrated that the level of antigen in blood correlated with germination of A. fumigatus conidia and the presence of A. flavus hyphae in the tissues.
The immune-compromised mouse model used within this study allowed for the development of IA using A. fumigatus and A. flavus conidia, which are the two most common species to cause human disease. Invasive infection in the mice was preceded by tissue injury that may be partially related to the cyclophosphamide-induced neutropenia, although mycotoxins produced by the Aspergillus species may have contributed to this. The described mouse model was less useful for A. niger and A. terreus as significant tissue invasion was not detected, though evidence of inflammation was detected. This may have been because tissues were harvested 48 h after inoculation, and tissue invasion may have occurred at later time points, reflecting the susceptibility of the mouse to the strain used [18]. In addition, 12 h post-inoculation with Aspergillus, the liver tissue showed cytoplasmic vacuolation. Forty-eight hours after inoculation with A. fumigatus and A. flavus, necrosis of hepatocytes was more severe, indicating a progressive inflammation and injury in the liver in mice. However, we did not detect significant abnormity in lung tissues in this study; this might have been because we induced the IA model via mouse tail vein, and 48 h is not enough time for Aspergillus to penetrate the blood-air barrier to cause pulmonary infection and injury.
The 1D2 and 4E4-biotin pair assay was able to detect the antigen in extracellular fractions in culture media from A. niger and A. terreus as well as A. fumigatus and A. flavus, although the amount of antigen in the supernatant of A. niger and A. terreus was lower compared to A. fumigatus and A. flavus [11]. In a similar way, this pair of mAb-based ELISA detected antigens in the blood from the early stage of IA. Eighteen hours after inoculation with A. fumigatus, Aspergillus antigens were released into the bloodstream. This suggested that soluble antigens were more rapidly secreted into the blood after inoculation with A. fumigatus compared to A. flavus. Moreover, the antigen was detected in the blood 18 h after inoculation, earlier than other studies that report detection at least 24 h after inoculation using monoclonal antibodies to galactomannan and (1→3)-β-D-glucan [19,20,21]. On the other hand, in A. flavus-infected mouse samples, this double-sandwich ELISA detected A. flavus antigens in blood 48 h post-inoculation. Interestingly, this signal was significantly higher compared to A. fumigatus, indicating that A. flavus starts to secrete abundant soluble antigens into the blood 48 h post-inoculation, as there were no antigens detected before that time. The blood analysis studies of samples from mice inoculated with A. niger or A. terreus showed no evidence of tissue invasion or antigen in the blood, which was consistent with IHC staining of tissues. Our previous study showed immunofluorescent staining of hyphae were negative despite the antigen being detectable in the supernatant of cultures. This may have been due to lower levels of antigen in the hyphal or masking of the epitope from different orientations of the antigen. In the current study, the negative results may result from the less pathogenic characteristics of both A. niger and A. terreus when compared to A. fumigatus and A. flavus, which failed to establish a significant infection in this study [18]. It is also possible that the structure of antigens may vary between different species of Aspergillus, and this might lead to the different response time or its negative results in some species. Though this study has demonstrated more usefulness of 1D2 and 4E4 in diagnosing IA in mice infected by different Aspergillus species at different time points when compared to the previous study [11], future work needs to be done to observe mice infected with established infection with A. niger and A. terreus for a longer period of time and to determine if antigens can then be detected in the blood and tissue samples. Overall, these results suggest that the ELISA assay in the plasma performed well, and they reported a positive result early in the course of infection that was also easy to perform. This relatively non-invasive diagnostic test may have potential for the early diagnosis of IA in human patients who are the high-risk IA group.
We were unable to detect the antigen in urine samples from any of the mice. Immunofluorescence in kidney sections was performed, and positive fluorescence was detected in the glomerulus by 1D2 and 4E4, indicating the antigens might get “stuck” in the kidney and be unable to cross the glomerular wall. The glomerular capillary wall, the essential barrier for kidney filtration, is principally composed of three layers—the capillary endothelial cells, the basement membrane, and the epithelial cells called podocytes [22]. The distinctive pore dimension and negative charge of each layer together account for the selective permeability of the glomerular filtration membrane [22,23]. The molecular weight cut-off value of the glomerular filtration is between 30 kDa and 50 kDa [24,25], and for Aspergillus antigens with molecular weight less than 30 kDa, these can cross the glomerular capillary wall so that they can be detected in a urine sample, such as galactofuranose and galactomannan, as has been reported [10,26,27,28]. However, a second mechanism may have contributed to the absence of antigens between 30 kDa and 37 kDa in the urine. Most proteins in the blood are negatively charged and are repelled by the negatively charged glomerular filtration membrane. It is also conceivable that some glycoprotein antigens may penetrate the three-layer filtration barrier but are resorbed in the proximal tubule back into the bloodstream [24,29].
These studies demonstrate that the antibodies readily detect invasive hyphae of A. fumigatus and A. flavus early in the course of infection and may have potential in the histological diagnosis of human IA with these two important pathogens from biopsy specimens. We were unable to demonstrate hyphae in mice inoculated A. niger or A. terreus conidia, but these may be detectable later in the course of infection, which we were unable to do. This mAb-based assay is worthy of investigation for application to the diagnosis of IA as the antigen is detectable early in the course of infection with A. fumigatus.
5. Conclusions
Collectively, 1D2 and 4E4-biotin double-sandwich ELISA detected circulating antigens as early as 18 h after mice were infected with A. fumigatus and 48 h after inoculation with A. flavus. Moreover, both 1D2 and 4E4 also identified hyphae or swollen conidia in the liver, kidney, and spleen samples from IA mice. Finally, there was no positive antigen in the urine of IA mice that could be detected by mAb 1D2 and 4E4-biotin-based ELISA. These mAbs may have application for detecting circulating antigen in blood, rather than urine, and might have value for the early diagnosis of IA in humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10120832/s1, Figure S1: HE staining of liver sections of A. niger and A. terreus infected mice.
Author Contributions
Conceptualisation, X.L., A.S.-T., J.G.L., M.B. and S.T.C.; methodology, X.L., A.S.-T. and J.G.L.; software, X.L.; validation, A.S.-T.; formal analysis, X.L.; investigation, X.L., A.S.-T., J.G.L., M.B. and S.T.C.; resources, X.L., A.S.-T., J.G.L., M.B. and S.T.C.; data curation, X.L. and A.S.-T.; writing—original draft preparation, X.L.; writing—review and editing, X.L., A.S.-T., J.G.L., M.B. and S.T.C.; visualisation, X.L. and A.S.-T.; supervision, A.S.-T., J.G.L., M.B. and S.T.C.; project administration, X.L., A.S.-T., J.G.L., M.B. and S.T.C.; funding acquisition, X.L., A.S.-T., J.G.L., M.B. and S.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the University of Otago Sandy Smith Scholarship/Grants-in-Aid, McGee Fellowship 2023, Otago Research Grant (No. 21943) and Joint Funds for the Innovation of Science and Technology, Fujian province (No. 2023Y9264).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
We would like to extend our sincere gratitude to Zhixing Zhu and Anthony Mitchell for their valuable laboratory support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and Outcome of Invasive Fungal Diseases after Allogeneic Stem Cell Transplantation: A Prospective Study of the Gruppo Italian Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transplant. 2014, 20, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C. How to make a fast diagnosis in invasive aspergillosis. Med. Mycol. 2019, 57, S155–S160. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Nabet, C.; Lampros, A.; Marcelin, A.G.; Thellier, M.; Piarroux, R.; Demoule, A.; Fekkar, A. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Lian, X.; Scott-Thomas, A.; Lewis, J.G.; Bhatia, M.; MacPherson, S.A.; Zeng, Y.; Chambers, S.T. Monoclonal Antibodies and Invasive Aspergillosis: Diagnostic and Therapeutic Perspectives. Int. J. Mol. Sci. 2022, 23, 5563. [Google Scholar] [CrossRef]
- Gunzer, M.; Thornton, C.R.; Beziere, N. Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis. J. Fungi 2020, 6, 338. [Google Scholar] [CrossRef]
- Marr, K.A.; Datta, K.; Mehta, S.; Ostrander, D.B.; Rock, M.; Francis, J.; Feldmesser, M. Urine Antigen Detection as an Aid to Diagnose Invasive Aspergillosis. Clin. Infect. Dis. 2018, 67, 1705–1711. [Google Scholar] [CrossRef]
- Lian, X.; Chambers, S.; Lewis, J.G.; Scott-Thomas, A.; Bhatia, M. Two Monoclonal Antibodies That Specifically Recognize Aspergillus Cell Wall Antigens and Can Detect Circulating Antigens in Infected Mice. Int J Mol Sci 2021, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Rolle, A.M.; Maurer, A.; Spycher, P.R.; Schillinger, C.; Solouk-Saran, D.; Hasenberg, M.; Weski, J.; Fonslet, J.; Dubois, A.; et al. Towards Translational ImmunoPET/MR Imaging of Invasive Pulmonary Aspergillosis: The Humanised Monoclonal Antibody JF5 Detects Aspergillus Lung Infections In Vivo. Theranostics 2017, 7, 3398–3414. [Google Scholar] [CrossRef] [PubMed]
- Rolle, A.M.; Hasenberg, M.; Thornton, C.R.; Solouk-Saran, D.; Mann, L.; Weski, J.; Maurer, A.; Fischer, E.; Spycher, P.R.; Schibli, R.; et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E1026–E1033. [Google Scholar] [CrossRef] [PubMed]
- Amich, J.; Mokhtari, Z.; Strobel, M.; Vialetto, E.; Sheta, D.; Yu, Y.; Hartweg, J.; Kalleda, N.; Jarick, K.J.; Brede, C.; et al. Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions. mBio 2020, 11, e02752-19. [Google Scholar] [CrossRef]
- Sakita, K.M.; Capoci, I.R.G.; Conrado, P.C.V.; Rodrigues-Vendramini, F.A.V.; Faria, D.R.; Arita, G.S.; Becker, T.C.A.; Bonfim-Mendonca, P.S.; Svidzinski, T.I.E.; Kioshima, E.S. Efficacy of Ebselen Against Invasive Aspergillosis in a Murine Model. Front. Cell. Infect. Microbiol. 2021, 11, 684525. [Google Scholar] [CrossRef]
- Kim, D.Y.; Pyo, A.; Ji, S.; You, S.H.; Kim, S.E.; Lim, D.; Kim, H.; Lee, K.H.; Oh, S.J.; Jung, Y.R.; et al. In vivo imaging of invasive aspergillosis with (18)F-fluorodeoxysorbitol positron emission tomography. Nat. Commun. 2022, 13, 1926. [Google Scholar] [CrossRef]
- Lewis, J.G.; Fredericks, R.; Fee, C.J.; Elder, P.A. Corticosteroid-binding globulin (CBG) reactive centre loop antibodies and surface plasmon resonance interrogate the proposed heat dependent “flip-flop” mechanism of human CBG. J. Steroid Biochem. Mol. Biol. 2016, 158, 38–45. [Google Scholar] [CrossRef]
- Paulussen, C.; Hallsworth, J.E.; Alvarez-Perez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef]
- White, P.L.; Wiederhold, N.P.; Loeffler, J.; Najvar, L.K.; Melchers, W.; Herrera, M.; Bretagne, S.; Wickes, B.; Kirkpatrick, W.R.; Barnes, R.A.; et al. Comparison of Nonculture Blood-Based Tests for Diagnosing Invasive Aspergillosis in an Animal Model. J. Clin. Microbiol. 2016, 54, 960–966. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cai, J.P.; Qiu, L.W.; Hao, W.; Pan, Y.X.; Tung, E.T.K.; Lau, C.C.Y.; Woo, P.C.Y.; Lau, S.K.P.; Yuen, K.Y.; et al. Development of monoclonal antibody-based galactomannoprotein antigen-capture ELISAs to detect Aspergillus fumigatus infection in the invasive aspergillosis rabbit models. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2943–2950. [Google Scholar] [CrossRef]
- Petraitiene, R.; Petraitis, V.; Bacher, J.D.; Finkelman, M.A.; Walsh, T.J. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1-->3)-beta-D-glucan in experimental invasive pulmonary aspergillosis. Med. Mycol. 2015, 53, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, B.; Nystrom, J.; Deen, W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; Morais, M.R.P.T.; Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 2021, 17, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.C.; Karnovsky, M.J. Glomerular Permeability—Ultrastructural Cytochemical Studies Using Peroxidases as Protein Tracers. J. Exp. Med. 1966, 124, 1123–1134. [Google Scholar] [CrossRef]
- Roksnoer, L.C.W.; Heijnen, B.F.J.; Nakano, D.; Peti-Peterdi, J.; Walsh, S.B.; Garrelds, I.M.; van Gool, J.M.G.; Zietse, R.; Struijker-Boudier, H.A.J.; Hoorn, E.J.; et al. On the Origin of Urinary Renin A Translational Approach. Hypertension 2016, 67, 927–933. [Google Scholar] [CrossRef]
- Jensen, H.E.; Stynen, D.; Sarfati, J.; Latge, J.P. Detection of galactomannan and the 18 kDa antigen from Aspergillus fumigatus in serum and urine from cattle with systemic aspergillosis. Zentralbl. Veterinarmed. B 1993, 40, 397–408. [Google Scholar] [CrossRef]
- Hoenigl, M.; Orasch, T.; Faserl, K.; Prattes, J.; Loeffler, J.; Springer, J.; Gsaller, F.; Reischies, F.; Duettmann, W.; Raggam, R.B.; et al. Triacetylfusarinine C: A urine biomarker for diagnosis of invasive aspergillosis. J. Infect. 2019, 78, 150–157. [Google Scholar] [CrossRef]
- Dufresne, S.F.; Datta, K.; Li, X.; Dadachova, E.; Staab, J.F.; Patterson, T.F.; Feldmesser, M.; Marr, K.A. Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS ONE 2012, 7, e42736. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Altenburg, M.K.; Sanford, R.; Willett, J.D.; Bleasdale, B.; Ballou, B.; Wilder, J.; Li, F.; Miner, J.H.; Berg, U.B.; et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc. Natl. Acad. Sci. USA 2017, 114, 2958–2963. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).