AaSlt2 Is Required for Vegetative Growth, Stress Adaption, Infection Structure Formation, and Virulence in Alternaria alternata
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Growth Conditions
2.2. Bioinformatics Analysis
2.3. Deletion and Complementation of AaSlt2
2.4. Determination of Spore Morphology, Vegetative Growth and Sporulation
2.5. Gene Expression Analysis
2.6. Infection Structure Formation Assays
2.7. Stresses Adaption Assays
2.8. Assays for Pathogenicity
2.9. Assessment of Cell Wall Degrading Enzyme Activity
2.10. Measurement of Melanin
2.11. Measurement of Toxins
2.12. Statistical Analysis
3. Results
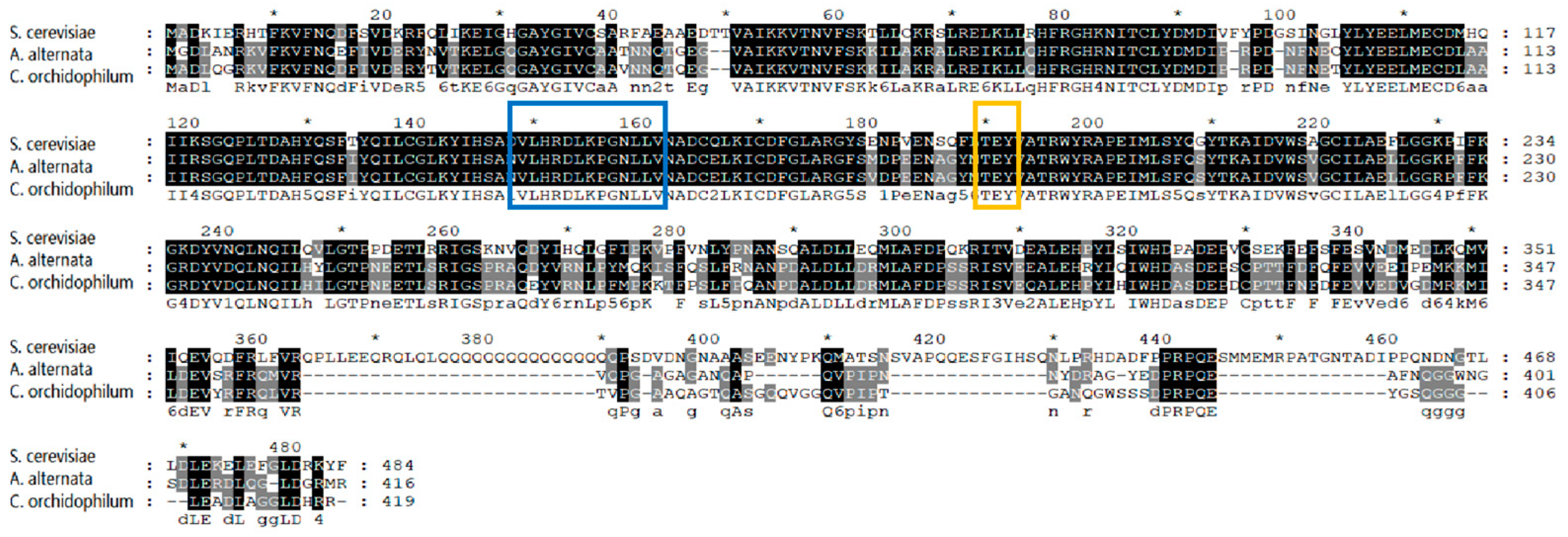
3.1. Identification and Bioinformatics Analysis of AaSlt2
3.2. AaSlt2 Is Involved in Spore Morphology Development, Vegetative Growth and Sporulation of A. alternata
3.3. AaSlt2 Is Essential for Infection Structure Formation of A. alternata
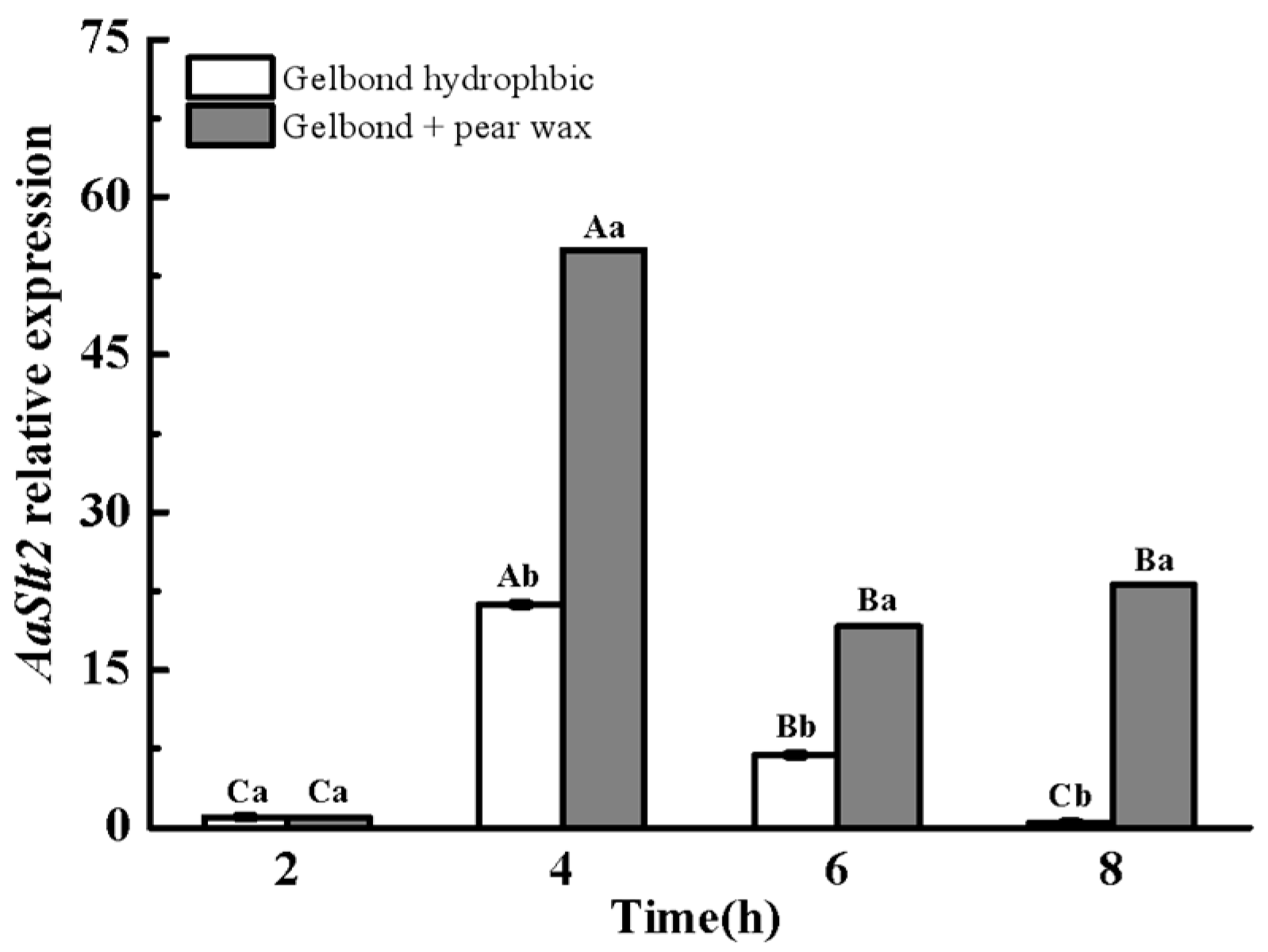
3.3.1. The Expression of the AaSlt2 Gene Is Up-Regulated During Infection Structure Formation
3.3.2. AaSlt2 Regulates the Infection Structure Formation of A. alternata on Onion Epidermis
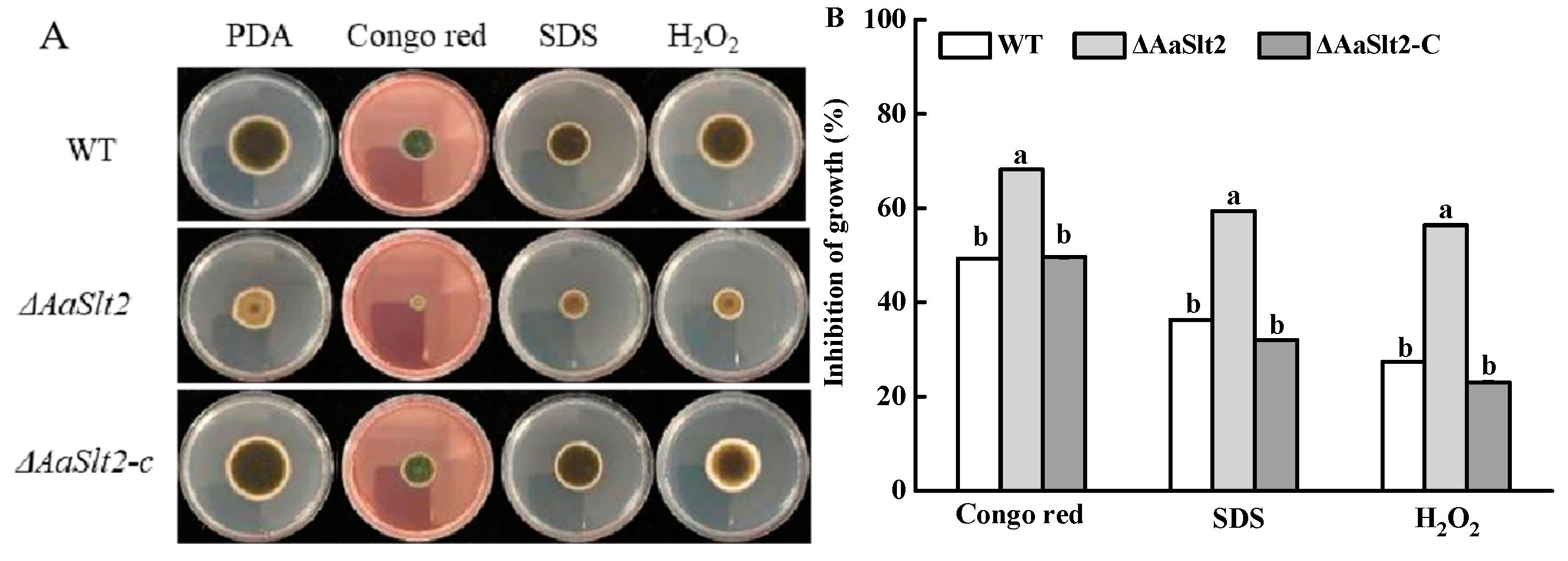
3.4. AaSlt2 Is Susceptible to Cell Wall Inhibitor Agents and Oxidative Stressors
3.5. AaSlt2 Is Essential for the Pathogenicity of A. alternata on Pear Fruit
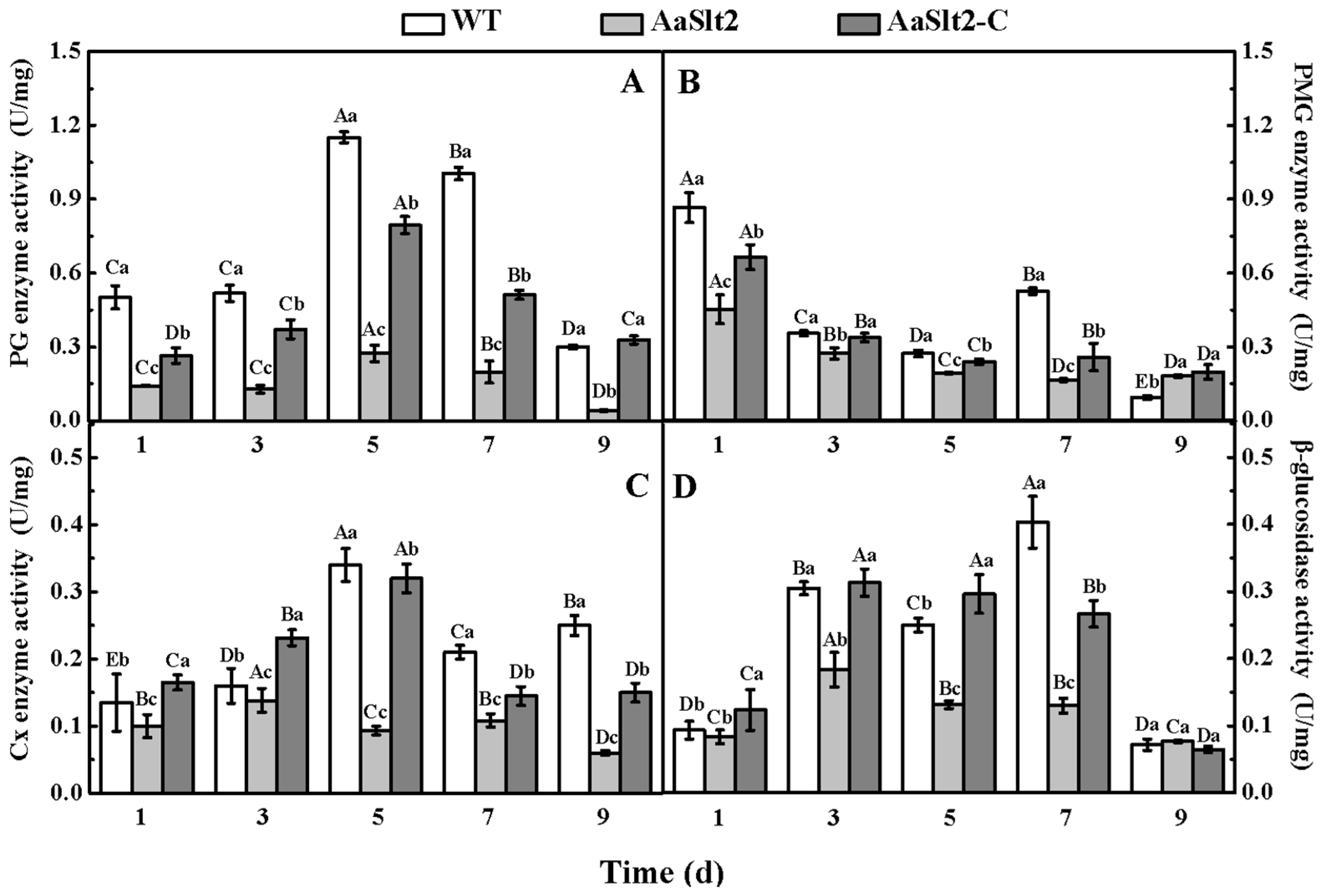
3.6. Deletion of AaSlt2 Results in a Decrease of CWDEs Activity
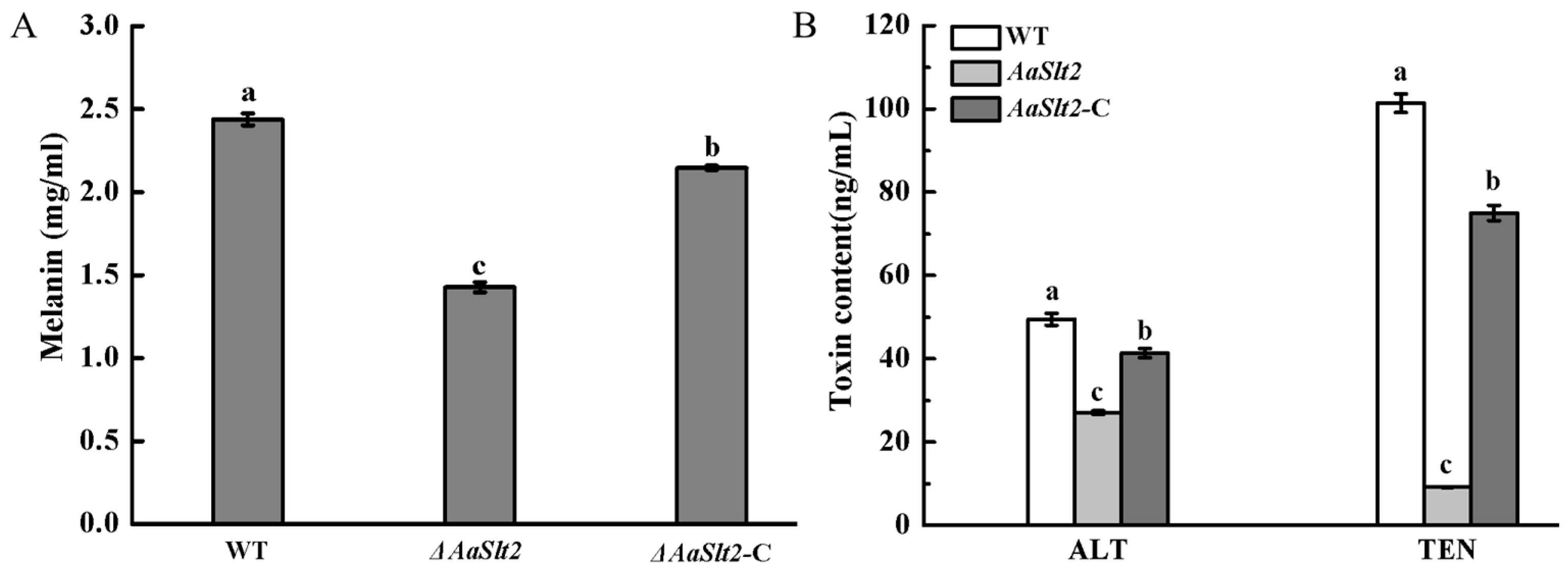
3.7. Deletion of AaSlt2 Results in a Reduction of Melanin Accumulation and Toxin Production of A. alternata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, T.T.; Chyngyz, E.; Sun, D.W.; Paliwal, J.; Pu, H.B. Pathogenetic process monitoring and early detection of pear black spot disease caused by Alternaria alternata using hyperspectral imaging. Postharvest Biol. Technol. 2019, 154, 96–104. [Google Scholar] [CrossRef]
- Zhang, S.J.; Wang, Q.; Guo, Y.J.; Kang, L.; Yu, Y.W. Carbon monoxide enhances the resistance of jujube fruit against postharvest Alternaria rot. Postharvest Biol. Technol. 2020, 101, 1040. [Google Scholar] [CrossRef]
- Ahmad, T.; Liu, Y.; Huang, S.J.; Moosa, A. First record of Alternaria alternata causing postharvest fruit rot of sweet cherry (Prunus avium) in China. Plant Dis. 2020, 104, 2030. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, R.; Wang, Z.; Yue, T.; Cui, L. Advances in detection and control of Alternaria toxin in fruits and vegetables. J. Food Saf. Food Qual. 2020, 11, 114–120. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.; Xiao, C. Fungicide resistance in Alternaria alternata from blueberry in California and its impact on control of Alternaria rot. Plant Dis. 2022, 106, 1446–1453. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Wang, Y.; Bajpai, V.; Huang, L.; Chen, Y.; Baek, K. The mitogen-activated protein kinase signal transduction pathways in Alternaria species. Plant Pathol. J. 2012, 28, 227–238. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK cascades and transcriptional factors: Regulation of heavy metal tolerance in plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.G.; Han, Z.; Liang, Y.M.; Tian, C.M. The mitogen-activated protein kinase gene CcPmk1 is required for fungal growth, cell wall integrity and pathogenicity in Cytospora chrysosperma. Fungal Genet. Biol. 2019, 128, 1–13. [Google Scholar] [CrossRef]
- Cong, J.; Xiao, K.Q.; Jiao, W.L.; Zhang, C.; Zhang, X.H.; Liu, J.L.; Zhang, Y.H.; Pan, H.Y. The coupling between cell wall integrity mediated by MAPK kinases and SsFkh1 is involved in sclerotia formation and pathogenicity of Sclerotinia sclerotiorum. Front. Microbiol. 2022, 13, 816091. [Google Scholar] [CrossRef]
- Cao, H.; Gong, H.; Song, T.; Yu, M.; Pan, X.; Yu, J.; Qi, Z.; Du, Y.; Liu, Y. The adaptor protein UvSte50 governs fungal pathogenicity of Ustilaginoidea virens via the MAPK signaling pathway. J. Fungi 2022, 8, 954. [Google Scholar] [CrossRef]
- He, P.; Wang, Y.; Wang, X.; Zhang, X.; Tian, C. The mitogen-activated protein kinase CgMK1 governs appressorium formation, melanin synthesis, and plant infection of Colletotrichum gloeosporioides. Front. Microbiol. 2017, 8, 2216. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Cid, V.; Martín, H. Fine regulation of Saccharomyces cerevisiae MAPK pathways by post-translational modifications. Yeast 2010, 27, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Han, L.; Xia, Y. MaMk1, a FUS3/KSS1-type mitogen-activated protein kinase gene, is required for appressorium formation, and insect cuticle penetration of the entomopathogenic fungus Metarhizium acridum. J. Invertebr. Pathol. 2014, 115, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, J.; Huang, P.; Liu, X.; Lu, J.; Lin, F. PoRal2 is involved in appressorium formation and virulence via Pmk1 MAPK pathways in the rice blast fungus Pyricularia oryzae. Front. Plant Sci. 2021, 12, 702368. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, W.; Cao, S.; Hou, R.; Li, X.; Ming, L.; Kan, J.; Zhao, Y.; Liu, F. The CfMK1 gene regulates reproduction, appressorium formation, and pathogenesis in a pear anthracnose-causing fungus. J. Fungi 2022, 8, 77. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Bi, W.; Mi, Q.L.; Kang, Z.T.; Liu, C.J.; Yang, J.K.; Luo, Y.Y. Conserved and divergent roles of the HOG1 kinase of Alternaria longipes in mycelial and conidial development, multi-stress responses, melanin production and pathogenicity. Eur. J. Plant Pathol. 2016, 147, 415–430. [Google Scholar] [CrossRef]
- Zheng, D.W.; Wang, Y.; Han, Y.; Xu, J.R.; Wang, C.F. UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci. Rep. 2016, 6, 24824. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.L.; Li, Y.C.; Bi, Y.; Li, R.; Yuan, J.; Xu, W.Y.; Prusky, D.B. AaHog1 regulates infective structural differentiation mediated by physicochemical signals from pear fruit cuticular wax, stress response, and Alternaria alternata pathogenicity. J. Fungi 2022, 8, 266. [Google Scholar] [CrossRef]
- Yago, J.I.; Lin, C.H.; Chung, K.R. The SLT2 mitogenactivated protein kinase-mediated signaling pathway governs conidiation, morphogenesis, fungal virulence and production of toxin and melanin in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2011, 12, 653–665. [Google Scholar] [CrossRef]
- Luo, X.; Keyhani, N.; Yu, X.; He, Z.; Luo, Z.; Pei, Y.; Zhang, Y. The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet. Biol. 2012, 49, 544–555. [Google Scholar] [CrossRef]
- Li, A.; Zhang, M.; Wang, Y.; Li, D.; Liu, X.; Tao, K.; Ye, W.; Wang, Y. PsMPK1, an SLT2-type mitogen-activated protein kinase, is required for hyphal growth, zoosporogenesis, cell wall integrity, and pathogenicity in Phytophthora sojae. Fungal Genet. Biol. 2014, 65, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Shi, L.; Yue, S.N.; Zhang, T.J.; Wang, S.L.; Liu, Y.N.; Ren, A.; Zhu, J.; Yu, H.S.; Zhao, M.W. The Slt2-MAPK pathway is involved in the mechanism by which target of rapamycin regulates cell wall components in Ganoderma lucidum. Fungal Genet. Biol. 2019, 123, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, L.; Deng, J.; Tan, C.; Geng, L.; Liao, Y.; Yuan, J.; Wang, S. A cell wall integrity-related MAP kinase kinase kinase AflBck1 is required for growth and virulence in fungus Aspergillus flavus. Mol. Plant-Microbe Interact. 2020, 33, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Geng, L.P.; Deng, J.L.; Huang, L.H.; Zhong, H.; Xin, S.J.; Fasoyin, O.E.; Wang, S.H. The MAP kinase AflSlt2 modulates aflatoxin biosynthesis and peanut infection in the fungus Aspergillus flavus. Int. J. Food Microbiol. 2020, 322, 108576. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-type MAP kinase Bmp3 in Botrytis cinerea by application of exogenous dsRNA affects fungal growth and virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Wang, T.L.; Zhang, M.; Li, Y.C.; Bi, Y.; Zhang, T.T.; Zheng, X.Y. MAPK signal cascade pathway is involved in sening and responding process of Alternaria alternata to cues from pear fruit surface. J. Gansu Agric. Univ. 2019, 54, 159–168+174. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.C.; Bi, Y.; Wang, Y. Role of pear fruit cuticular wax and surface hydrophobicity in regulating the prepenetration phase of Alternaria alternata infection. J. Phytopathol. 2017, 165, 313–322. [Google Scholar] [CrossRef]
- Jia, M.; Liu, X.T.; Zhao, H.; Ni, Y.X.; Liu, H.Y.; Tian, B.M. Cell-wall-degrading enzymes produced by sesame leaf spot pathogen Corynespora cassiicola. J. Phytopathol. 2020, 169, 186–192. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.C.; Wang, T.L.; Bi, Y.; Li, R.; Huang, Y.; Mao, R.Y.; Jiang, Q.Q.; Liu, Y.X.; Prusky, D.B. AaPKAc regulates differentiation of infection structures induced by physicochemical signals from pear fruit cuticular wax, secondary metabolism, and pathogenicity of Alternaria alternata. Front. Plant Sci. 2021, 12, 642601. [Google Scholar] [CrossRef]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crop. Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Chen, X.X.; Xu, C.; Qian, Y.; Liu, R.; Zhang, Q.Q.; Zeng, G.H.; Zhang, X.; Zhao, H.; Fang, W.G. MAPK cascade-mediated regulation of pathogenicity, conidiation and tolerance to abiotic stresses in the entomopathogenic fungus Metarhizium robertsii. Environ. Microbiol. 2016, 18, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Osés-Ruiz, M.; Cruz-Mireles, N.; Martin-Urdiroz, M.; Soanes, D.M.; Eseola, A.B.; Tang, B.; Derbyshire, P.; Nielsen, M.; Cheema, J.; Were, V.; et al. Appressorium-mediated plant infection by Magnaporthe oryzae is regulated by a Pmk1-dependent hierarchical transcriptional network. Nat. Microbiol. 2021, 6, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.H.; Wang, H.F.; Lu, Z.Y.; Huang, S.S.; Luo, Z.B.; Zhang, L.Y.; Lv, T.; Tang, X.H.; Zhang, Y.J. Fus3/Kss1-MAP kinase and Ste12-like control distinct biocontrol-traits besides regulation of insect cuticle penetration via phosphorylation cascade in a filamentous fungal pathogen. Pest Manag. Sci. 2023, 79, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Martín, H.; Rodríguez-Pachón, J.M.; Ruiz, C.; Nombela, C.; Molina, M. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 1511–1519. [Google Scholar] [CrossRef]
- Turrà, D.; Segorbe, D.; Pietro, A.D. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar] [CrossRef]
- Leng, G.; Song, K. Direct interaction of Ste11 and Mkk1/2 through Nst1 integrates high-osmolarity glycerol and pheromone pathways to the cell wall integrity MAPK pathway. FEBS Lett. 2016, 590, 148–160. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Liu, H.Q.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef]
- Mehrabi, R.; Van, D.; Lee, T.; Waalwijk, C.; Kema, G.H.J. MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol. Plant-Microbe Interact. 2006, 19, 389–398. [Google Scholar] [CrossRef]
- Zhen, Z.; Xing, X.; Xie, M.; Yang, L.; Yang, X.; Zheng, Y.; Chen, Y.; Ma, N.; Li, Q.; Zhang, K.; et al. MAP kinase Slt2 orthologs play similar roles in conidiation, trap formation, and pathogenicity in two nematode-trapping fungi. Fungal Genet. Biol. 2018, 116, 42–50. [Google Scholar] [CrossRef]
- Bashi, Z.D.; Gyawali, S.; Bekkaoui, D.; Coutu, C.; Lee, L.; Poon, J.; Rimmer, S.R.; Khachatourians, G.G.; Hegedus, D.D. The Sclerotinia sclerotiorum Slt2 mitogen-activated protein kinase ortholog, SMK3, is required for infection initiation but not lesion expansion. Can. J. Microbiol. 2016, 62, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Naqvi, N.I. Surface sensing and signaling networks in plant pathogenic fungi. Semin. Cell Dev. Biol. 2016, 57, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.B.D.; Lopes, M.D.S.; Bini, A.P.; Tschoeke, B.A.P.; Verssani, B.A.W.; Figureueredo, E.F.; Cataldi, T.R.; Marques, J.P.R.; Silva, L.D.; Labate, C.A.; et al. The eucalyptus cuticular waxes contribute in preformed defense against Austropuccinia psidii. Front. Plant Sci. 2019, 9, 1978. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Y.X.; Li, Y.C.; Zong, Y.Y.; Bi, Y.; Prusky, D.B. The sensor protein AaSln1 is involved in differentiation of infection structures, osmotic stress tolerance and virulence in Alternaria alternata. Postharvest Biol. Technol. 2024, 209, 112697. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, G.; Li, L.; Kong, L.; Wang, C.; Zhang, H.; Xu, J. Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog. 2011, 7, e1001261. [Google Scholar] [CrossRef]
- Rui, O.; Hahn, M. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 2007, 8, 173–184. [Google Scholar] [CrossRef]
- Schroeder, L.; Ikui, A.E. Tryptophan confers resistance to SDS-associated cell membrane stress in Saccharomyces cerevisiae. PLoS ONE 2019, 14, e0199484. [Google Scholar] [CrossRef]
- Huang, S.; He, Z.; Zhang, S.; Keyhani, N.; Song, Y.; Yang, Z.; Jiang, Y.; Zhang, W.; Pei, Y.; Zhang, Y. Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation and virulence in the insect fungal pathogen Beauveria bassiana. Fungal Genet. Biol. 2015, 83, 78–91. [Google Scholar] [CrossRef]
- So, K.K.; Ko, Y.H.; Chun, T.; Kim, J.M.; Kim, D.H. Mutation of the Slt2 ortholog from Cryphonectria parasitica results in abnormal cell wall integrity and sectorization with impaired pathogenicity. Sci. Rep. 2017, 7, 9038. [Google Scholar] [CrossRef]
- Jacobson, E.S. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef]
- Kustrzeba-Wójcicka, I.; Siwak, E.; Terlecki, G.; Wolańczyk-Mędrala, A.; Mędrala, W. Alternaria alternata and its allergens: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Eliahu, N.; Igbaria, A.; Rose, M.S.; Horwitz, B.A.; Lev, S. Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, CHk1 and MPs1, and the transcription factor cmr1. Eukaryotic Cell 2007, 6, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, W.Y.; Zong, Y.Y.; Wang, X.J.; Li, Y.C.; Bi, Y.; Prusky, D.B. Melanin synthesis gene Aapks contributes to appressorium formation, stress response, cell well integrity and virulence in Alternaria alternata. Postharvest Biol. Technol. 2023, 198, 112247. [Google Scholar] [CrossRef]
- Hou, Z.; Xue, C.; Peng, Y.; Katan, T.; Kistler, H.C.; Xu, J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 2002, 15, 1119–1127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Wang, T.; Li, Y.; Bi, Y.; Zhang, M.; Wang, X.; Prusky, D.B. AaSlt2 Is Required for Vegetative Growth, Stress Adaption, Infection Structure Formation, and Virulence in Alternaria alternata. J. Fungi 2024, 10, 774. https://doi.org/10.3390/jof10110774
Jiang Q, Wang T, Li Y, Bi Y, Zhang M, Wang X, Prusky DB. AaSlt2 Is Required for Vegetative Growth, Stress Adaption, Infection Structure Formation, and Virulence in Alternaria alternata. Journal of Fungi. 2024; 10(11):774. https://doi.org/10.3390/jof10110774
Chicago/Turabian StyleJiang, Qianqian, Tiaolan Wang, Yongcai Li, Yang Bi, Miao Zhang, Xiaojing Wang, and Dov B. Prusky. 2024. "AaSlt2 Is Required for Vegetative Growth, Stress Adaption, Infection Structure Formation, and Virulence in Alternaria alternata" Journal of Fungi 10, no. 11: 774. https://doi.org/10.3390/jof10110774
APA StyleJiang, Q., Wang, T., Li, Y., Bi, Y., Zhang, M., Wang, X., & Prusky, D. B. (2024). AaSlt2 Is Required for Vegetative Growth, Stress Adaption, Infection Structure Formation, and Virulence in Alternaria alternata. Journal of Fungi, 10(11), 774. https://doi.org/10.3390/jof10110774








