Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida Strains
2.2. Antifungal Susceptibility Testing
2.3. Biofilm Formation and Inhibition
2.4. The Effect of Rosmarinic Acid on Adhesion, Hyphae and Biofilm-Related Gene Expression
2.5. Field Emission Scanning Electron Microscopy (FESEM)
2.6. Statistical Analysis
3. Results
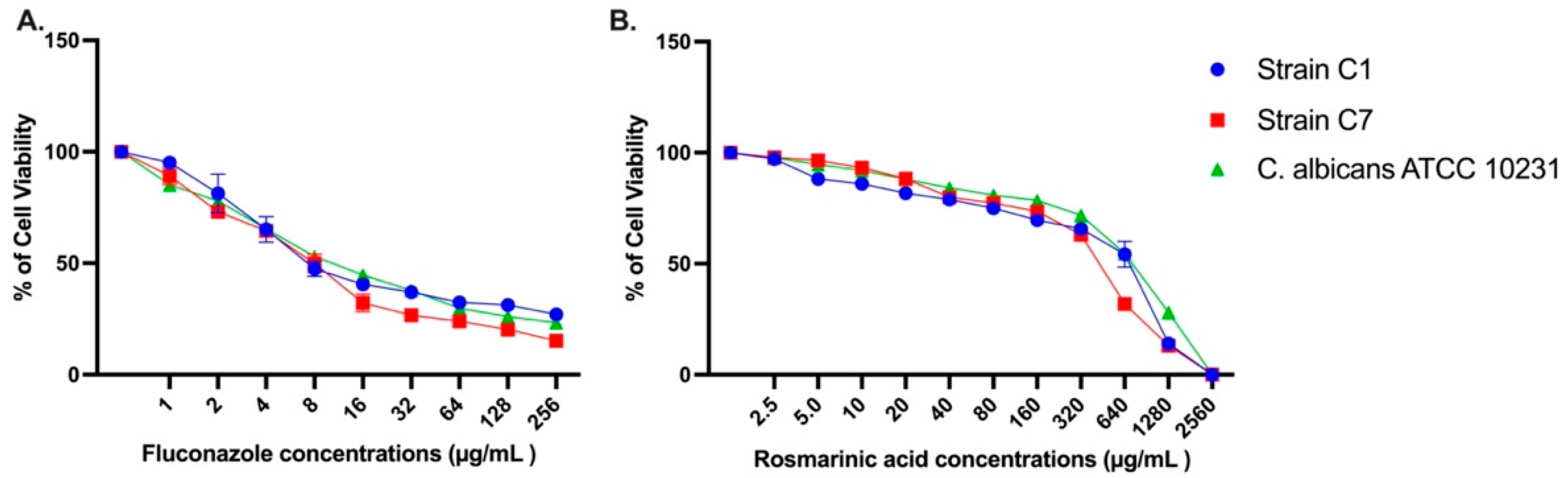
3.1. Antifungal Activity
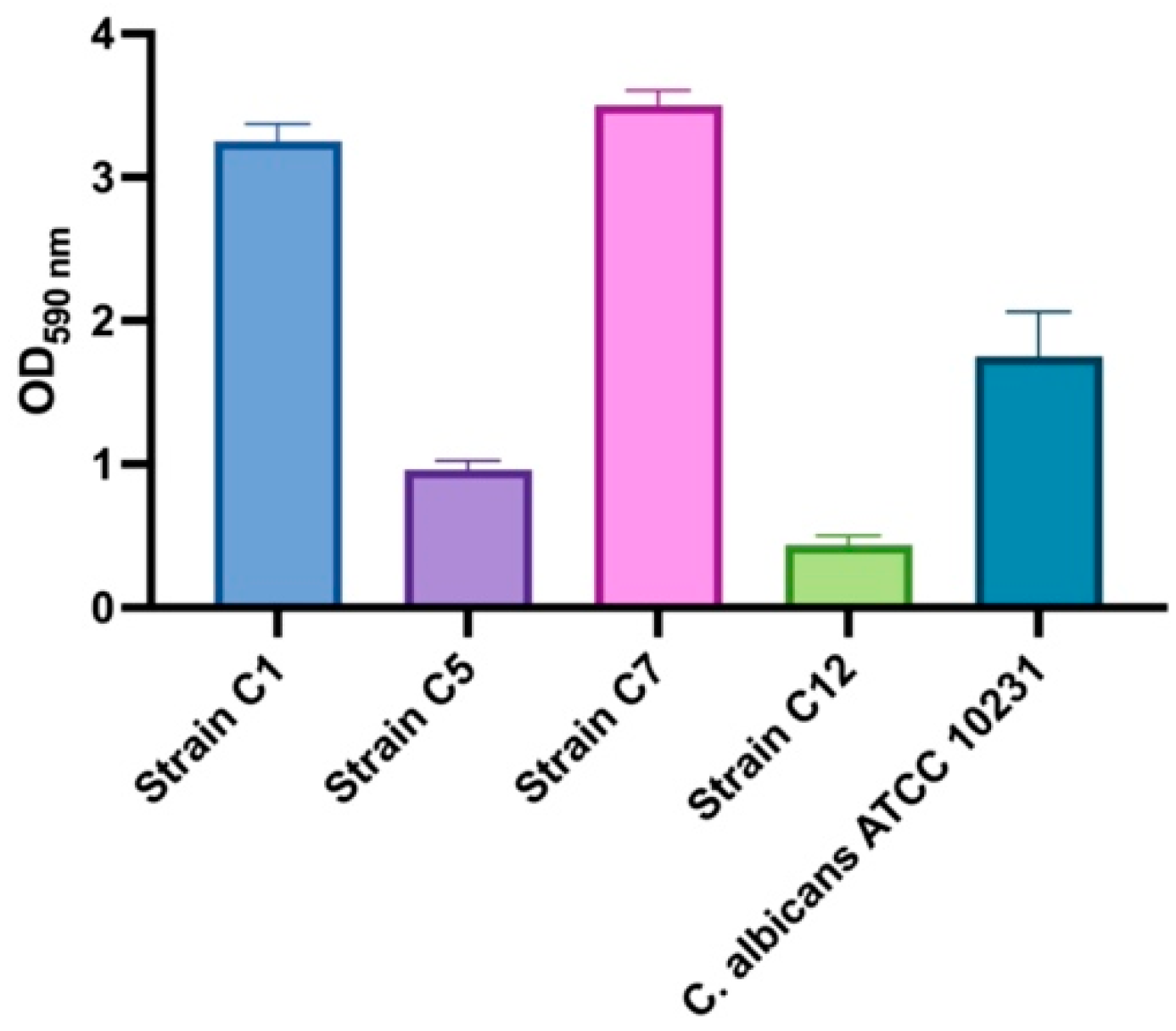
3.2. Biofilm-Forming Capacity and Anti-Biofilm Activity
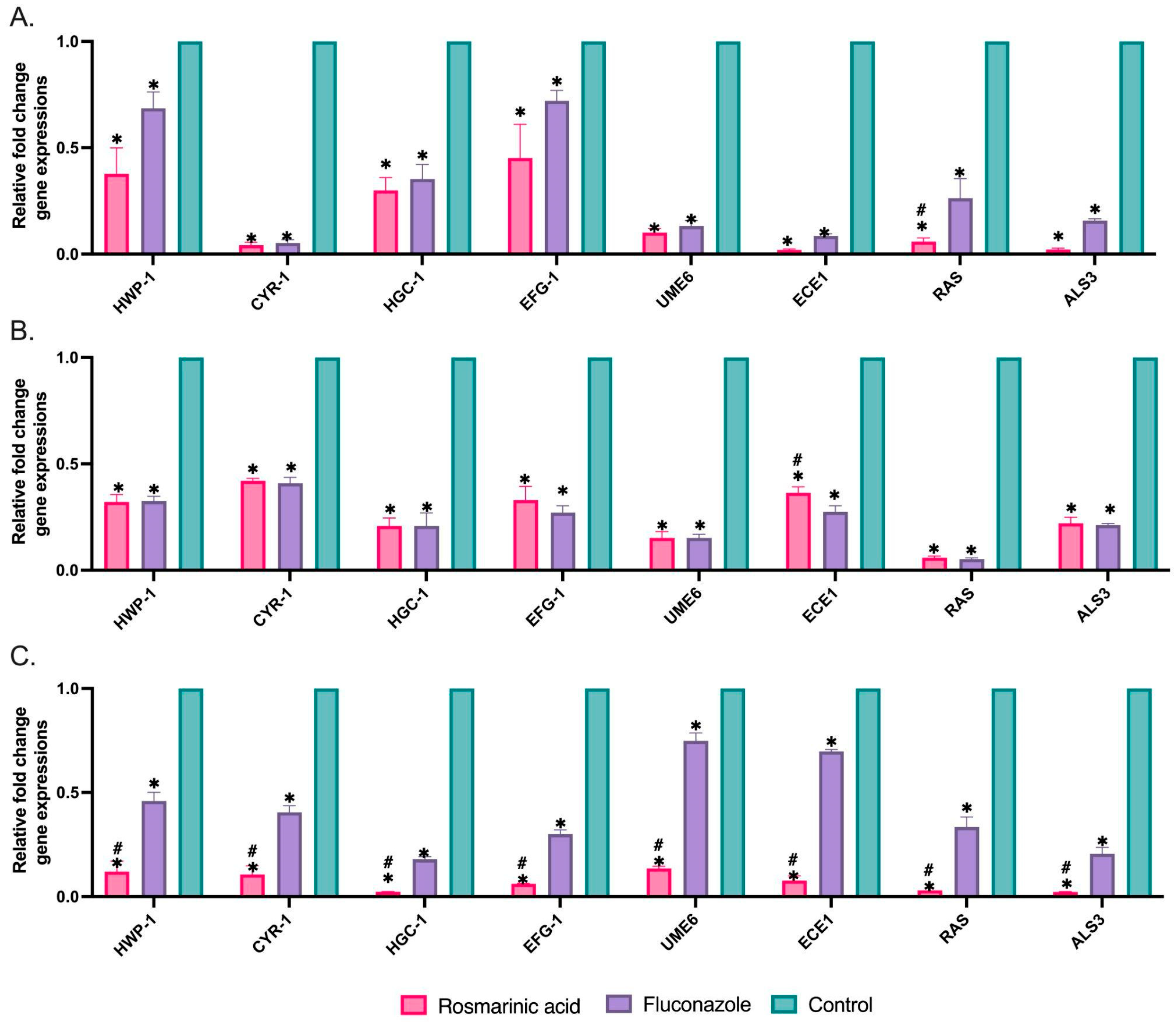
3.3. Biofilm-Related Gene Expressions
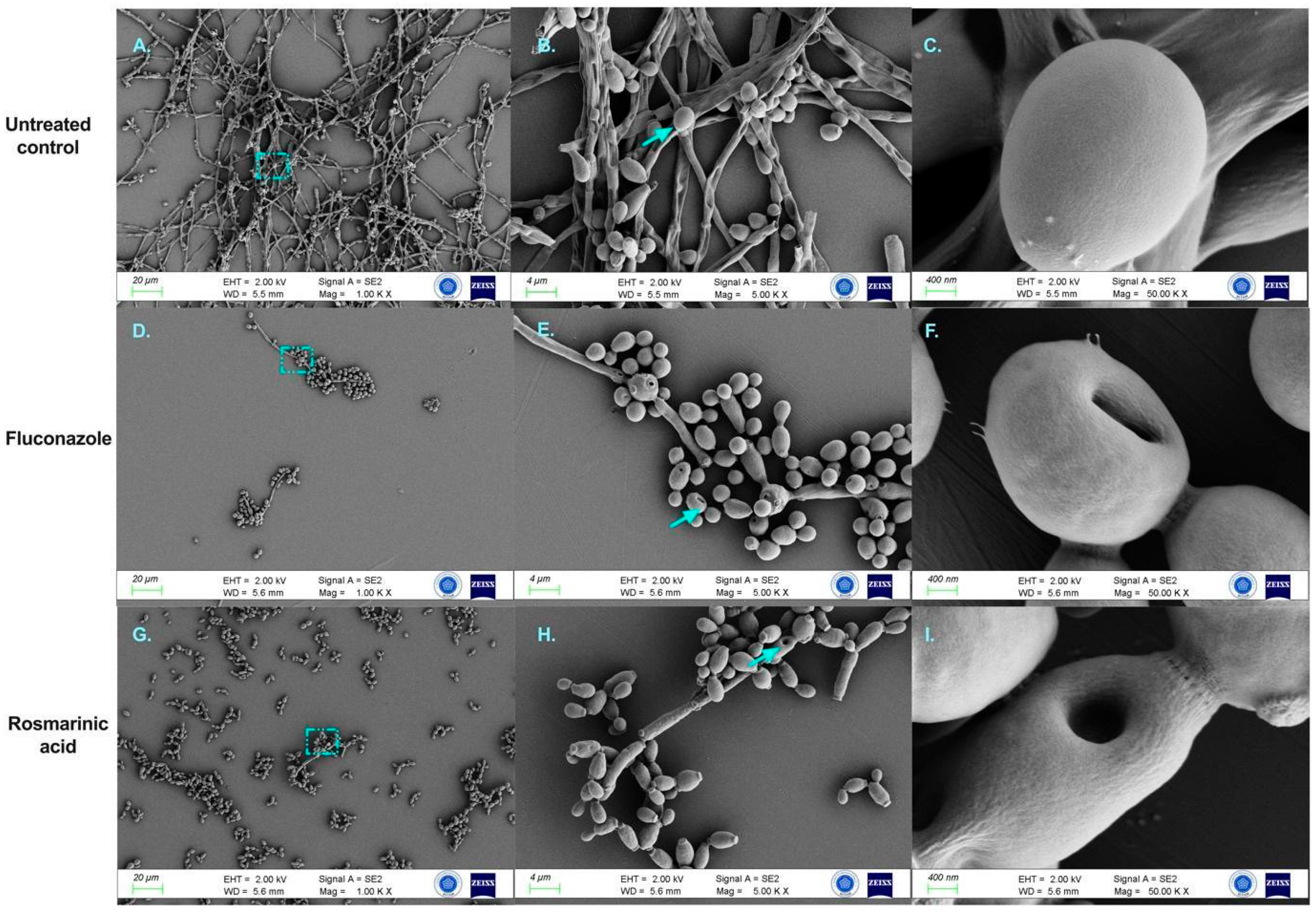
3.4. FESEM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Miao, M.X.; Jia, C.L.; Cao, Y.B.; Yan, T.H.; Jiang, Y.Y.; Yang, F. Interactions between Candida albicans and the resident microbiota. Front. Microbiol. 2022, 13, 930495. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.B.; Simões, R.S.; Pedro, N.A.; Pinheiro, M.J.; Carvalho, M.F.N.N.; Mira, N.P. An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. J. Fungi 2020, 6, 23. [Google Scholar] [CrossRef]
- Hernández-Pabón, J.C.; Tabares, B.; Gil, Ó.; Lugo-Sánchez, C.; Santana, A.; Barón, A.; Firacative, C. Candida Non-albicans and Non-auris Causing Invasive Candidiasis in a Fourth-Level Hospital in Colombia: Epidemiology, Antifungal Susceptibility, and Genetic Diversity. J. Fungi 2024, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Kitaya, S.; Kanamori, H.; Katori, Y.; Tokuda, K. Clinical Features and Outcomes of Persistent Candidemia Caused by Candida albicans versus Non-albicans Candida Species: A Focus on Antifungal Resistance and Follow-Up Blood Cultures. Microorganisms 2023, 11, 928. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef]
- Silva, L.N.; de Mello, T.P.; de Souza Ramos, L.; Branquinha, M.H.; Dos Santos, A.L.S. New and Promising Chemotherapeutics for Emerging Infections Involving Drug-resistant Non-albicans Candida Species. Curr. Top. Med. Chem. 2019, 19, 2527–2553. [Google Scholar] [CrossRef]
- Chow, E.W.L.; Pang, L.M.; Wang, Y. From Jekyll to Hyde: The Yeast-Hyphal Transition of Candida albicans. Pathogens 2021, 10, 859. [Google Scholar] [CrossRef]
- Watchaputi, K.; Jayasekara, L.A.C.B.; Ratanakhanokchai, K.; Soontorngun, N. Inhibition of cell cycle-dependent hyphal and biofilm formation by a novel cytochalasin 19,20-epoxycytochalasin Q in Candida albicans. Sci. Rep. 2023, 13, 9724. [Google Scholar] [CrossRef] [PubMed]
- Poon, Y.; Hui, M. Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis. Front. Microbiol. 2023, 14, 1105949. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Matsubara, V.H.; Samaranayake, L.P. Future therapies targeted towards eliminating Candida biofilms and associated infections. Expert. Rev. Anti-Infect. Ther. 2017, 15, 299–318. [Google Scholar] [CrossRef]
- Mota Fernandes, C.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, e01719-20. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid-From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kernou, O.N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef]
- Cheah, H.L.; Lim, V.; Sandai, D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 2014, 9, e95951. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kello, M.; Čoma, M.; Slobodníková, L.; Drobná, E.; Holková, I.; Garajová, M.; Mrva, M.; Zachar, V.; Lukáč, M. Derivatization of Rosmarinic Acid Enhances its in vitro Antitumor, Antimicrobial and Antiprotozoal Properties. Molecules 2019, 24, 1078. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Fourth Informational Supplement; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Sumlu, E.; Aydin, M.; Korucu, E.N.; Alyar, S.; Nsangou, A.M. Artemisinin May Disrupt Hyphae Formation by Suppressing Biofilm-Related Genes of Candida albicans: In Vitro and In Silico Approaches. Antibiotics 2024, 13, 310. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiterplates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.P.; Guinoiseau, E.; Luciani, A.; Yang, Y.; Battesti, M.J.; Paolini, J.; Costa, J.; Quilichini, Y.; Berti, L.; Lorenzi, V. Key role of hydrogen peroxide in antimicrobial activity of spring, Honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018, 66, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zheng, D.; Bai, Y.; Yang, L.; Yong, J.; Li, Y. Insights into the anti-Candida albicans properties of natural phytochemicals: An in vitro and in vivo investigation. Phytother. Res. 2024, 38, 2518–2538. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Zhao, Y.; Si, Y.; Mei, L.; Shao, J.; Wang, T.; Yan, G.; Wang, C. Sodium New Houttuyfonate Inhibits Candida albicans Biofilm Formation by Inhibiting the Ras1-cAMP-Efg1 Pathway Revealed by RNA-seq. Front. Microbiol. 2020, 11, 2075. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, A.; Chen, F.; Zhao, Y.; Zhu, X.; Zhang, T.; Ni, G.; Wang, R. Synthesis, structure-activity relationship and biological evaluation of indole derivatives as anti-Candida albicans agents. Bioorg. Chem. 2024, 146, 107293. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Sharma, A.; Mitchell, A.P. Strain variation in gene expression impact of hyphal cyclin Hgc1 in Candida albicans. G3 2023, 13, jkad151. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, P.L.; Kadosh, D. Candida albicans Ume6, a filament-specific transcriptional regulator, directs hyphal growth via a pathway involving Hgc1 cyclin-related protein. Eukaryot. Cell 2010, 9, 1320–1328. [Google Scholar] [CrossRef]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef]
- Garbe, E.; Thielemann, N.; Hohner, S.; Kumar, A.; Vylkova, S.; Kurzai, O.; Martin, R. Functional analysis of the Candida albicans ECE1 Promoter. Microbiol. Spectr. 2023, 11, e0025323. [Google Scholar] [CrossRef] [PubMed]
| Genes | Primer | Sequence (5′→3′) |
|---|---|---|
| HWP1 | Forward | GCTCCTGCTCCTGAAATGAC |
| Reverse | CTGGAGCAATTGGTGAGGTT | |
| CYR1 | Forward | CCAACAAACGACCAAAAGGT |
| Reverse | TCTTGAACTGCCAGACGATG | |
| HGC1 | Forward | GCTTCCTGCACCTCATCAAT |
| Reverse | AGCACGAGAACCAGCGATAC | |
| EFG1 | Forward | GCCTCGAGCACTTCCACTGT |
| Reverse | TTTTTTCATCTTCCCACATGGTAGT | |
| UME6 | Forward | ACCACCACTACCACCACCAC |
| Reverse | TATCCCCATTTCCAAGTCCA | |
| ECE1 | Forward | TTGCTAATGCCGTCGTCAGA |
| Reverse | GAACGACCATCTCTCTTGGCAT | |
| RAS1 | Forward | TGGATGTTGTGTTATTGTTTGAGC |
| Reverse | GTCTTGAATTGTTCATCTTCTCCCA | |
| ALS3 | Forward | TCGTCCTCATTACACCAACCA |
| Reverse | TGAAGTTGCAGATGGGGCTT | |
| 18S rRNA | Forward | AGAAACGGCTACCACATCCA |
| Reverse | AGCCCAAGGTTCAACTACGA |
| Species (Number) | MIC50 Range of FLC (μg/mL) | MIC50 Range of RA (μg/mL) |
|---|---|---|
| C. albicans [4] | 1–4 | 640–1280 |
| C. lusitaniae [4] | 2–64 | 640–1280 |
| C. glabrata [4] | 16–32 | 160–320 |
| C. krusei [4] | 32 | 1280 |
| C. kefyr [4] | 1–4 | 160–1280 |
| C. parapsilosis [4] | 2–64 | 640–1280 |
| C. albicans ATCC 10231 | 1 | 640 |
| C. glabrata ATCC 90030 | 16 | 160 |
| C. krusei ATCC 6258 | 64 | 320 |
| C. parapsilosis ATCC 22019 | 2 | 640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, M.; Unusan, N.; Sumlu, E.; Korucu, E.N. Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes. J. Fungi 2024, 10, 751. https://doi.org/10.3390/jof10110751
Aydin M, Unusan N, Sumlu E, Korucu EN. Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes. Journal of Fungi. 2024; 10(11):751. https://doi.org/10.3390/jof10110751
Chicago/Turabian StyleAydin, Merve, Nurhan Unusan, Esra Sumlu, and Emine Nedime Korucu. 2024. "Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes" Journal of Fungi 10, no. 11: 751. https://doi.org/10.3390/jof10110751
APA StyleAydin, M., Unusan, N., Sumlu, E., & Korucu, E. N. (2024). Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes. Journal of Fungi, 10(11), 751. https://doi.org/10.3390/jof10110751






