Molecular Mechanism of Mok I Gene Overexpression in Enhancing Monacolin K Production in Monascus pilosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Construction of a Eukaryotic Expression Vector for Mok I Gene
2.3. Geneticin Susceptibility Assay for M. pilosus
2.4. Agrobacterium-Mediated Transformation of M. pilosus
2.5. PCR Identification of M. pilosus Transformants
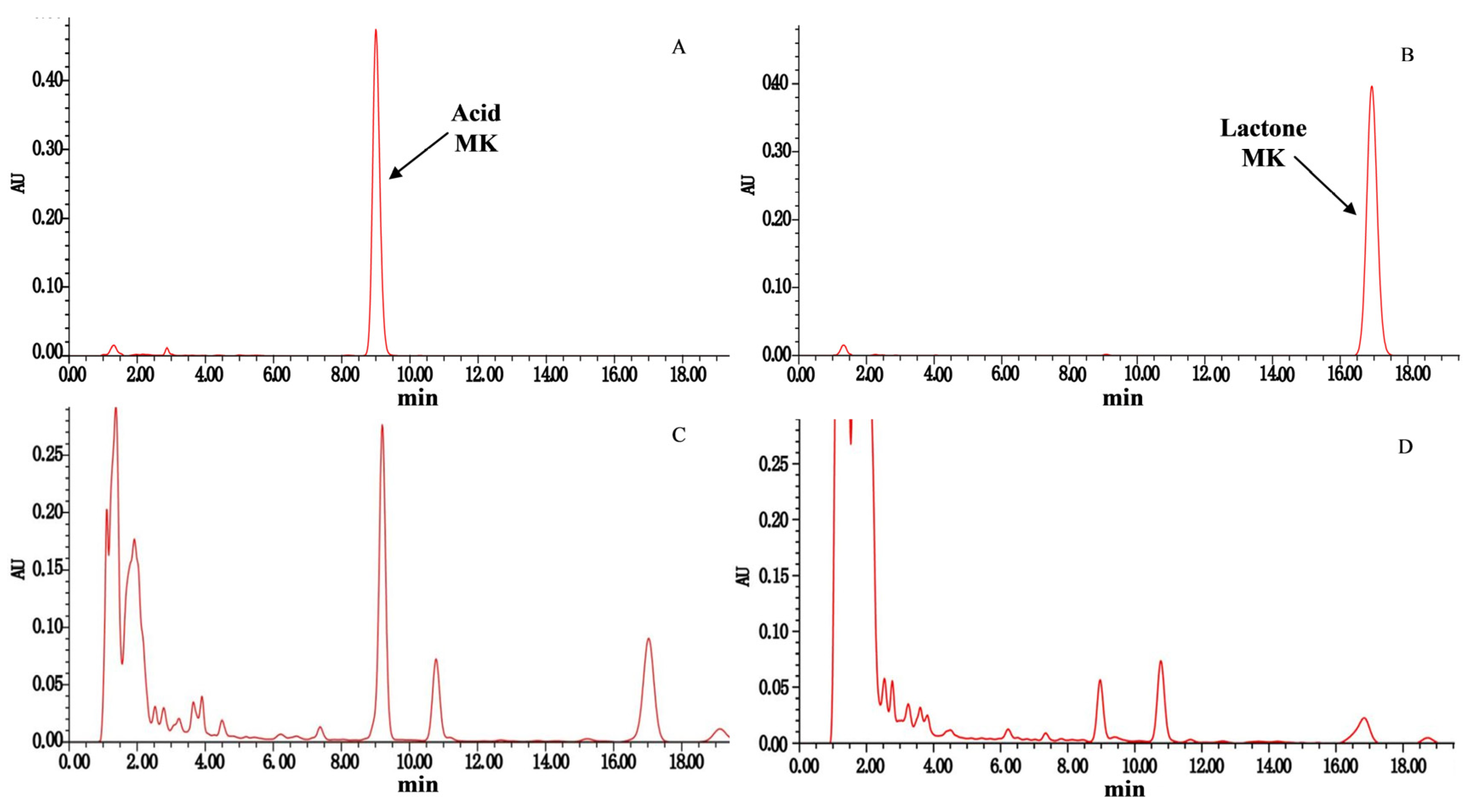
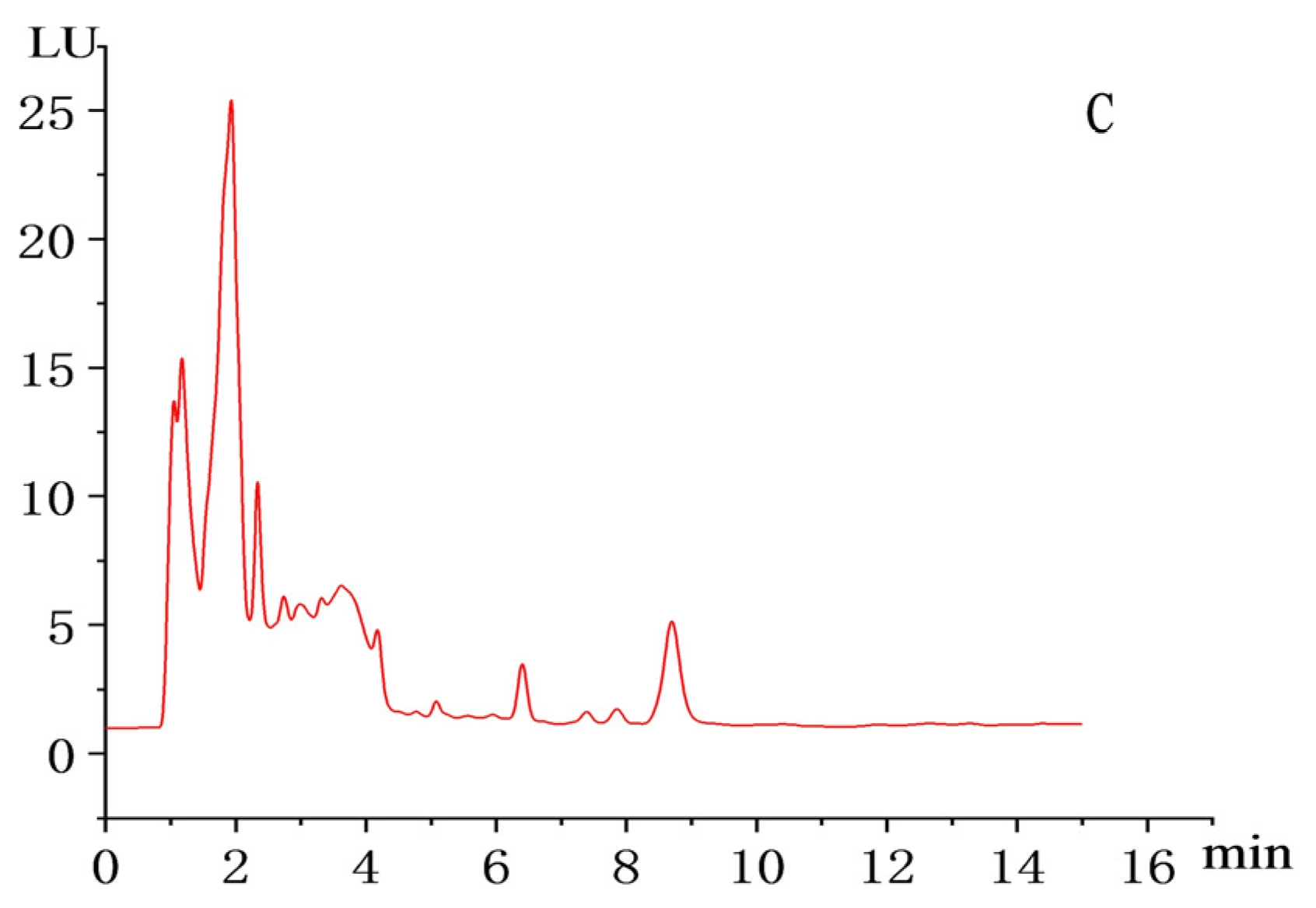
2.6. HPLC Detection of MK Yield of the Transformed Strains
2.6.1. Solid Fermentation of the Transformed Strains
2.6.2. HPLC Detection Method for MK Content
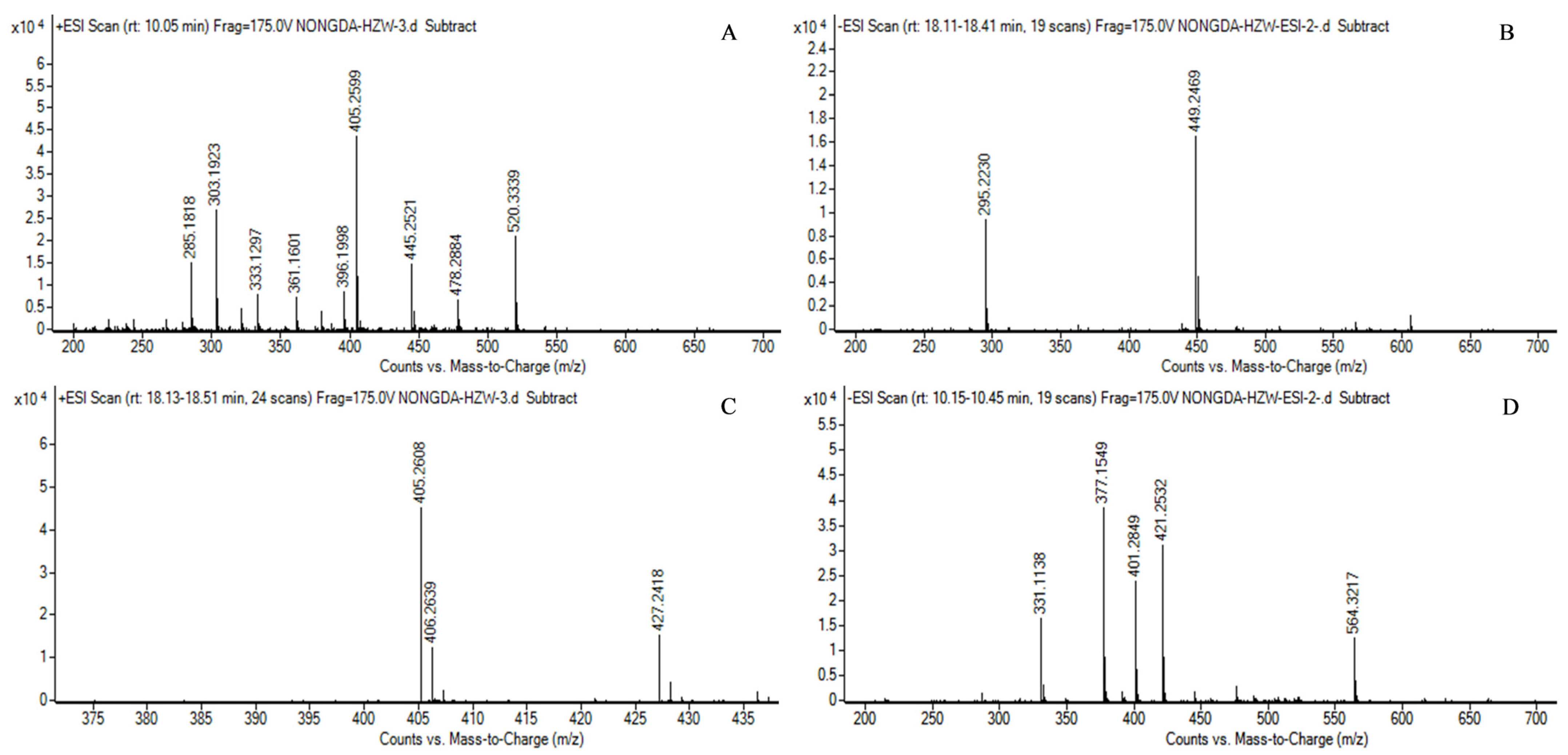
2.7. Mass Spectrometry Identification of MK in Transformed Strains
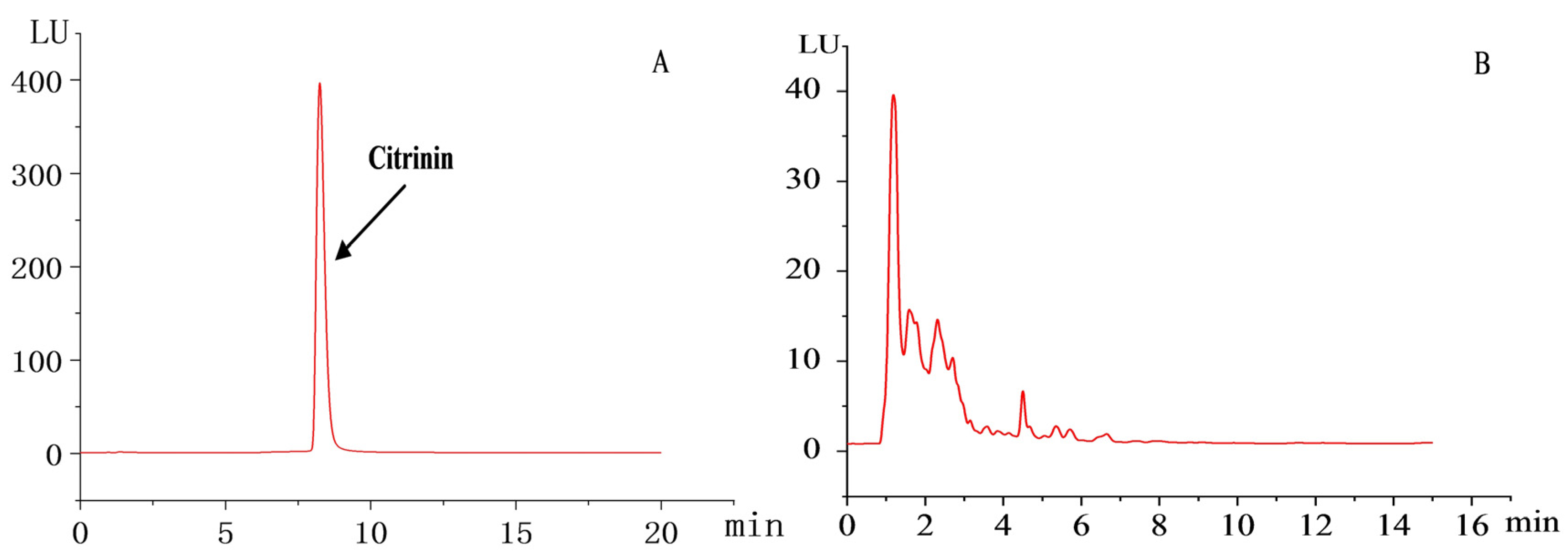
2.8. HPLC Detection of Citrinin Content in Transformed Strains
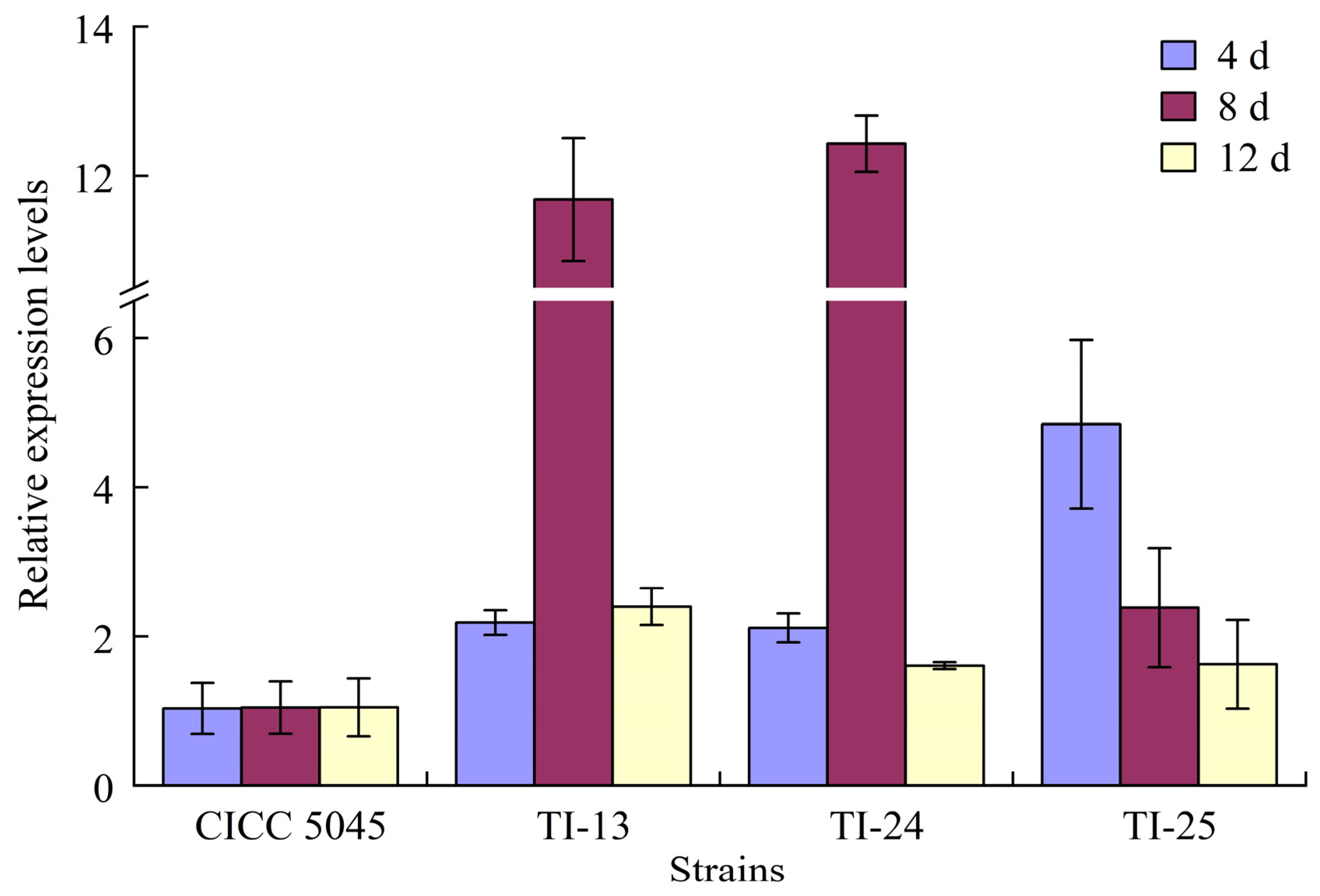
2.9. Detection and Analysis of Expression Levels of Mok I Gene in Transformed Strains
2.10. Multi-Omics Analysis of the Molecular Mechanism of High MK Production in Transformed Strains
2.10.1. Transcriptomic Analysis of the Molecular Mechanism of High MK Production in Transformed Strains
2.10.2. Metabolomic Analysis of the Molecular Mechanism of High MK Production in Transformed Strains
2.10.3. Multi-Omics Joint Analysis of the Molecular Mechanism of High MK Production in Transformed Strains
3. Results
3.1. Obtaining Transgenic Strains Overexpressing Mok I
3.1.1. Construction of Eukaryotic Expression Vectors for Mok I
3.1.2. Results of Agrobacterium-Mediated Transformation of M. pilosus
3.1.3. PCR Identification Results of M. pilosus Transformants
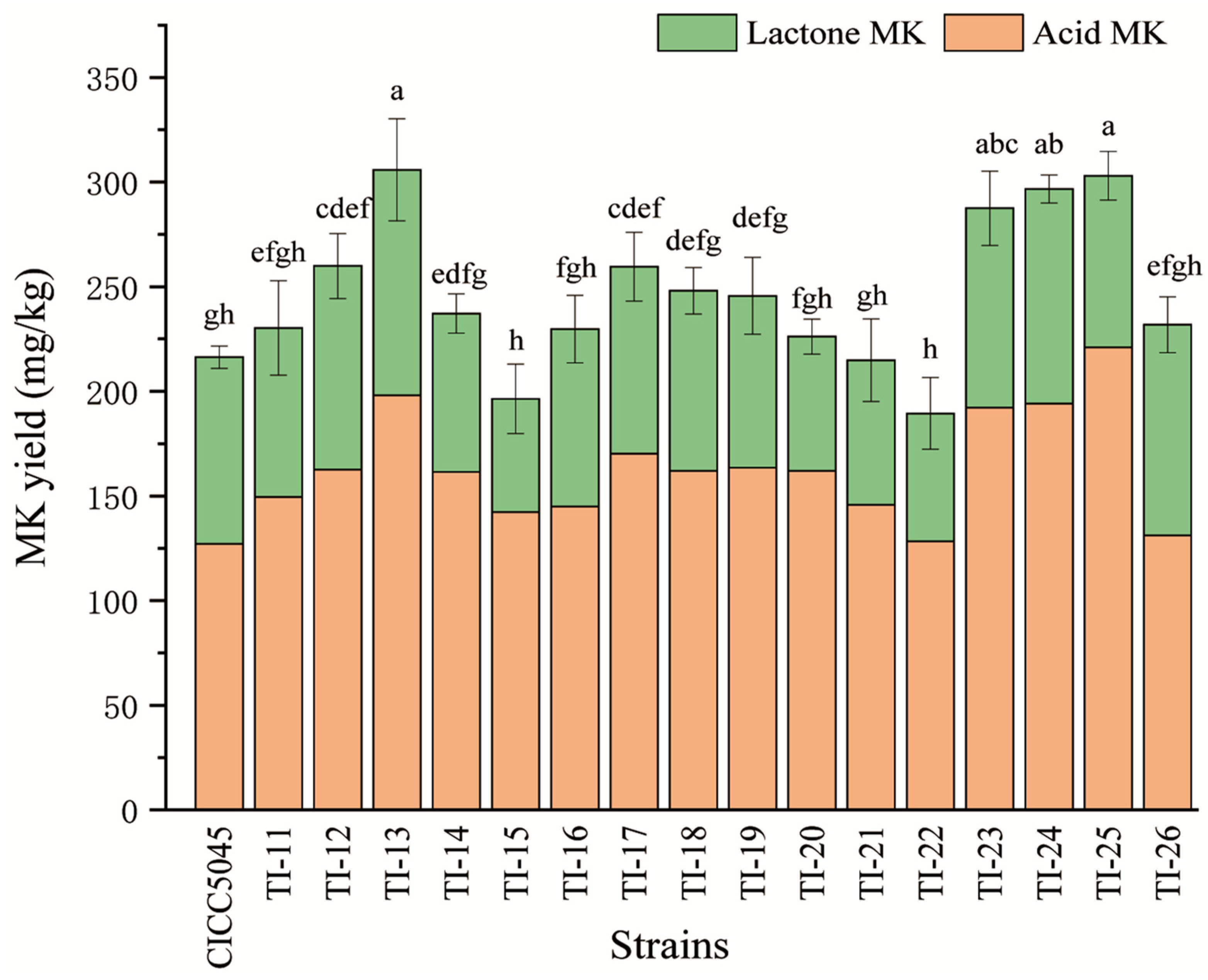
3.2. Results of MK Content Detection in Transformed Strains
3.3. Mass Spectrometry Identification Results of MK in Transformed Strains
3.4. HPLC Test Results of Citrinin Content in Transformed Strains
3.5. Detection Results of Expression Levels of Mok I Gene in Transformed Strains
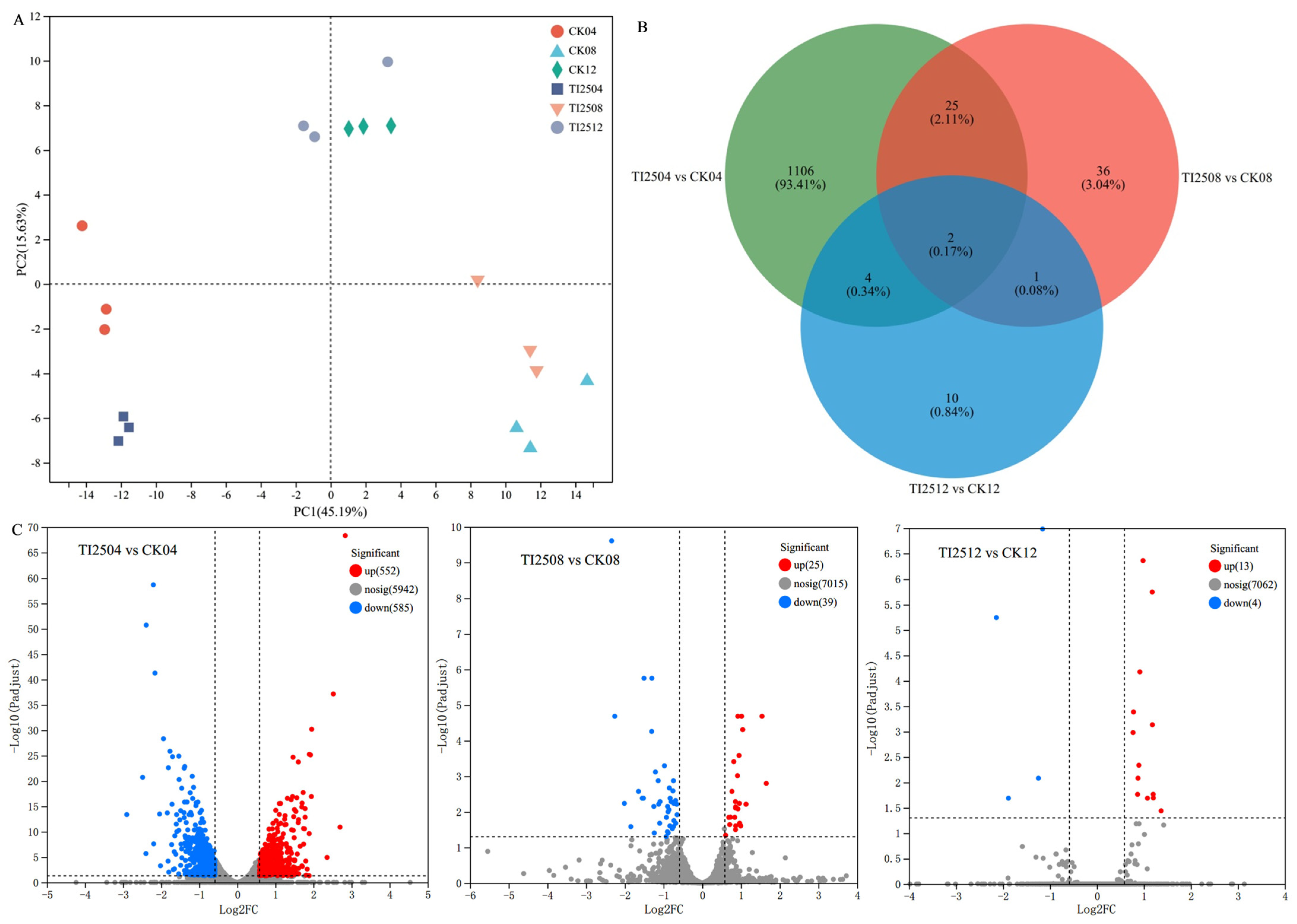
3.6. Transcriptomic Analysis Results of the Molecular Mechanism of High MK Production in Transformed Strains
3.6.1. Differential Gene Expression Analysis of Transformed Strains
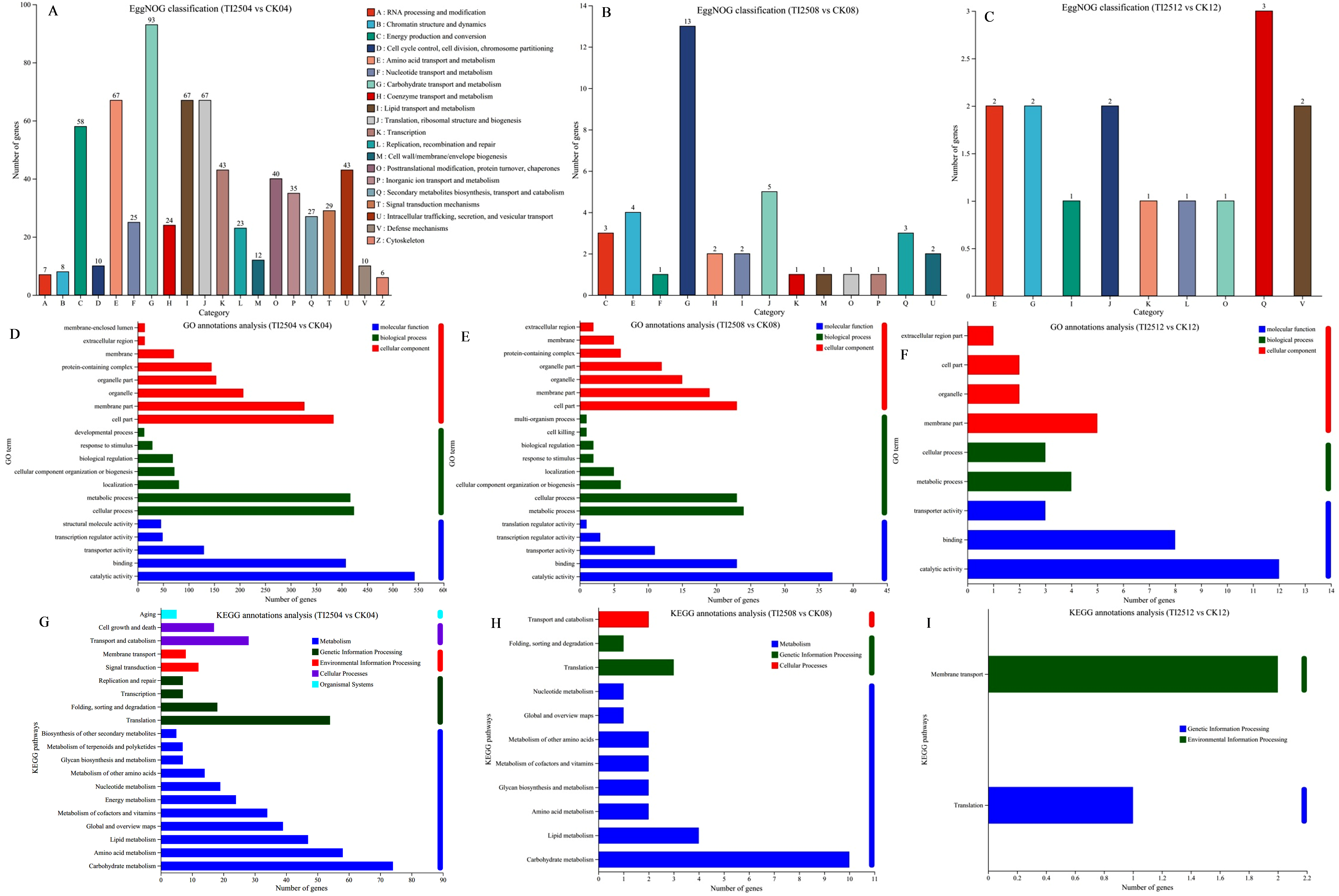
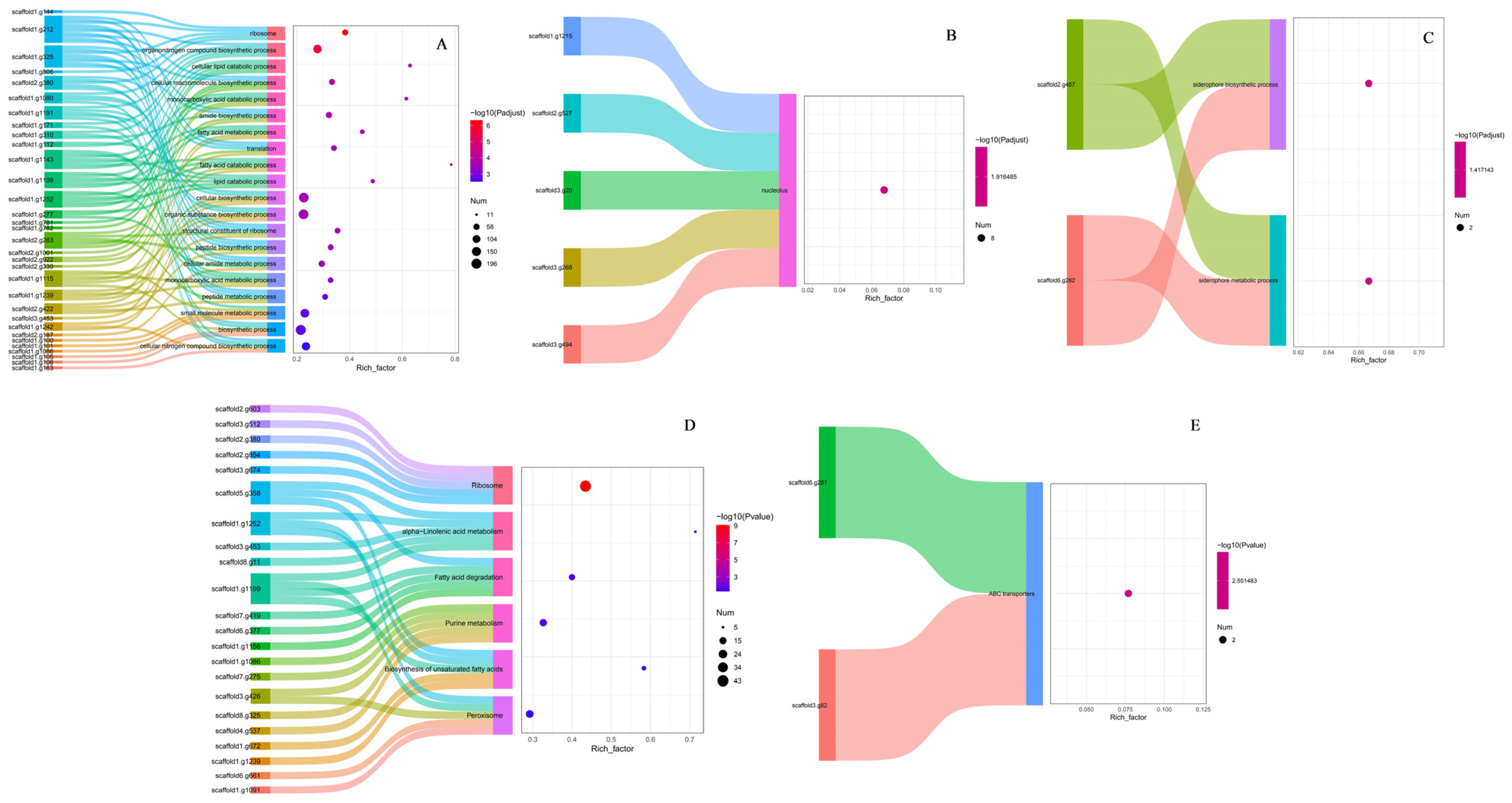
3.6.2. EggNOG, GO, and KEGG Functional Annotation Analysis of Differentially Expressed Genes in Transformed Strains
3.6.3. GO and KEGG Functional Enrichment Analysis of Differentially Expressed Genes in Transformed Strains
3.7. Metabolomic Analysis Results of the Molecular Mechanism of High MK Production in Transformed Strains
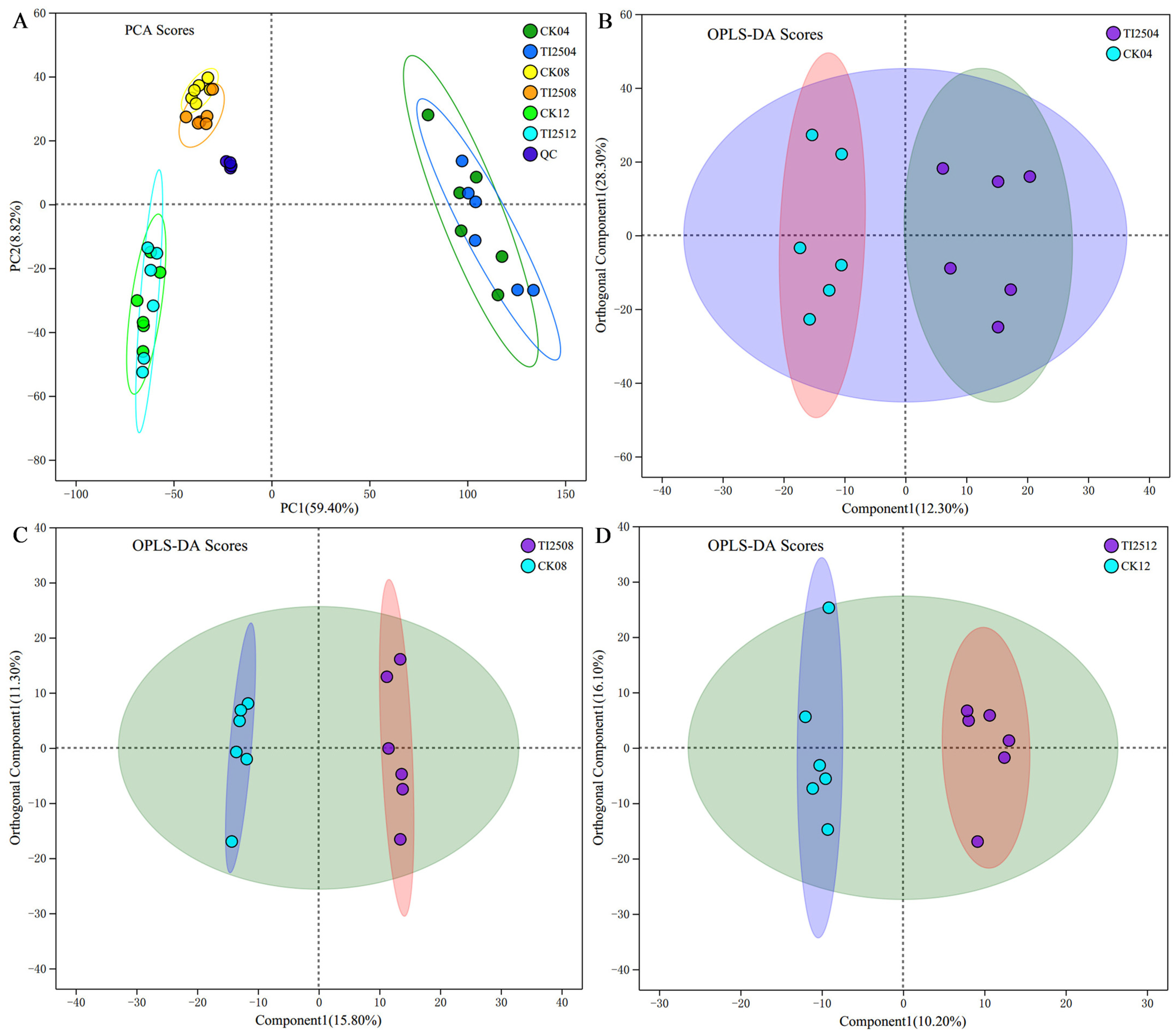
3.7.1. Metabolome Detection Results and Multivariate Statistical Analysis of RYR Samples
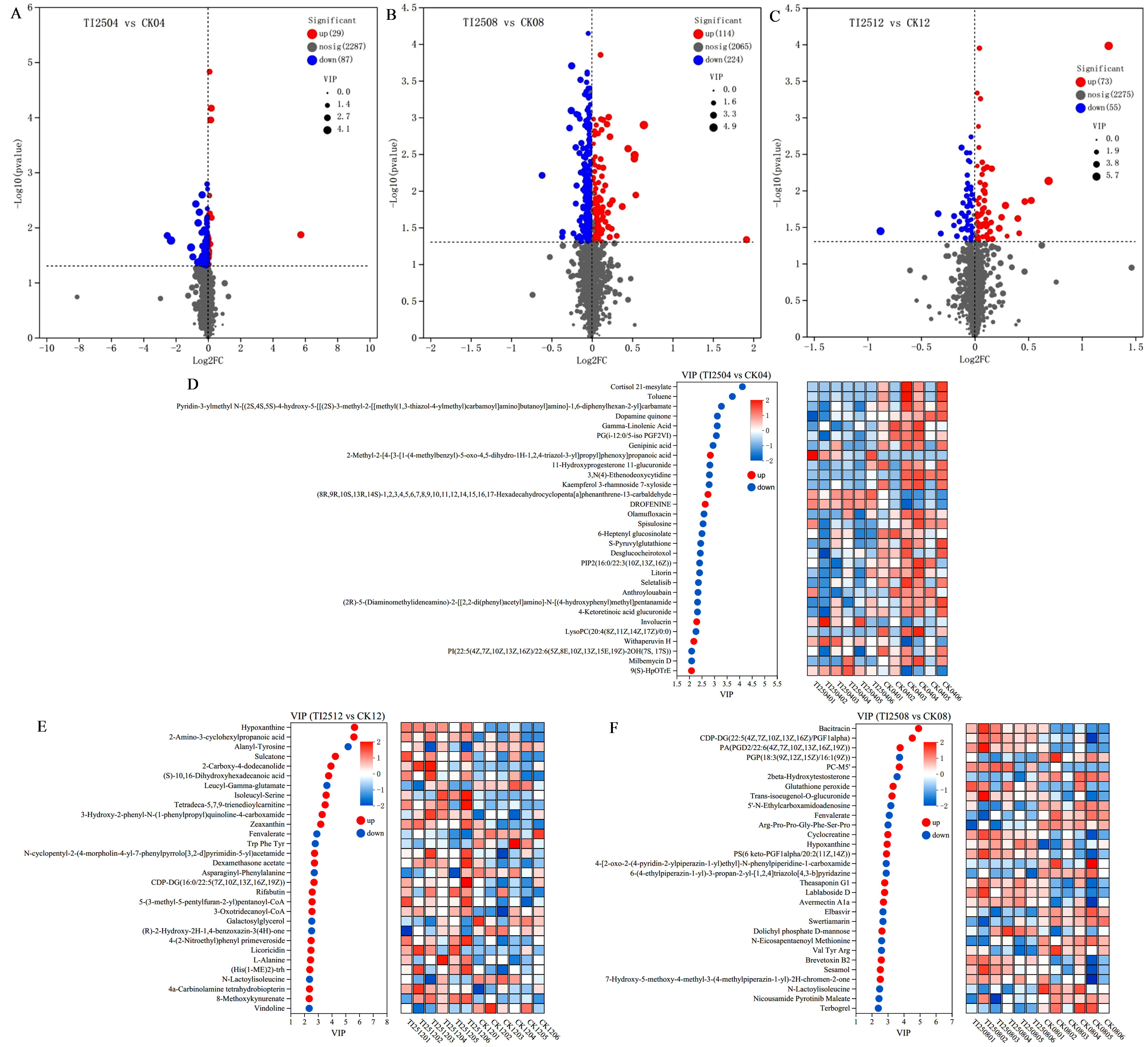
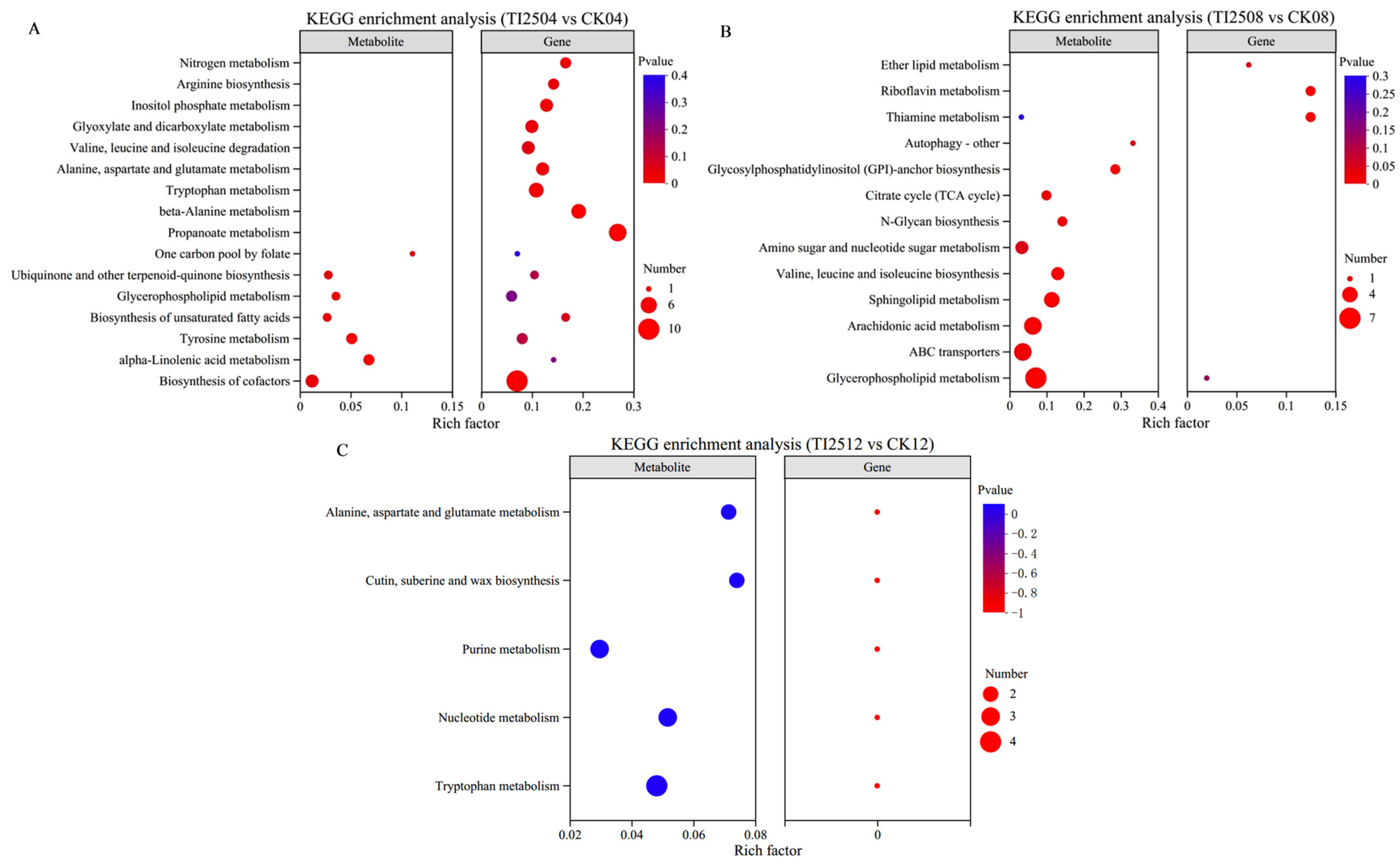
3.7.2. Differential Metabolites and Pathway Enrichment Analysis of RYR Samples
3.8. Multi-Omics Joint Analysis Results of the Molecular Mechanism of High MK Production in Transformed Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dikshit, R.; Tallapragada, P. Statistical optimization of pigment production by Monascus sanguineus under stress condition. Prep. Biochem. Biotech. 2014, 44, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Long, P.C.; Zhu, L.S.; Lai, H.F.; Xu, S.Y.; Dong, X.X.; Shao, Y.C.; Wang, L.L.; Cheng, S.Y.; Liu, G.; He, J.R.; et al. Monascus red pigment liposomes: Microstructural characteristics, stability, and anticancer activity. Foods 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Pan, Q.L.; Xue, Y.N.; Dong, Y.P.; Chen, Y.H.; Huang, L.G.; Zhang, B.; Liu, Z.Q.; Zheng, Y.G. Synthetic biology for Monascus: From strain breeding to industrial production. Biotechnol. J. 2024, 19, e2400180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.B.; Guo, R.; Guo, W.L.; Hong, J.L.; Li, L.; Ni, L.; Sun, J.Y.; Liu, B.; Rao, P.F.; Lv, X.C. Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high-fat diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Chen, M.; Guo, W.L.; Li, T.T.; Liu, B.; Bai, W.D.; Ai, L.Z.; Rao, P.F.; Ni, L.; Lv, X.C. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020, 136, 109511. [Google Scholar] [CrossRef]
- Huang, Z.W.; Chen, L.C.; Xiao, L.S.; Ye, Y.F.; Mo, W.L.; Zheng, Z.H.; Li, X.Y. Monascus-fermented quinoa alleviates hyperlipidemia in mice by regulating the amino acid metabolism pathway. Food Funct. 2024, 15, 9210–9223. [Google Scholar] [CrossRef]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biot. 2013, 40, 169–181. [Google Scholar] [CrossRef]
- Buzzelli, L.; Segreti, A.; Di Gioia, D.; Lemme, E.; Squeo, M.R.; Nenna, A.; Di Gioia, G. Alternative lipid lowering strategies: State-of-the-art review of red yeast rice. Fitoterapia 2024, 172, 105719. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Toth, P.P. Red yeast rice for the improvement of lipid profiles in mild-to-moderate hypercholesterolemia: A narrative review. Nutrients 2023, 15, 2288. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.A.; Al-Zahrani, H.A.A.; El-Bondkly, A.M.A. Genome shuffling of mangrove endophytic Aspergillus luchuensis MERV10 for improving the cholesterol-lowering agent lovastatin under solid state fermentation. Mycobiology 2016, 44, 171–179. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yuan, G.F.; Hsieh, S.Y.; Lin, Y.S.; Wang, W.Y.; Liaw, L.L.; Tseng, C.P. Identification of the mok H gene encoding transcription factor for the upregulation of monacolin K biosynthesis in Monascus pilosus. J. Agric. Food Chem. 2010, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.W.; Hong, H.S. Research progress on gene synthesis and anticancer and lipid-lowering mechanism of monacolin K. Anti-Cancer Agent. Me. 2023, 23, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Li, M.; Huang, Q.M.; Xie, L.M.; Huang, Z.B. Monacolin K induces apoptosis of human glioma U251 cells by triggering ROS-mediated oxidative damage and regulating MAPKs and NF-κB Pathways. Acs Chem. Neurosci. 2023, 14, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Chen, Z.T.; Wen, Q.Y.; Xiong, Z.X.; Cao, X.H.; Zheng, Z.H.; Zhang, Y.X.; Huang, Z.W. An overview on the biosynthesis and metabolic regulation of monacolin K/lovastatin. Food Funct. 2020, 11, 5738–5748. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Gonzalez, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biot. 2010, 85, 869–883. [Google Scholar] [CrossRef]
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhaes, B.S.; Parachin, N.S. Lovastatin production: From molecular basis to industrial process optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef]
- Chen, Y.P.; Tseng, C.P.; Liaw, L.L.; Wang, C.L.; Chen, I.C.; Wu, W.J.; Wu, M.D.; Yuan, G.F. Cloning and characterization of monacolin K biosynthetic gene cluster from Monascus pilosus. J. Agric. Food Chem. 2008, 56, 5639–5646. [Google Scholar] [CrossRef]
- Sakai, K.; Kinoshita, H.; Nihira, T. Identification of mok B involved in monacolin K biosynthesis in Monascus pilosus. Biotechnol. Lett. 2009, 31, 1911–1916. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, J.; Zhang, A.A.; Hao, S.; Zhang, H.; Zhu, Q.Q.; Sun, B.G.; Wang, C.T. Overexpression of monacolin K biosynthesis genes in the Monascus purpureus azaphilone polyketide pathway. J. Agric. Food Chem. 2019, 67, 2563–2569. [Google Scholar] [CrossRef]
- Huang, X.N.; Tang, S.; Zheng, L.H.; Teng, Y.; Yang, Y.; Zhu, J.W.; Lu, X.F. Construction of an efficient and robust Aspergillus terreus cell factory for monacolin J production. Acs Synth. Biol. 2019, 8, 818–825. [Google Scholar] [CrossRef]
- Li, J.H.; Jia, C.J.; Lu, Q.H.; Hungate, B.A.; Dijkstra, P.; Wang, S.Q.; Wu, C.Y.; Chen, S.H.; Li, D.Q.; Shim, H. Mechanistic insights into the success of xenobiotic degraders resolved from metagenomes of microbial enrichment cultures. J. Hazard. Mater. 2021, 418, 126384. [Google Scholar] [CrossRef] [PubMed]
- Boulant, E.; Cambon, E.; Vergalli, J.; Bernard, R.; Neulat-Ripoll, F.; Nolent, F.; Gorgé, O.; Girleanu, M.; Favier, A.; Leonetti, J.; et al. Tolerance engineering in Deinococcus geothermalis by heterologous efflux pumps. Sci. Rep-Uk 2021, 11, 4280. [Google Scholar] [CrossRef] [PubMed]
- Vasylkivska, M.; Patakova, P. Role of efflux in enhancing butanol tolerance of bacteria. J. Biotechnol. 2020, 320, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ley, A.; Coumou, H.C.; Frandsen, R.J.N. Heterologous expression of mlc E in Saccharomyces cerevisiae provides resistance to natural and semi-synthetic statins. Metab. Eng. Commun. 2015, 2, 117–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, X.K.; Guo, T.L.; Xu, X.Q.; Lin, J. Multiplex metabolic pathway engineering of Monascus pilosus enhances lovastatin production. Appl. Microbiol. Biotechnol. 2023, 107, 6541–6552. [Google Scholar] [CrossRef]
- Xie, L.; Xie, J.H.; Chen, X.X.; Tao, X.; Xie, J.Y.; Shi, X.Y.; Huang, Z.B. Comparative transcriptome analysis of Monascus purpureus at different fermentation times revealed candidate genes involved in exopolysaccharide biosynthesis. Food Res. Int. 2022, 160, 111700. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, M.; Gong, Z.H.; Liu, X.Y.; Zhang, C.Y.; Liang, Y.; Lin, Q.L.; Zhou, B.; Guo, T.; Liu, J. Enhancing extracellular Monascus pigment production in submerged fermentation with engineered microbial consortia. Food Microbiol. 2024, 121, 104499. [Google Scholar] [CrossRef]
- Zhu, L.S.; Long, P.C.; Hu, M.; Wang, L.L.; Shao, Y.C.; Cheng, S.Y.; Dong, X.X.; He, Y. Insight into selenium biofortification and the selenite metabolic mechanism of Monascus ruber M7. Food Chem. 2024, 455, 139740. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Science Press: Beijing, China, 2017. [Google Scholar]
- Liu, M.M.; Zeng, X.; Xie, J.; Liang, Y.M.; Tao, J.J.; Qin, T.Q.; Long, C.N.; Zeng, B. Heterologous expression of the lytic polysaccharide monooxygenasesgene AoAA13 gene to improve Monascus pigment production in Monascus ruber. J. Jiangxi Sci. Technol. Norm. Univ. 2018, 6, 75–81. (In Chinese) [Google Scholar]
- Zhang, C.; Chai, S.Y.; Hao, S.; Zhang, A.A.; Zhu, Q.Q.; Zhang, H.; Wang, C.T. Effects of glutamic acid on the production of monacolin K in four high-yield monacolin K strains in Monascus. Appl. Microbiol. Biot. 2019, 103, 5301–5310. [Google Scholar] [CrossRef]
- Long, C.N.; Zeng, X.; Xie, J.; Liang, Y.M.; Tao, J.J.; Tao, Q.Q.; Liu, M.M.; Cui, J.J.; Huang, Z.W.; Zeng, B. High-level production of Monascus pigments in Monascus ruber CICC41233 through ATP-citrate lyase overexpression. Biochem. Eng. J. 2019, 146, 160–169. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ye, F.Y.; Zhou, B.; Liang, Y.; Lin, Q.L.; Lu, D.; Zhou, X.; Liu, J. Comparative analysis of different rice substrates for solid-state fermentation by a citrinin-free Monascus purpureus mutant strain with high pigment production. Food Biosci. 2023, 56, 103245. [Google Scholar] [CrossRef]
- Huang, Z.W.; Lin, J.C.; Cheng, Z.X.; Xu, M.; Huang, X.Y.; Yang, Z.J.; Zheng, J.G. Production of dammarane-type sapogenins in rice by expressing the dammarenediol-II synthase gene from Panax ginseng C.A. Mey. Plant Sci. 2015, 239, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, C.L.; Li, Z.J.; Chen, M.H.; Wu, S.F.; Ren, Z.Y. Effect of mok E overexpression on monacolin K production and morphology of mycelia and spores in Monascus. Food Sci. 2018, 39, 45–49. [Google Scholar]
- Han, C.; Shi, C.P.; Liu, L.M.; Han, J.C.; Yang, Q.Q.; Wang, Y.; Li, X.D.; Fu, W.Y.; Gao, H.; Huang, H.S.; et al. Majorbio Cloud 2024: Update single-cell and multiomics workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef]
- Yang, X.L.; Xiang, L.B.; Zhang, C.; Cao, Y.P.; Wang, C.T. Promotion of monacolin K production in Monascus extractive fermentation: The variation in fungal morphology and in the expression levels of biosynthetic gene clusters. J. Sci. Food Agric. 2021, 101, 5652–5659. [Google Scholar] [CrossRef]
- Shi, R.Y.; Luo, Q.Q.; Liu, Y.T.; Meng, G.N.; Chen, W.; Wang, C.T. Effect of γ-butyrolactone, a quorum sensing molecule, on morphology and secondary metabolism in Monascus. Lwt-Food Sci. Technol. 2022, 172, 114225. [Google Scholar] [CrossRef]
- Li, S.F.; Cai, Q.H.; Liu, Q.R.; Gong, Y.X.; Zhao, D.Q.; Wan, J.; Wang, D.J.; Shao, Y.C. Effective enhancement of the ability of Monascus pilosus to produce lipid-lowering compound monacolin K via perturbation of metabolic flux and histone acetylation modification. Food Res. Int. 2024, 195, 114961. [Google Scholar] [CrossRef]
- Rahm, J.J.; Holman, R.T. Effect of linoleic acid upon the metabolism of linolenic acid. J. Nutr. 1964, 84, 15–19. [Google Scholar] [CrossRef]
- Kraboun, K.; Rojsuntornkitti, K.; Jittrepotch, N.; Kongbangkerd, T.; Uthai, N.; Pechyen, C. Formation analysis of primary and secondary metabolites during angkak fermentation based on GC-TOF-MS, GC-FID, and HPLC and metabolomics analysis. Microbe 2023, 1, 100006. [Google Scholar] [CrossRef]
- Mei, Q.; Xu, Z.N.; Wu, Q.Y.; Qin, L.K.; Zeng, H.Y.; Zhu, Y. Analysis of metabolites of coix seed fermented by Monascus purpureus. Food Biosci. 2022, 50, 102054. [Google Scholar] [CrossRef]
- Qiao, J.; Zeng, H.W.; Ye, W.J.; Qiu, R.; Zeng, X.; Xin, B.Y.; Xie, T.Y. Integrative addition of sucrose esters and immobilisation technology for enhancing yellow pigment yield of Monascus purpureus HBSD08 under submerged fermentation conditions and its molecular mechanism. Lwt-Food Sci. Technol. 2023, 186, 115233. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Xiao, L.; Mo, W.; Zhang, Y.; Cai, Y.; Huang, S.; Chen, Z.; Long, C. Molecular Mechanism of Mok I Gene Overexpression in Enhancing Monacolin K Production in Monascus pilosus. J. Fungi 2024, 10, 721. https://doi.org/10.3390/jof10100721
Huang Z, Xiao L, Mo W, Zhang Y, Cai Y, Huang S, Chen Z, Long C. Molecular Mechanism of Mok I Gene Overexpression in Enhancing Monacolin K Production in Monascus pilosus. Journal of Fungi. 2024; 10(10):721. https://doi.org/10.3390/jof10100721
Chicago/Turabian StyleHuang, Zhiwei, Lishi Xiao, Wenlan Mo, Yaru Zhang, Yiyang Cai, Simei Huang, Zhiting Chen, and Chuannan Long. 2024. "Molecular Mechanism of Mok I Gene Overexpression in Enhancing Monacolin K Production in Monascus pilosus" Journal of Fungi 10, no. 10: 721. https://doi.org/10.3390/jof10100721
APA StyleHuang, Z., Xiao, L., Mo, W., Zhang, Y., Cai, Y., Huang, S., Chen, Z., & Long, C. (2024). Molecular Mechanism of Mok I Gene Overexpression in Enhancing Monacolin K Production in Monascus pilosus. Journal of Fungi, 10(10), 721. https://doi.org/10.3390/jof10100721






