A Treasure Trove of Urban Microbial Diversity: Community and Diversity Characteristics of Urban Ancient Ginkgo biloba Rhizosphere Microorganisms in Shanghai
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
2.2. Experimental Methods
2.2.1. Soil Chemical Analyses
2.2.2. PLFA Analysis
2.2.3. High-Throughput Sequencing Methods for AMF
2.3. Data Analysis
3. Results
3.1. Soil Environmental Traits
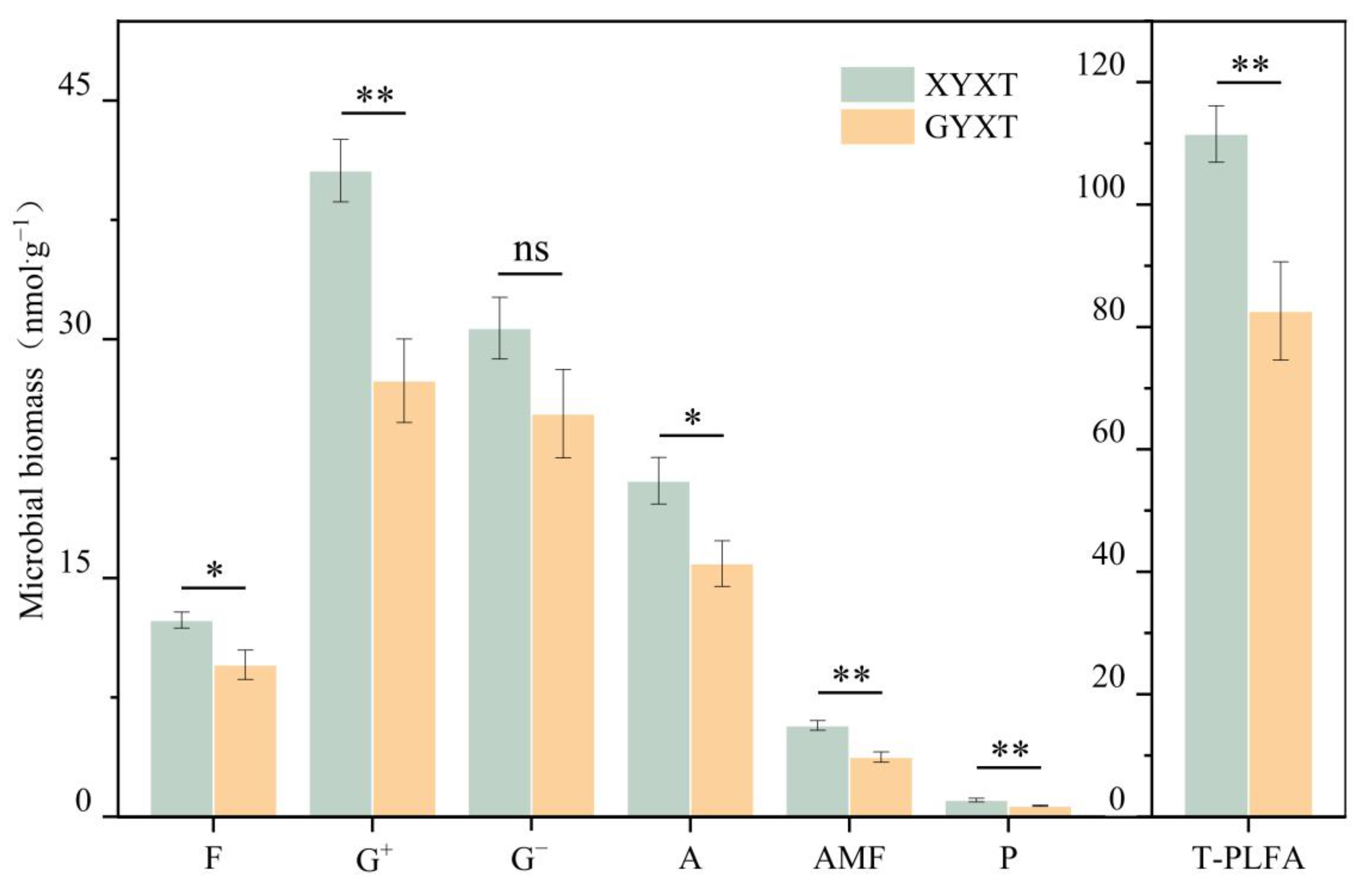
3.2. Microbial Biomass of the Rhizosphere Soil of G. biloba
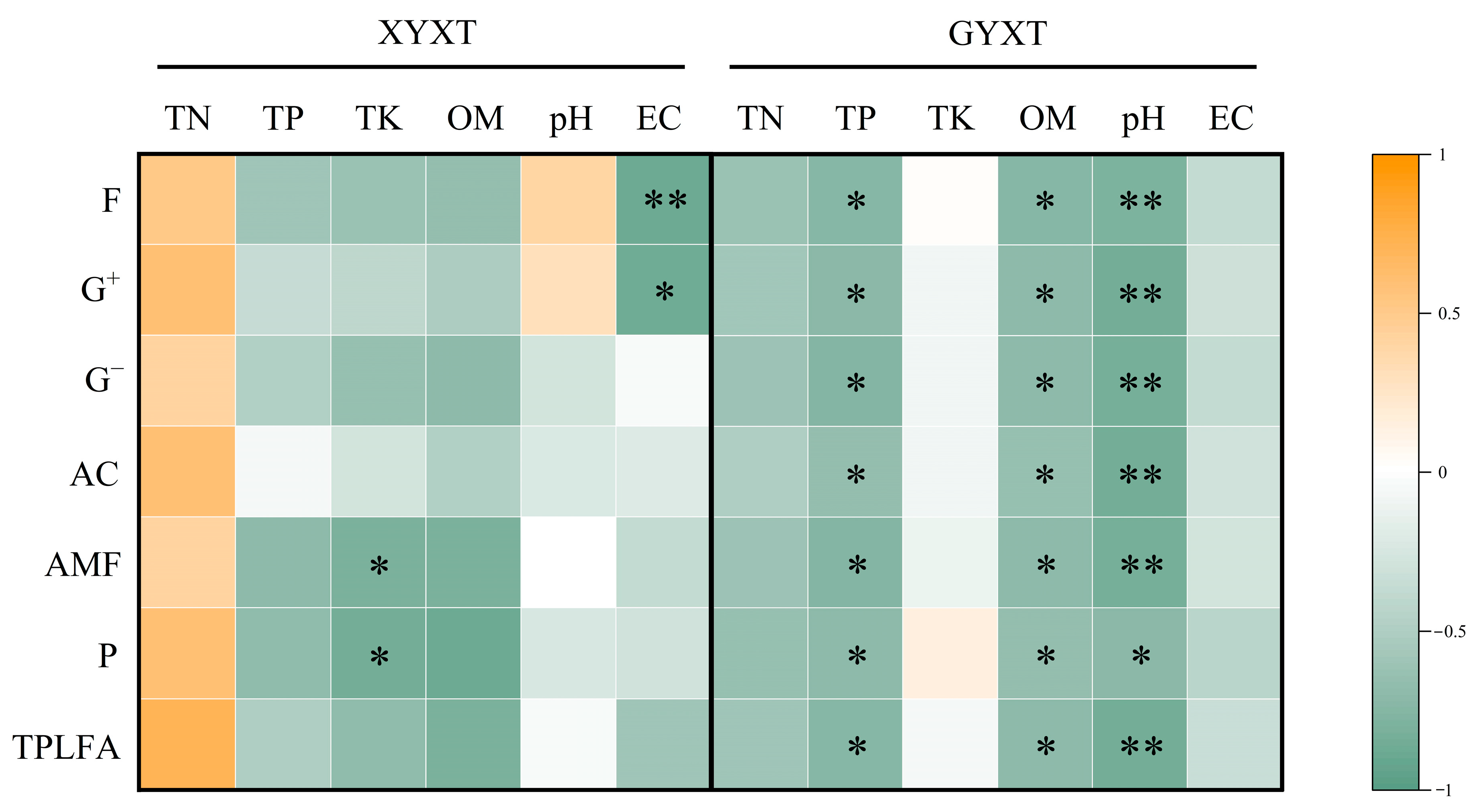
3.3. Relationship Between Soil Environmental Traits and Microbial Biomass
3.4. Analysis of the AMF Community in the Roots and Rhizosphere Soil of G. biloba
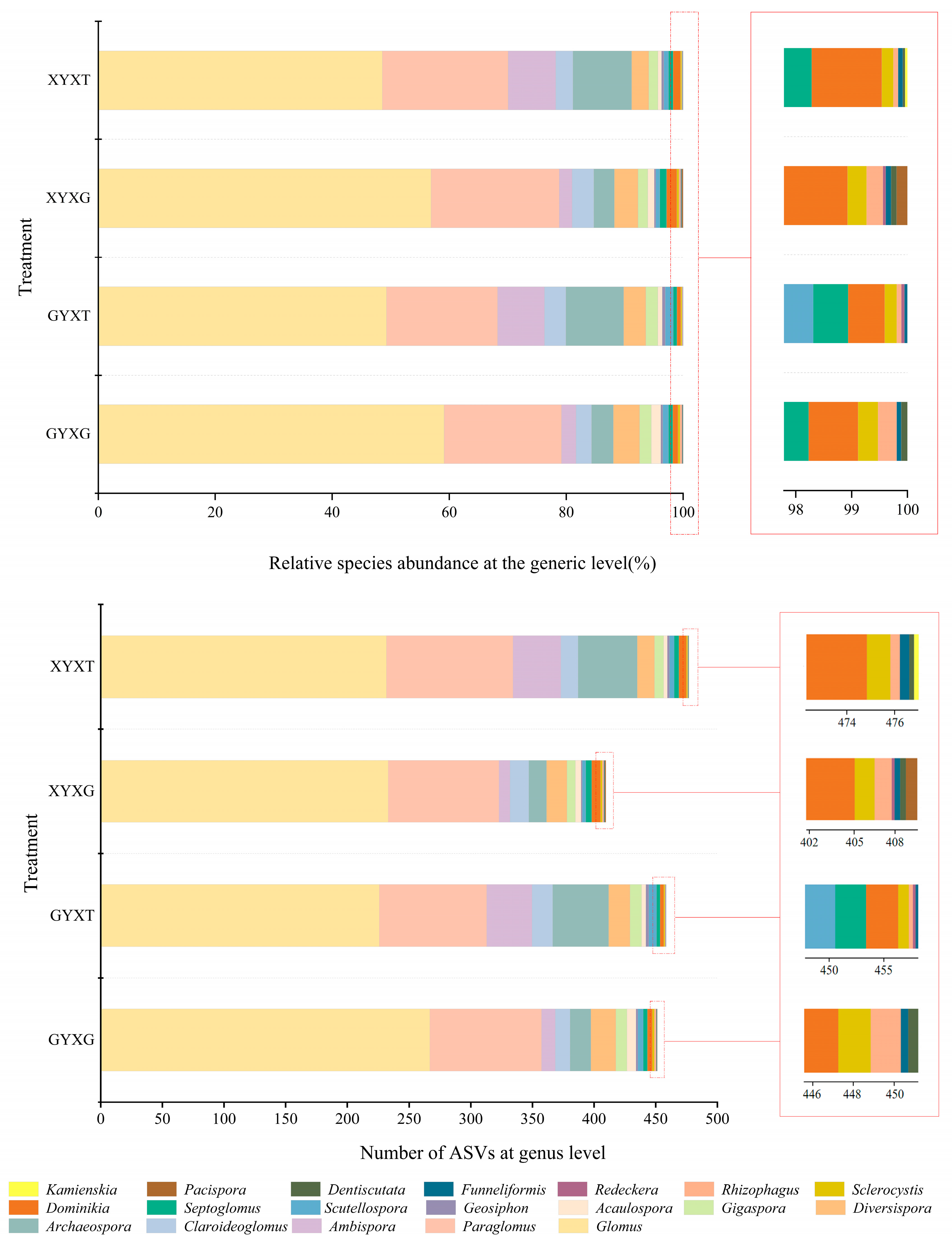
3.4.1. Composition of the AMF Community
3.4.2. Abundance Analysis of AMF Community
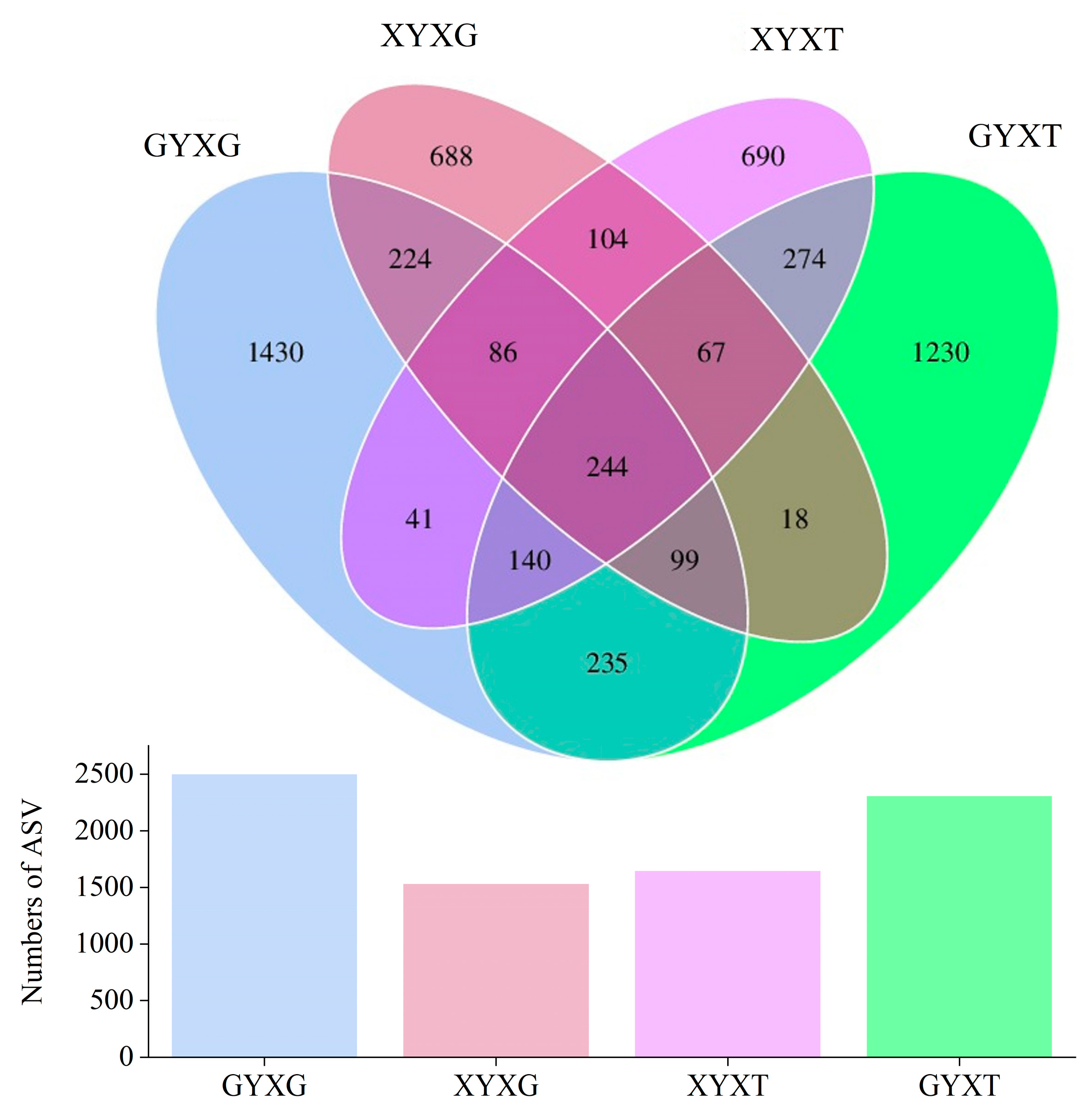
3.4.3. Venn Diagram Analysis of AMF Community
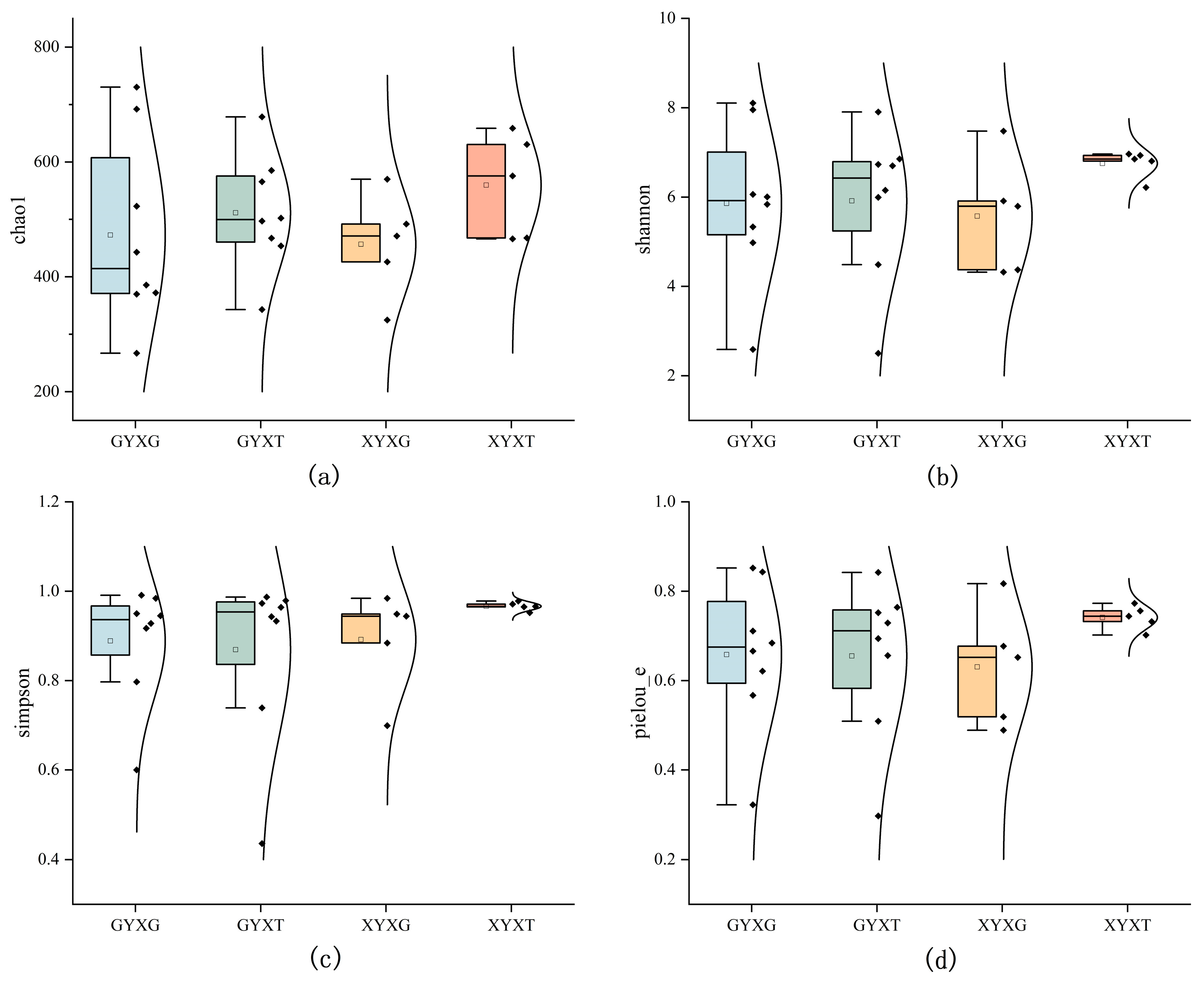
3.4.4. Diversity of AMF Community
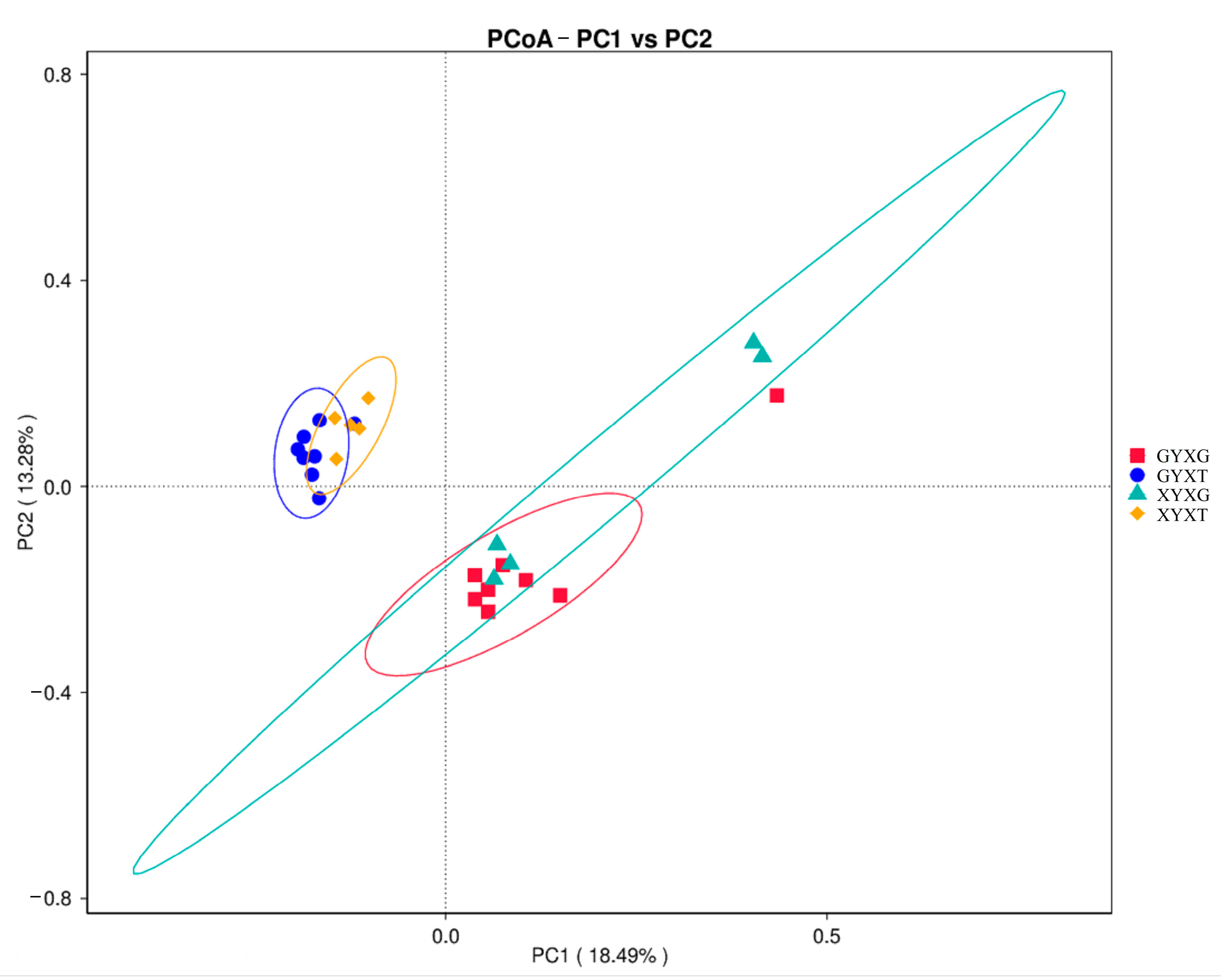
3.4.5. PCoA of the AMF Community
3.4.6. Cluster Analysis of AMF Community
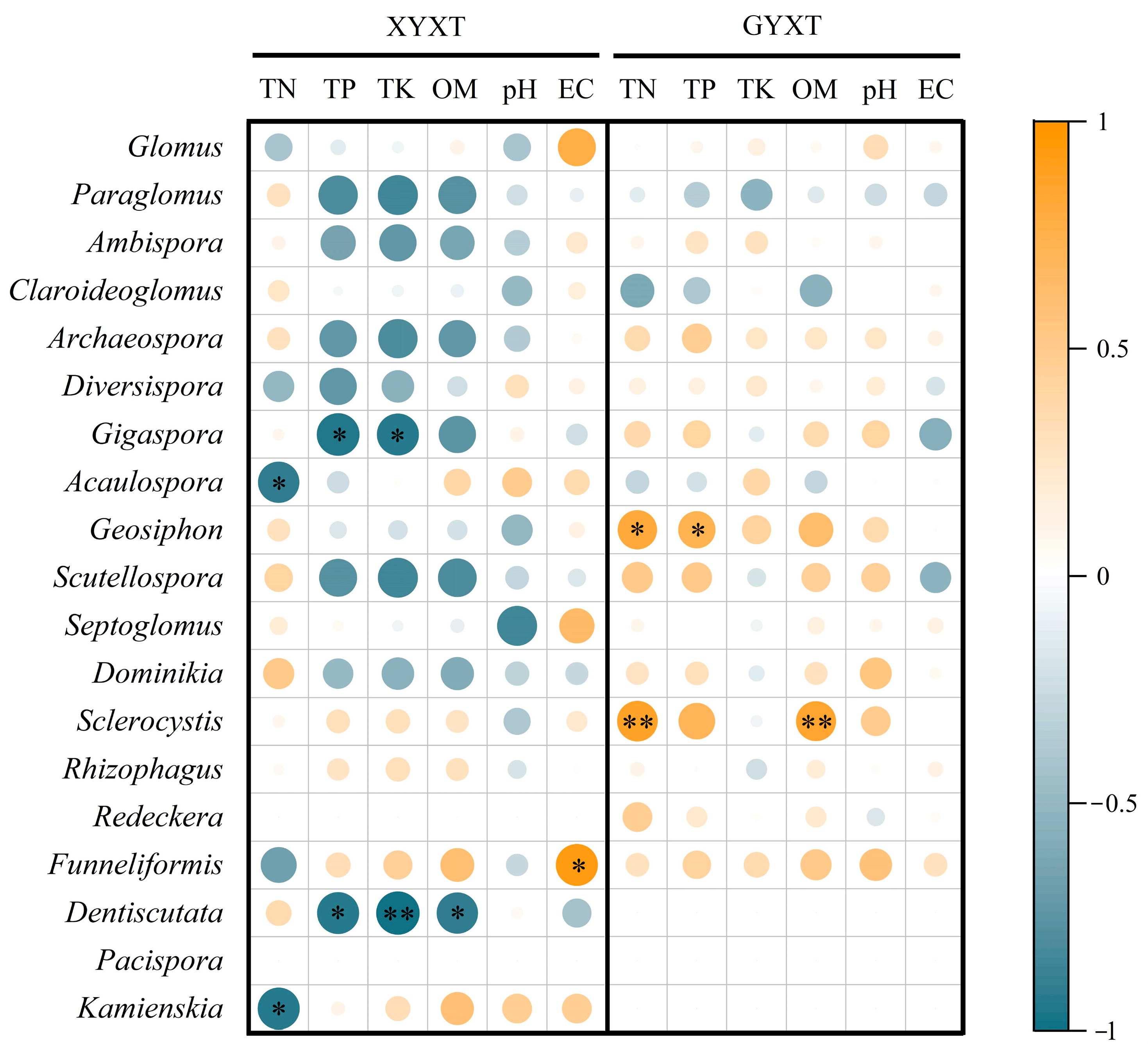
3.4.7. Relationship between Soil Environmental Traits and the AMF Community
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.J.; Lindenmayer, D.B.; Yang, W.J.; Ren, Y.; Campbell, M.J.; Wu, C.P.; Luo, Y.Q.; Zhong, L.; Yu, M.J. Diversity and density patterns of large old trees in China. Sci. Total Environ. 2019, 655, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.; Reader, T.; Gilbert, F.; Atkinson, N. Historical maps confirm the accuracy of zero-inflated model predictions of ancient tree abundance in english wood-pastures. J. Appl. Ecol. 2021, 58, 2661–2672. [Google Scholar] [CrossRef]
- Wu, C.F.; Guo, J.; Wang, G.B. Morphological and physiological responses of male and female Ginkgo biloba to temperature changes. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 150. [Google Scholar]
- Dunberg, A. Jubenility, maturation, ageing and rejuvenation in woodyplants. In Vegetative Propagation of Forest Tree-Physiology and Practice; Symp: Uppsala, Sweden, 1977; pp. 55–64. [Google Scholar]
- Liang, Z.J.; Le, X.W.; Li, X.S. Research on health evaluation index system of ancient Ginkgo biloba in Shanghai based on analytic hierarchy process. For. Environ. Sci. 2023, 39, 89–96. [Google Scholar]
- Comer, J.; Perkins, L. Resistance of the soil microbial community to land-surface disturbances of high-intensity winter grazing and wildfire. J. Environ. Manag. 2021, 279, 111596. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.J.; Jiang, Z.F.; Wu, Y.H.; Xu, M.J.; Ni, J. High-throughput sequence analysis reveals variation in the relative abundance of components of the bacterial and fungal microbiota in the rhizosphere of Ginkgo biloba. PeerJ 2019, 7, e8051. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. Structural diversity in (vesicular)–arbuscular mycorrhizal symbioses. New Phytol. 1997, 137, 373–388. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Fontana, A. Vesicular-Arbuscular mycorrhizas of Ginkgo biloba L. in natural and controlled conditions. New Phytol. 1985, 99, 441–447. [Google Scholar] [CrossRef]
- Wu, Q.S.; Qa, W. Arbuscular mycorrhizae of Ginkgo biloba and its correlation with soil available phosphorus. J. Yangtze Univ. (Nat. Sci. Ed.) Agric. Sci. Vol. 2008, 3, 49–51+112. [Google Scholar]
- Yuan, Z.M.; Cheng, X.F.; Wang, J.P.; Zhu, L.J.; Ma, S.L.; Zhang, J.C.; Chu, D.S. Effects of edaphic factors on arbuscular mycorrhizal fungi colonization in muddy coastal area of northern Jiangsu province. J. Northeast. For. Univ. 2019, 47, 123–129. [Google Scholar] [CrossRef]
- Soares, C.R.F.S.; Siqueira, J.O. Mycorrhiza and phosphate protection of tropical grass species against heavy metal toxicity in multi-contaminated soil. Biol. Fertil. Soils 2008, 44, 833–841. [Google Scholar] [CrossRef]
- Huang, Z.; Zou, Z.R.; He, C.X.; He, Z.Q.; Zhang, Z.B.; Li, J.M. Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 2011, 339, 391–399. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.L.; Liu, Y.J.; Yi, Q.; You, F.; Ma, Y.Y.; Thomsen, L.; Chan, T.S.; Lu, Y.R.; Hall, M.; et al. Arbuscular mycorrhizal symbiosis enhances water stable aggregate formation and organic matter stabilization in Fe ore tailings. Geoderma 2022, 406, 115528. [Google Scholar] [CrossRef]
- Pauwels, R.; Graefe, J.; Bitterlich, M. An arbuscular mycorrhizal fungus alters soil water retention and hydraulic conductivity in a soil texture specific way. Mycorrhiza 2023, 33, 165–179. [Google Scholar] [CrossRef]
- Tang, Y.H.; Le, X.W.; Liu, J.L. Research on endogenous mycorrhizal fungi to Shanghai ancient Ginkgo soil function. Shanghai Constr. Sci. Technol. 2021, 93, 113–115. [Google Scholar]
- Labidi, S.; Ben Jeddi, F.; Tisserant, B.; Yousfi, M.; Sanaa, M.; Dalpe, Y.; Sahraoui, A.L.-H. Field application of mycorrhizal bio-inoculants affects the mineral uptake of a forage legume (Hedysarum coronarium L.) on a highly calcareous soil. Mycorrhiza 2015, 25, 297–309. [Google Scholar] [CrossRef]
- Gao, X. Research Progress on the Identification Methodsof the Age of Ancient Trees. J. Green Sci. Technol. 2023, 25, 163–168+172. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of soil microbial communities: Effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Jia, P.L.; Wang, Y.; Wang, H.L.; Li, C.C.; An, S.S. The local environment regulates biogeographic patterns of soil fungal communities on the Loess Plateau. Catena 2019, 183, 104220. [Google Scholar] [CrossRef]
- Willers, C.; van Rensburg, P.J.J.; Claassens, S. Phospholipid fatty acid profiling of microbial communities-a review of interpretations and recent applications. J. Appl. Microbiol. 2015, 119, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.D.; Wang, W.; Zeng, H. Application of phospholipid fatty acid method in analyzing soil microbial community composition. Microbiol. China 2016, 43, 2086–2095. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Abe, B.T.; Wesselhoeft, R.A.; Chen, R.; Anderson, D.G.; Chang, H.Y. Circular RNA migration in agarose gel electrophoresis. Mol. Cell 2022, 82, 1768–1777.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, P.F.; Wang, C.; Chen, J.; Wang, X.; Hu, B.; Yuan, Q.S. Ecological insights into the disturbances in bacterioplanktoncommunities due to emerging organic pollutants from differentanthropogenic activities along an urban river. Sci. Total Environ. 2021, 796, 148973. [Google Scholar] [CrossRef] [PubMed]
- Lumini, E.; Orgiazzi, A.; Borriello, R.; Bonfante, P.; Bianciotto, V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ. Microbiol. 2010, 12, 2165–2179. [Google Scholar] [CrossRef]
- Lin, X.G.; Feng, Y.Z.; Zhang, H.Y.; Chen, R.R.; Wang, J.H.; Zhang, J.B.; Chu, H.Y. Long-Term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in north China revealed by 454 pyrosequencing. Environ. Sci. Technol. 2012, 46, 5764–5771. [Google Scholar] [CrossRef]
- Bi, Y.L.; Xiao, L.; Guo, C.; Christie, P. Revegetation type drives rhizosphere arbuscular mycorrhizal fungi and soil organic carbon fractions in the mining subsidence area of northwest China. Catena 2020, 195, 104791. [Google Scholar] [CrossRef]
- Li, M.J.; Shao, D.T.; Zhou, J.C.; Gu, J.H.; Qin, J.J.; Chen, W.; Wei, W.Q. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2020, 32, 755–767. [Google Scholar] [CrossRef]
- Koskey, G.; Avio, L.; Turrini, A.; Cristiana, S.; Paolo, B. Durum wheat-lentil relay intercropping enhances soil mycorrhizal activity but does not alter structure of arbuscular mycorrhizal fungal community within roots. Agric. Ecosyst. Environ. 2023, 357, 108696. [Google Scholar] [CrossRef]
- John, D.; Mari, M.; Marina, S.; Binte, A.S.; Talaat, A.; Asem, A.A.; Juha, M.A.; Saleh, A.-Q.; Elena, A.; Sten, A.; et al. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Davison, J. Uniting species-and community-oriented approaches to understand arbuscular mycorrhizal fungal diversity. Fungal Ecol. 2016, 24, 106–113. [Google Scholar] [CrossRef]
- Deng, J.J.; Yu, D.P.; Zhou, W.M.; Li, Z.; Zhu, W.X. Variations of Phyllosphere and Rhizosphere Microbial Communities of Pinus koraiensis Infected by Bursaphelenchus xylophilus. Microb. Ecol. 2022, 84, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, F.; Wang, Q.; Luo, J.; Zhang, Q.; Pan, Y.Y.; Wu, C.L.; Liu, W. The Effect of Different Remediation Treatments on Soil Fungal Communities in Rare Earth Tailings Soil. Forests 2022, 13, 1987. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Fu, X.Q.; Huang, W.X. Analysis on microbial diversity in the rhizosphere of Nanfeng tangerine of different tree-age. Acta Hortic. Sin. 2014, 41, 631–640. [Google Scholar] [CrossRef]
- de Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Hammesfahr, U.; Heuer, H.; Manzke, B.; Smalla, K.; Thiele-Bruhn, S. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biol. Biochem. 2008, 40, 1583–1591. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Acea, M.J.; Carballas, T. Seasonal changes in microbial biomass and nutrient flush in forest soils. Biol. Fertil. Soils 1995, 19, 220–226. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Liu, G.Q.; Bian, X.M.; Zhang, W.; Li, S. Eficets of long-term continuous cropping of colon and retuming cotton stalk into field on soil biological activities. Chin. J. Appl. Ecol. 2008, 19, 1027–1032. [Google Scholar]
- Cai, X.B.; Feng, G.; Qian, C.; Gai, J.P. Effects of AM fungi on steppe plants and soil environment in Tibet plateau. Acta Pedol. Sin. 2007, 44, 63–72. [Google Scholar] [CrossRef]
- Ruan, R.J.; Wang, L.J.; Ni, J. Application of high-throughput sequencing in the research of Ginkgo biloba. J. Hangzhou Norm. Univ. (Nat. Sci. Ed.) 2021, 20, 137–142. [Google Scholar] [CrossRef]
- Pei, Y.; Yin, M.; Li, Q.H.; Zhang, Y.F.; Zhong, Y.; Chen, X.; Zhang, Y.P.; Huang, B.; Ren, Z. Diversity and community structure of arbuscular mycorrhizal fungi (AMF) in the rhizospheric soil of Panax notoginseng in different ages. Eurasian Soil Sci. 2023, 56, 329–339. [Google Scholar] [CrossRef]
- Jing, Y.B.; Li, T.; Cui, H.L.; Li, L.F.; Allen, S.C.; Chen, L.; Li, Y.P.; Zhao, Z.W. Shifts in the arbuscular mycorrhizal fungal community composition of Betula alnoides along young, middle-aged plantation and adjacent natural forest. Iforest-Biogeosci. For. 2020, 13, 447–455. [Google Scholar] [CrossRef]
- Alimi, A.A.; Ezeokoli, O.T.; Adeleke, R.; Moteetee, A. Arbuscular mycorrhizal fungal communities colonising the roots of indigenous legumes of South Africa as revealed by high-throughput DNA metabarcoding. Rhizosphere 2021, 19, 100405. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Wang, B.-T.; Jin, L.; Ruan, H.-H.; Lee, H.-G.; Jin, F.-J. Impacts of biogas slurry fertilization on arbuscular mycorrhizal fungal communities in the rhizospheric soil of poplar plantations. J. Fungi 2022, 8, 1253. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ma, K.; Cui, H.Z.; Li, G.W.; Yu, H.Q.; Jiang, Q. Correlations between arbuscular mycorrhizal fungi and distribution of main grassland types in Ningxia. Acta Pratacult. Sin. 2020, 29, 150–160. [Google Scholar]
- Gao, T.T.; Wang, Z.K.; He, Y.L.; Li, G.F.; Lv, X.H.; Zhuang, L. Comparative study of rhizospheric arbuscular mycorrhizal fungi from three medicinal licorice plants of Xinjiang. Acta Bot. Boreali-Occident. Sin. 2021, 41, 643–653. [Google Scholar]
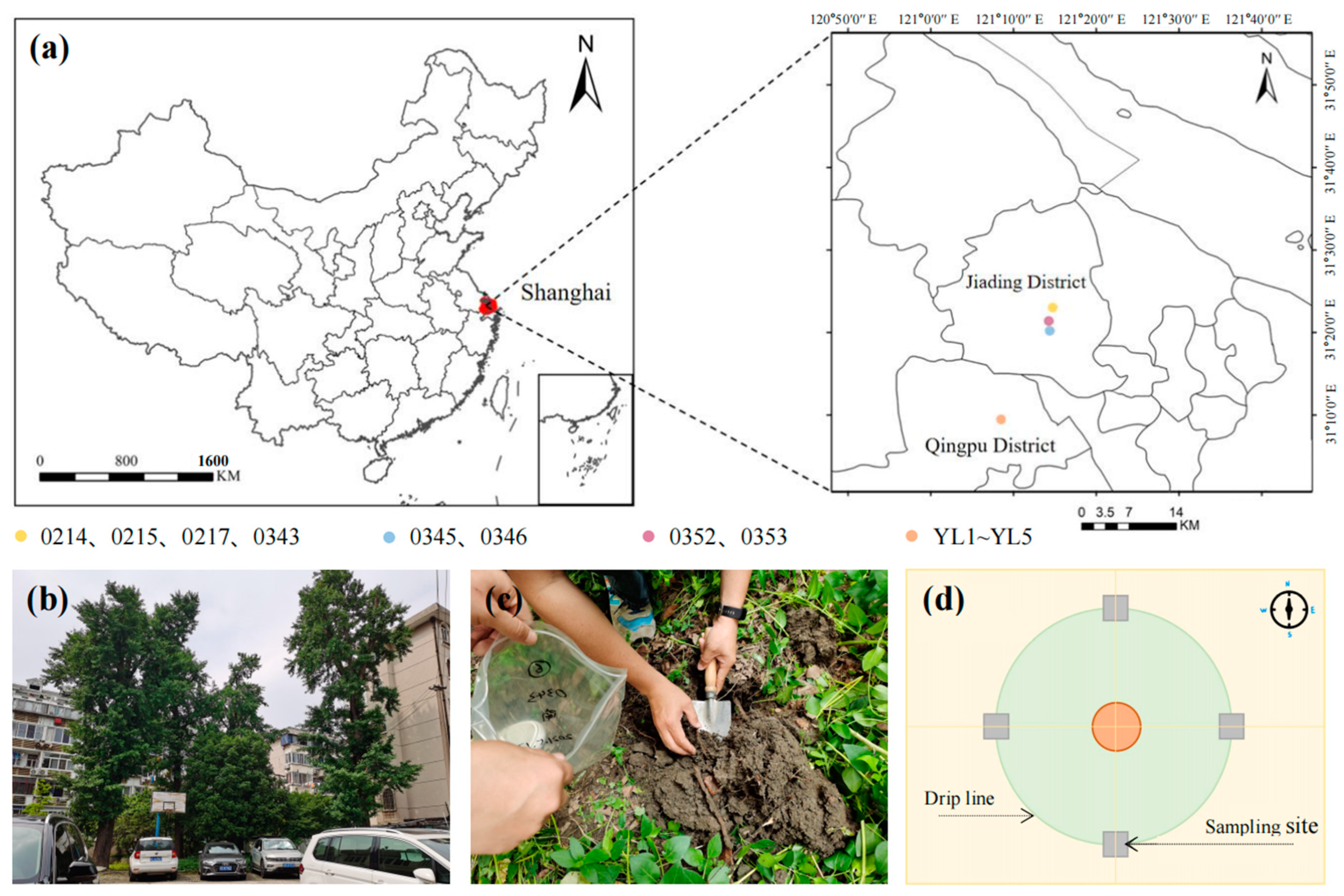

| Category | Number | Tree Age/Years | Longitude and Latitude | Site | Growth Status | Growth Environment Characteristics |
|---|---|---|---|---|---|---|
| Ancient G. biloba | 0214 | 250 | 31°23′3″ N, 121°14′46″ E | Liuyi Community | normal | Within the green area, there is a cluster of ancient trees, surrounded by residential areas and parking lots. There is a considerable amount of construction and household waste in the vicinity. |
| 0215 | 250 | normal | ||||
| 0217 | 250 | normal | ||||
| 0343 | 180 | normal | ||||
| 0345 | 180 | 31°20′37″ N, 121°14′11″ E | Ziqi Donglai Park | normal | In the park, grass is regularly removed under the trees, and the surrounding area is covered with white clover vegetation. | |
| 0346 | 180 | normal | ||||
| 0352 | 180 | 31°21′24″ N, 121°14′6″ E | Near Shengxin Road Suspension Binhai Bridge | normal | Lakeside area with exposed ground, protected by a fence. | |
| 0353 | 180 | normal | ||||
| Adult G. biloba | YL1 | 50 | 31°9′14′′ N, 121°8′35″ E | Hua Le Road | normal | Adjacent to the main urban thoroughfare, limited growing space. |
| YL2 | 50 | normal | ||||
| YL3 | 50 | normal | ||||
| YL4 | 50 | normal | ||||
| YL5 | 50 | normal |
| Characterization of Microbial Taxa | PLFA Species |
|---|---|
| Fungi (F) | 18:1ω9c, 18:2ω6c, and 18:3ω6c |
| Gram-positive bacteria (G+) | i14:0, i15:0, a15:0, i16:0, a17:0, and i17:0 |
| Gram-negative bacteria (G−) | 16:1ω7c, cy 17:0c, 18: 1ω7c, and cy 19:0c |
| Actinomycetes (A) | 10Me16:0, 10Me17:0, and 10Me18:0 |
| Arbuscular mycorrhizal fungi (AMF) | 16:1ω5c |
| Protozoa (P) | 20:3ω6c and 20:4ω6c |
| Soil | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | OM (g·kg−1) | pH | EC (mS·cm−1) |
|---|---|---|---|---|---|---|
| XYXT | 1.34 ± 0.08 a | 2.02 ± 0.15 a | 4.10 ± 0.32 b | 30.85 ± 3.51 a | 8.23 ± 0.03 a | 0.17 ± 0.014 a |
| GYXT | 2.00 ± 0.28 a | 1.39 ± 0.22 b | 5.78 ± 0.19 a | 29.29 ± 4.32 a | 7.79 ± 0.05 b | 0.13 ± 0.005 b |
| Treatments | F/B | G+/G− | cy17:0c/16:1ω7c |
|---|---|---|---|
| XYXT | 0.173 ± 0.004 a | 1.346 ± 0.122 a | 0.599 ± 0.006 a |
| GYXT | 0.182 ± 0.004 a | 1.100 ± 0.027 a | 0.360 ± 0.012 b |
| Order | Family | Genus | Virtual Taxa |
|---|---|---|---|
| Glomerales | Glomeraceae | Glomus | G. microaggregatum VTX00104, G. hoi VTX00199 |
| VTX00053, VTX00066, VTX00068, VTX00070, VTX00072–VTX00074, VTX00076, VTX00077, VTX00079, VTX00080, VTX00082–VTX00084, VTX00088, VTX00089, VTX00093, VTX00096, VTX00098, VTX00109, VTX00112, VTX00120–VTX00122, VTX00124–VTX00127, VTX00137, VTX00140, VTX00143, VTX00150, VTX00151, VTX00154, VTX00156, VTX00159, VTX00166, VTX00167, VTX00174, VTX00175, VTX00178–VTX00184, VTX00186, VTX00188, VTX00189, VTX00191, VTX00194, VTX00197, VTX00200, VTX00202, VTX00206, VTX00209, VTX00212, VTX00214, VTX00216, VTX00219, VTX00223, VTX00224, VTX00234, VTX00235, VTX00253, VTX00259, VTX00270, VTX00290–VTX00292, VTX00294, VTX00301, VTX00304, VTX00305, VTX00309, VTX00312, VTX00316, VTX00317, VTX00319, VTX00322–VTX00327, VTX00329, VTX00331, VTX00333, VTX00342–VTX00344, VTX00359, VTX00360, VTX00362, VTX00364, VTX00366, VTX00368–VTX00373, VTX00382–VTX00384, VTX00386–VTX00390, VTX00392, VTX00393, VTX00395, VTX00397–VTX00399, VTX00404, VTX00409, VTX00411–VTX00413, VTX00416, VTX00419, VTX00420, VTX00422, VTX00423, VTX00426, VTX00427, VTX00432, VTX00436, VTX00437, VTX00441–VTX00443, VTX00448, VTX00452, and VTX00453 | |||
| Glomerales | Rhizophagus | R. clarus VTX00090, R. proliferus VTX00099, R. intraradices VTX00105, and R. irregularis VTX00114 | |
| Septoglomus | S. viscosum VTX00063 and S. constrictum VTX00064 | ||
| Sclerocystis | S. sinuosa VTX00069 and S. coremioides VTX00268 | ||
| Funneliformis | F. mosseae VTX00067 | ||
| Dominikia | D. indica VTX00222 | ||
| Kamienskia | K. perpusilla VTX00287 | ||
| Claroideoglomeraceae | Claroideoglomus | C. lamellosum VTX00193 and C. claroideum VTX00225 and VTX00279 | |
| VTX00055–VTX00057, VTX00237, VTX00276–VTX00278, VTX00297, VTX00340, VTX00357, VTX00358, and VTX00402 | |||
| Paraglomerales | Paraglomeraceae | Paraglomus | P. brasilianum VTX00239, P. laccatum VTX00281, and P. majewskii VTX00335 |
| VTX00001, VTX00002, VTX00308, VTX00337, VTX00348–VTX00352, VTX00375, VTX00433, VTX00435, VTX00444, and VTX00446 | |||
| Archaeosporales | Ambisporaceae | Ambispora | A. leptoticha VTX00242, A. fennica VTX00283, A. granatensis VTX00339 |
| VTX00405 | |||
| Archaeosporales | Archaeosporaceae | Archaeospora | VTX00004–VTX00009, VTX00051, VTX00338, VTX00376, and VTX00450 |
| Geosiphonaceae | Geosiphon | G. pyriformis VTX00241 | |
| Diversisporales | Diversisporaceae | Diversispora | D. epigaea VTX00061 |
| VTX00040, VTX00306, VTX00354–VTX00356, VTX00380, and VTX00401 | |||
| Redeckera | R. fulvum VTX00262 | ||
| Acaulosporaceae | Acaulospora | A. spinosa VTX00026 and A. longula VTX00028 | |
| VTX00013, VTX00015, VTX00016, VTX00227, VTX00231, VTX00272, and VTX00328 | |||
| Gigasporaceae | Scutellospora | S. dipurpurescens VTX00049, S. nodosa VTX00052, S. spinosissima VTX00254, S. projecturata VTX00260, and S. nodosa VTX00261 | |
| VTX00318 | |||
| Gigaspora | G. decipiens VTX00039 | ||
| Dentiscutata | D. heterogama VTX00255 | ||
| Pacisporaceae | Pacispora | P. scintillans VTX00284 | |
| VTX00011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Wang, Q.; Yang, Y.; Pan, F.; Zou, Z.; Su, X.; Wang, Y.; Liu, W.; Tang, Y. A Treasure Trove of Urban Microbial Diversity: Community and Diversity Characteristics of Urban Ancient Ginkgo biloba Rhizosphere Microorganisms in Shanghai. J. Fungi 2024, 10, 720. https://doi.org/10.3390/jof10100720
Mao J, Wang Q, Yang Y, Pan F, Zou Z, Su X, Wang Y, Liu W, Tang Y. A Treasure Trove of Urban Microbial Diversity: Community and Diversity Characteristics of Urban Ancient Ginkgo biloba Rhizosphere Microorganisms in Shanghai. Journal of Fungi. 2024; 10(10):720. https://doi.org/10.3390/jof10100720
Chicago/Turabian StyleMao, Jieying, Qiong Wang, Yaying Yang, Feng Pan, Ziwei Zou, Xiaona Su, Yi Wang, Wei Liu, and Yaohua Tang. 2024. "A Treasure Trove of Urban Microbial Diversity: Community and Diversity Characteristics of Urban Ancient Ginkgo biloba Rhizosphere Microorganisms in Shanghai" Journal of Fungi 10, no. 10: 720. https://doi.org/10.3390/jof10100720
APA StyleMao, J., Wang, Q., Yang, Y., Pan, F., Zou, Z., Su, X., Wang, Y., Liu, W., & Tang, Y. (2024). A Treasure Trove of Urban Microbial Diversity: Community and Diversity Characteristics of Urban Ancient Ginkgo biloba Rhizosphere Microorganisms in Shanghai. Journal of Fungi, 10(10), 720. https://doi.org/10.3390/jof10100720







