Predominance of Trichophyton soudanense as Agent of Dermatophytoses in Cape Verdean School-Age Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients and Ethical Issues
2.2. Sample and Data Collection
2.3. Culture and Identification of Dermatophytes
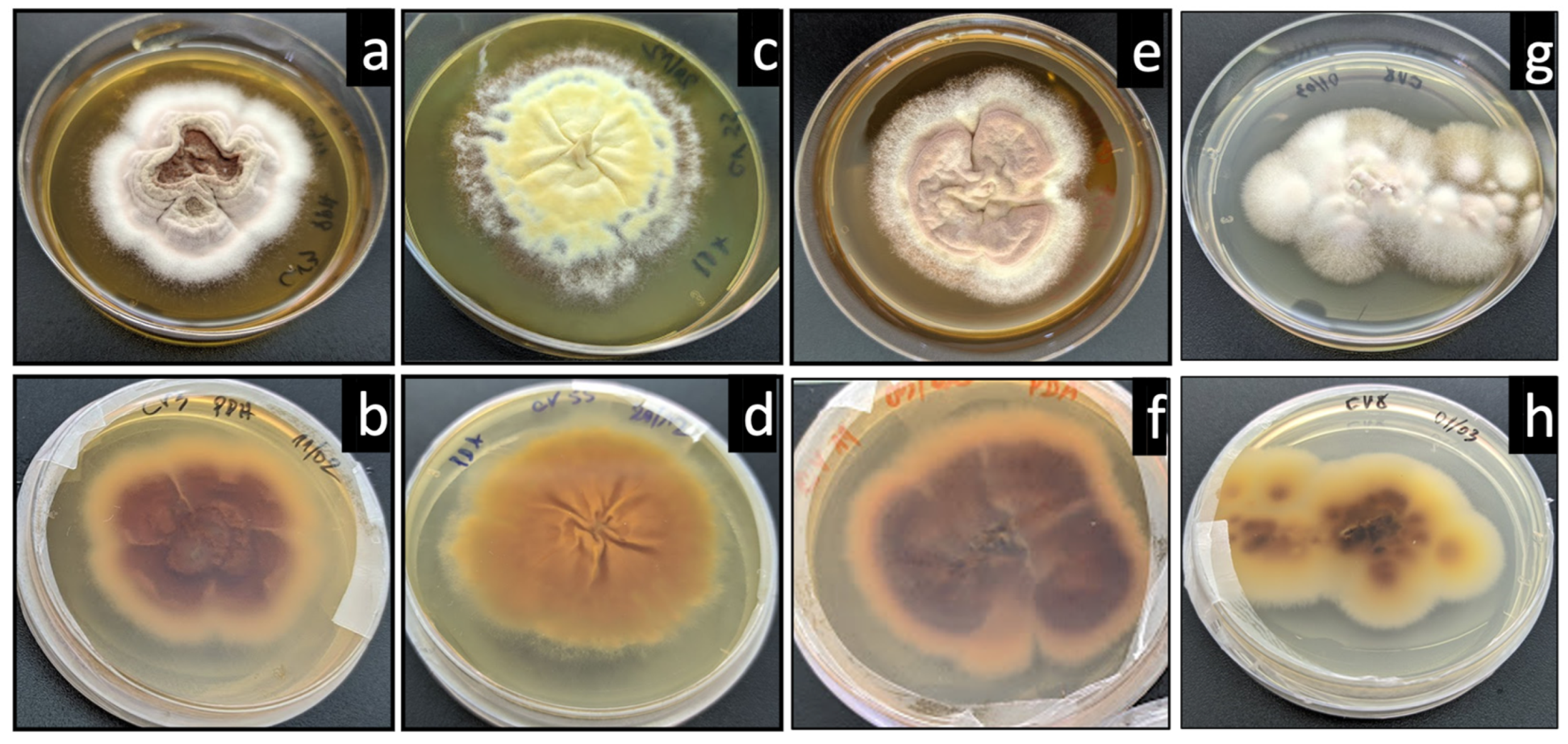
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achterman, R.R.; White, T.C. A Foot in the Door for Dermatophyte Research. PLoS Pathog. 2012, 8, 6–9. [Google Scholar] [CrossRef]
- Segal, E.; Elad, D. Human and Zoonotic Dermatophytoses: Epidemiological Aspects. Front. Microbiol. 2021, 1, 713532. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Frenkel, M. Dermatophyte infections in environmental contexts. Res. Microbiol. 2015, 166, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, O.; L’Ollivier, C.; Piarroux, R.; Ranque, S. Epidemiology of human dermatophytoses in Africa. Med. Mycol. 2018, 56, 145–161. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A. Genetic Predisposition and its Heredity in the Context of Increased Prevalence of Dermatophytoses. Mycopathologia 2021, 186, 163–176. [Google Scholar] [CrossRef]
- Underhill, P.A.; Shen, P.; Lin, A.A.; Jin, L.; Passarino, G.; Yang, W.H.; Kauffman, E.; Bonné-Tamir, B.; Bertranpetit, J.; Francalacci, P.; et al. Y chromosome sequence variation and the history of human populations. Nat. Genet. 2000, 26, 358–361. [Google Scholar] [CrossRef]
- Tuckman, A. The Potential Psychological Impact of Skin Conditions. Dermatol. Ther. 2017, 7, 53–57. [Google Scholar] [CrossRef]
- Chacon, A.; Franca, K.; Fernandez, A.; Nouri, K. Psychosocial impact of onychomycosis: A review. Int. J. Dermatol. 2013, 52, 1300–1307. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Marcoux, D.; Dang, J.; Auguste, H.; McCuaig, C.; Powell, J.; Hatami, A.; Maari, C.; Le Meur, J.-P. Emergence of African species of dermatophytes in tinea capitis: A 17-year experience in a Montreal pediatric hospital. Pediatr. Dermatol. 2018, 35, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Chabasse, D. Les dermatophytes: D’où viennent-ils? Comment sont-ils devenus des parasites? J. Mycol. Med. 2008, 18, 27–35. [Google Scholar] [CrossRef]
- Achterman, R.R.; White, T.C. Dermatophytes. Curr. Biol. 2013, 23, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Dabas, Y.; Xess, I.; Singh, G.; Pandey, M.; Meena, S. Molecular Identification and Antifungal Susceptibility Patterns of Clinical Dermatophytes Following CLSI and EUCAST Guidelines. J. Fungi 2017, 3, 17. [Google Scholar] [CrossRef]
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef] [PubMed]
- Hayette, M.; Sacheli, R. Dermatophytosis, Trends in Epidemiology and Diagnostic Approach. Curr. Fungal Infect. Rep. 2015, 9, 164–179. [Google Scholar] [CrossRef]
- Antuori, A.; Fernández, G.; Fernández, A.; Alcaide, M.; Boada, A.; Bielsa, M.I.; Romaní, N.; Matas, L. Epidemiology of dermatophytic infections between 2008 and 2017 in Barcelona, Spain. Enferm. Infecc. Microbiol. Clin. 2019, 37, 642–647. [Google Scholar] [CrossRef]
- Gits-Muselli, M.; Benderdouche, M.; Hamane, S.; Mingui, A.; De Chauvin, M.F.; Guigue, N.; Picat, M.Q.; Bourrat, E.; Petit, A.; Bagot, M.; et al. Continuous increase of Trichophyton tonsurans as a cause of tinea capitis in the urban area of Paris, France: A 5-year-long study. Med. Mycol. 2017, 55, 476–484. [Google Scholar] [CrossRef]
- Donghi, D.; Hauser, V.; Bosshard, P.P. Microsporum audouinii tinea capitis in a Swiss school: Assessment and management of patients and asymptomatic carriers. Med. Mycol. 2011, 49, 324–328. [Google Scholar] [CrossRef]
- Carrillo-Muñoz, A.J.; Tur-Tur, C.; Cárdenes, D.; Rojas, F.; Giusiano, G. Influence of the ecological group on the in vitro antifungal susceptibility of dermatophytic fungi. Rev. Iberoam. Micol. 2013, 30, 130–133. [Google Scholar] [CrossRef]
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2020, 2, 22–27. [Google Scholar] [CrossRef]
- Nweze, E.I.; Eke, I. Dermatophytosis in northern Africa. Mycoses 2016, 59, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nweze, E.I.; Eke, I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2018, 56, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, O.; Kone, A.K.; Niaré-Doumbo, S.; Goïta, S.; Gaudart, J.; Djimdé, A.A.; Piarroux, R.; Doumbo, O.K.; Thera, M.A.; Ranque, S. Dermatophytosis among Schoolchildren in Three Eco-climatic Zones of Mali. PLoS Negl. Trop. Dis. 2016, 10, e0004675. [Google Scholar] [CrossRef] [PubMed]
- Dogo, J.; Afegbua, S.L.; Dung, E.C. Prevalence of Tinea Capitis among School Children in Nok Community of Kaduna State, Nigeria. J. Pathog. 2016, 2016, 9601717. [Google Scholar] [CrossRef]
- Marcos-Tejedor, F.; Mota, M.; Iglesias-Sánchez, M.J.; Mayordomo, R.; Gonçalves, T. Identification of fungi involved in onychomycosis in patients of a spanish rural area. J. Fungi 2021, 7, 623. [Google Scholar] [CrossRef]
- Sacheli, R.; Cuypers, L.; Seidel, L.; Darfouf, R.; Adjetey, C.; Lagrou, K.; Hayette, M.P. Epidemiology of Dermatophytes in Belgium: A 5 Years’ Survey. Mycopathologia 2021, 186, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hainer, B.L. Dermatophyte Infections. Am. Fam. Physician 2003, 67, 101–108. [Google Scholar]
- Young, M.; Keane, C.; English, L. Misdiagnosed Dermatophytosis. J. Infect. 1982, 4, 127–129. [Google Scholar] [CrossRef]
- Ely, J.W.; Rosenfeld, S.; Stone, M.S. Diagnosis and Management of Tinea Infections. Am. Fam. Physician 2014, 90, 702–710. [Google Scholar]
- Libon, F.; Nikkels-Tassoudji, N.; Dezfoulian, B.; Arrese, J.E.; Nikkels, A.F. Non-dermatophyte Dermatoses Mimicking Dermatophytoses in Humans. Mycopathologia 2017, 182, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Maulingkar, S.V.; Pinto, M.J.; Rodrigues, S. A clinico-mycological study of dermatophytoses in Goa, India. Mycopathologia 2014, 178, 297–301. [Google Scholar] [CrossRef]
- Gräser, Y.; Kuijpers, A.F.; Presber, W.; de Hoog, G.S. Molecular taxonomy of the Trichophyton rubrum complex. J. Clin. Microbiol. 2000, 38, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Karabıçak, N.; Karatuna, O.; İlkit, M.; Akyar, I. Evaluation of the Bruker Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) System for the Identification of Clinically Important Dermatophyte Species. Mycopathologia 2015, 180, 165–171. [Google Scholar] [CrossRef]
- Packeu, A.; Stubbe, D.; Roesems, S.; Goens, K.; Van Rooij, P.; de Hoog, S.; Hendrickx, M. Lineages Within the Trichophyton rubrum Complex. Mycopathologia 2020, 185, 123–136. [Google Scholar] [CrossRef]
- Su, H.; Packeu, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Blechert, O.; Ilkit, M.; Hagen, F.; Gräser, Y.; Liu, W.; Deng, S.; et al. Species distinction in the Trichophyton rubrum complex. J. Clin. Microbiol. 2019, 57, e00352-19. [Google Scholar] [CrossRef]
- Cornet, L.; D’hooge, E.; Magain, N.; Stubbe, D.; Packeu, A.; Baurain, D.; Becker, P. The taxonomy of the Trichophyton rubrum complex: A phylogenomic approach. Microb. Genom. 2021, 7, 000707. [Google Scholar] [CrossRef] [PubMed]
- Diongue, K.; Bréchard, L.; Diallo, M.A.; Seck, M.C.; Ndiaye, M.; Badiane, A.S.; Ranque, S.; Ndiaye, D. A Comparative Study on Phenotypic versus ITS-Based Molecular Identification of Dermatophytes Isolated in Dakar, Senegal. Int. J. Microbiol. 2019, 2019, 6754058. [Google Scholar] [CrossRef]
- Coulibaly, O.; Thera, M.A.; Piarroux, R.; Doumbo, O.K.; Ranque, S. High dermatophyte contamination levels in hairdressing salons of a West African suburban community. Mycoses 2015, 58, 65–68. [Google Scholar] [CrossRef]
- Hogewoning, A.A.; Adegnika, A.A.; Bouwes Bavinck, J.N.; Yazdanbakhsh, M.; Kremsner, P.G.; van der Raaij-Helmer, E.M.; Staats, C.C.; Willemze, R.; Lavrijsen, A.P. Prevalence and causative fungal species of tinea capitis among schoolchildren in Gabon. Mycoses 2011, 54, e354–e359. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Olum, R.; Nsenga, L.; Namusobya, M.; Russell, L.; de Sousa, E.; Osaigbovo, I.I.; Kwizera, R.; Baluku, J.B. Estimation of the burden of tinea capitis among children in Africa. Mycoses 2021, 64, 349–363. [Google Scholar] [CrossRef]
- Dolenc-Voljč, M. Dermatophyte Infections in Humans: Current Trends and Future Prospects. In Medical Mycology: Current Trends and Future Prospects; Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Rai, M., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2015; pp. 3–27. [Google Scholar] [CrossRef]
- Jazdarehee, A.; Malekafzali, L.; Lee, J.; Lewis, R.; Mukovozov, I. Transmission of Onychomycosis and Dermatophytosis between Household Members: A Scoping Review. Fungi 2022, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Woldeamanuel, Y.; Mengistu, Y.; Chryssanthou, E.; Petrini, B. Dermatophytosis in Tulugudu Island, Ethiopia. Med. Mycol. 2005, 43, 79–82. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, E. Epidemiology of dermatophytoses in Crete, Greece: A 5-year survey. Med. Mycol. J. 2016, 57, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, B.; Mirhendi, H.; Shidfar, M.R.; Nouripour-Sisakht, S.; Jalalizand, N.; Geramishoar, M.; Shokoohi, G.R. A comparative study on morphological versus molecular identification of dermatophyte isolates. J. Mycol. Med. 2015, 25, 29–35. [Google Scholar] [CrossRef]
- Mochizuki, T.; Takeda, K.; Anzawa, K. Molecular markers useful for the epidemiology of dermatophytoses. J. Dermatol. 2015, 42, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Mukherjee, P.K.; Warshaw, E.M.; Evans, S.; Korman, N.J.; Tavakkol, A. Molecular analysis of dermatophytes suggests spread of infection among household members. Cutis 2013, 91, 237–245. [Google Scholar] [PubMed]
- Suzuki, S.; Mano, Y.; Furuya, N.; Fujitani, K. Molecular epidemiological analysis of the spreading conditions of Trichophyton in long-term care facilities in Japan. Jpn. J. Infect. Dis. 2018, 71, 462–466. [Google Scholar] [CrossRef]
- Ilkit, M.; Demirhindi, H. Asymptomatic dermatophyte scalp carriage: Laboratory diagnosis, epidemiology and management. Mycopathologia 2008, 165, 61–71. [Google Scholar] [CrossRef]
- Hill, R.C.; Gold, J.A.W.; Lipner, S.R. Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management. J. Fungi 2024, 10, 357. [Google Scholar] [CrossRef]


| Number/Percentage 1 | ||||
|---|---|---|---|---|
| Dermatophytosis 1 | ||||
| Yes | No | |||
| Mean age | 10.4 | |||
| Sex | Female | 26 (40.0%) | 6 (10.0%) | 18 (30%) |
| Male | 36 (60.0%) | 12 (20.0%) | 24 (40.0%) | |
| Lesion localization | Scalp | 5 (8.3%) | 1 (1.7%) | 4 (6.7%) |
| Ear | 1 (1.7%) | 0 | 1 (1.7%) | |
| Hand | 4 (6.7%) | 2 (3.3%) | 2 (3.3%) | |
| Toenail | 1 (1.7%) | 0 | 1 (1.7%) | |
| Leg | 6 (10.0%) | 2 (3.3%) | 4 (6.7%) | |
| Arm | 6 (10.0%) | 2 (3.3%) | 4 (6.7%) | |
| Body | 10 (16.7%) | 6 (10.0%) | 4 (6.7%) | |
| Foot | 1 (1.7%) | 0 | 1 (1.7%) | |
| Interdigital | 4 (6.7%) | 0 | 4 (6.7%) | |
| Nail | 15 (25.0%) | 4 (6.7%) | 11 (18.3%) | |
| Face | 5 (8.3%) | 1 (1.7%) | 4 (6.7%) | |
| Back | 1 (1.7%) | 0 | 1 (1.7%) | |
| Skin | 1 (1.7%) | 0 | 1 (1.7%) | |
| Barefoot | 28 (46.7%) | 9 (15.0%) | 19 (31.7%) | |
| Shared items 2 | 36 (60.0%) | 11 (18.3%) | 25 (41.7%) | |
| Shower/week | 3 | 12 (20.0%) | 3 (5.0%) | 9 (15.0%) |
| 4 | 20 (33.3%) | 7 (11.7%) | 13 (21.7%) | |
| 5 | 16 (26.7%) | 4 (6.7%) | 12 (20.0%) | |
| 6 | 4 (6.7%) | 1 (1.7%) | 3 (5.0%) | |
| 7 | 8 (13.3%) | 3 (5.0%) | 5 (8.3%) | |
| Fungal Identification | |||||
|---|---|---|---|---|---|
| Sample | Body Site | Antifungal Treatment | Morphology | ITS-5.8S Sequencing | NCBI Accession Number |
| CV3 | Body | Ketoconazole | T. violaceum | T. soudanense | OR168629 |
| CV4 | Hand | Ketoconazole | T. soudanense | T. soudanense | OR185564 |
| CV8 | Nail | None | T. soudanense | T. soudanense | OR206379 |
| CV10 | Leg | None | T. soudanense | T. soudanense | OR185566 |
| CV11 | Body | Ketoconazole | T. rubrum | T. soudanense | OR185565 |
| CV12 | Arm | None | T. violaceum | T. soudanense | OR185567 |
| CV15 | Face | Ketoconazole | T. violaceum | T. soudanense | OR206381 |
| CV20 | Body | None | T. violaceum | T. soudanense | OR185597 |
| CV24 | Leg | None | T. rubrum | T. soudanense | OR185598 |
| CV30 | Body | None | T. violaceum | T. soudanense | OR185599 |
| CV42 | Arm | Ketoconazole | T. soudanense | T. soudanense | OR185600 |
| CV45 | Body | Ketoconazole | T. soudanense | T. soudanense | OR185601 |
| CV47 | Body | None | T. soudanense | T. soudanense | OR185602 |
| CV50 | Toenail | None | T. rubrum | T. soudanense | OR185603 |
| CV52 | Hand | Clotrimazole | T. rubrum | T. soudanense | OR185607 |
| CV55 | Nail | None | T. rubrum | T. rubrum | OR249962 |
| CV58 | Nail | None | T. soudanense | T. soudanense | OR186345 |
| CV60 | Scalp | Griseofulvin | T. soudanense | T. soudanense | OR206380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, E.E.M.; Mota, M.; Veiga, L.V.A.d.M.; Fernandes, C.; Gonçalves, T. Predominance of Trichophyton soudanense as Agent of Dermatophytoses in Cape Verdean School-Age Children. J. Fungi 2024, 10, 693. https://doi.org/10.3390/jof10100693
Correia EEM, Mota M, Veiga LVAdM, Fernandes C, Gonçalves T. Predominance of Trichophyton soudanense as Agent of Dermatophytoses in Cape Verdean School-Age Children. Journal of Fungi. 2024; 10(10):693. https://doi.org/10.3390/jof10100693
Chicago/Turabian StyleCorreia, Edmilson Emanuel Monteiro, Marta Mota, Luciano Vagner Ascenção de Melo Veiga, Chantal Fernandes, and Teresa Gonçalves. 2024. "Predominance of Trichophyton soudanense as Agent of Dermatophytoses in Cape Verdean School-Age Children" Journal of Fungi 10, no. 10: 693. https://doi.org/10.3390/jof10100693
APA StyleCorreia, E. E. M., Mota, M., Veiga, L. V. A. d. M., Fernandes, C., & Gonçalves, T. (2024). Predominance of Trichophyton soudanense as Agent of Dermatophytoses in Cape Verdean School-Age Children. Journal of Fungi, 10(10), 693. https://doi.org/10.3390/jof10100693







