An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Generation and Detection of Mutant Strains
2.3. RT-qPCR Detection of lncRsn and Its Neighboring Gene
2.4. Plant Infection and DON Production Assays
2.5. Microscopic Examination
2.6. Sexual Reproduction Assays
2.7. Assays for Defects in Responses to Different Stresses and Cell Wall Integrity Analysis
3. Results
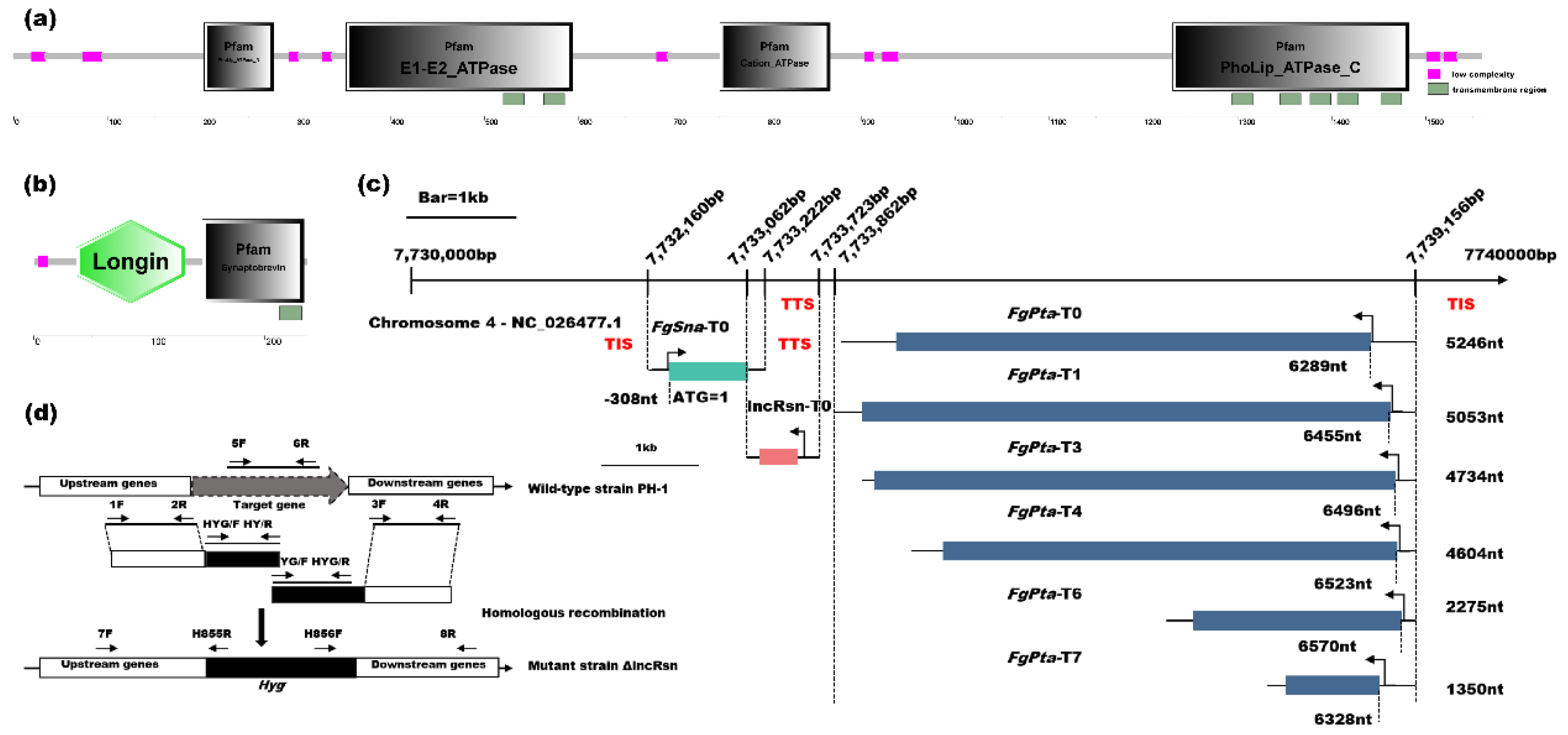
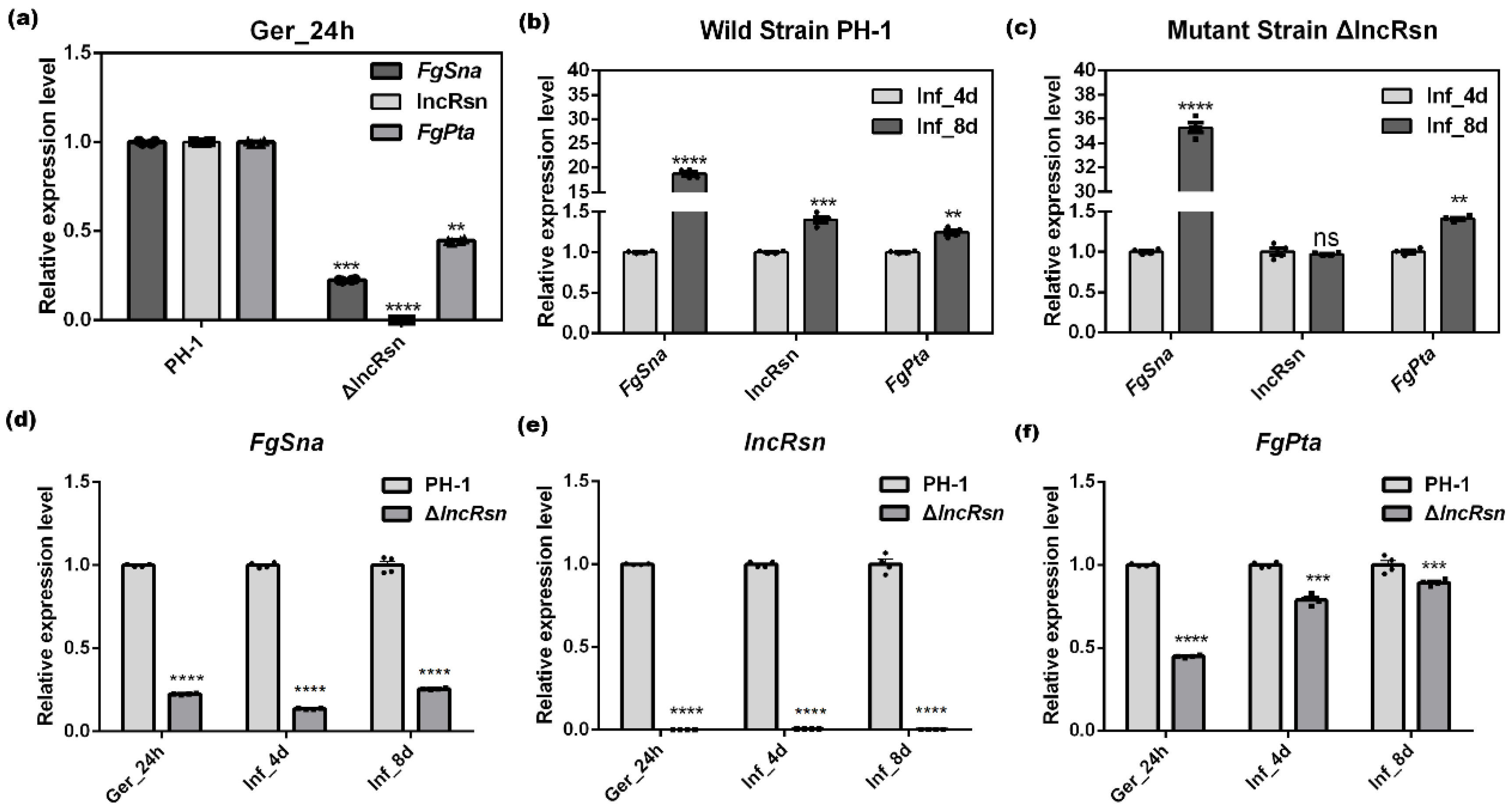
3.1. Analysis of lncRsn, FgSna and FgPta
3.2. lncRsn Is Essential for Sexual Reproduction in F. graminearum
3.3. lncRsn Is Essential for Pathogenicity in F. graminearum
3.4. Deletion of lncRsn Interferes with the Synthesis of DON
3.5. Deletion of lncRsn Is Not Necessary for Asexual Reproduction but Display Reduced Sensitivity under Stress Conditions
3.6. FgSna and lncRsn Negatively Regulate in F. graminearum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. J. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. J. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. J. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. J. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef]
- Ariel, F.; Romero-Barrios, N.; Jegu, T.; Benhamed, M.; Crespi, M. Battles and hijacks: Noncoding transcription in plants. J. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef]
- Zhao, T.; Tao, X.; Feng, S.; Wang, L.; Hong, H.; Ma, W.; Shang, G.; Guo, S.; He, Y.; Zhou, B.; et al. LncRNAs in polyploid cotton interspecific hybrids are derived from transposon neofunctionalization. J. Genome Biol. 2018, 19, 195. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Ham, B.; Zhang, S.; Fei, Z.; Lucas, W.J. Plant lncRNAs are enriched in and move systemically through the phloem in response to phosphate deficiency. J. Integr. Plant Biol. 2019, 61, 492–508. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Wang, M.B. Molecular functions of long non-coding RNAs in plants. J. Genes 2012, 3, 15. [Google Scholar] [CrossRef]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. J. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef]
- Liu, X.; Hao, L.L.; Li, D.Y.; Zhu, L.H.; Hu, S.N. Long non-coding RNAs and their biological roles in plants. J. Genom. Proteom. Bioinform. 2015, 13, 137–147. [Google Scholar] [CrossRef]
- Till, P.; Mach, R.L.; Mach-Aigner, A.R. A current view on long noncoding RNAs in yeast and filamentous fungi. J. Appl. Microbiol. Biotechnol. 2018, 102, 7319–7331. [Google Scholar] [CrossRef] [PubMed]
- Arthanari, Y.; Heintzen, C.; Griffiths-Jones, S.; Crosthwaite, S.K. Natural antisense transcripts and long non-coding RNA in Neurospora crassa. PLoS ONE 2014, 9, e91353. [Google Scholar] [CrossRef] [PubMed]
- Cemel, I.A.; Ha, N.; Schermann, G.; Yonekawa, S.; Brunner, M. The coding and noncoding transcriptome of Neurospora crassa. J. BMC Genom. 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Touat-Todeschini, L.; Shichino, Y.; Dangin, M.; Thierry-Mieg, N.; Gilquin, B.; Hiriart, E.; Sachidanandam, R.; Lambert, E.; Brettschneider, J.; Reuter, M.; et al. Selective termination of lncRNA transcription promotes heterochromatin silencing and cell differentiation. EMBO J. 2017, 36, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Shuman, S.; Schwer, B. Genetic screen for suppression of transcriptional interference reveals fission yeast 14–3–3 protein Rad24 as an antagonist of precocious Pol2 transcription termination. J. Nucleic Acids Res. 2022, 50, 803–819. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, W.; Xie, J.; Fu, Y.; Jiang, D.; Lin, Y.; Chen, W.; Cheng, J. A novel antisense long non-coding RNA participates in asexual and sexual reproduction by regulating the expression of GzmetE in Fusarium graminearum. J. Environ. Microbiol. 2021, 23, 4939–4955. [Google Scholar] [CrossRef]
- Huang, P.; Yu, X.; Liu, H.; Ding, M.; Wang, Z.; Xu, J.-R.; Jiang, C. Regulation of TRI5 expression and deoxynivalenol biosynthesis by a long non-coding RNA in Fusarium graminearum. J. Nat. Commun. 2024, 15, 1216. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. J. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. J. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Reemerging Disease of Devastating Impact. J. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. J. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. J. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, C.A.; Güldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, F.; Ding, M.; Geng, C.; Chen, L.; Zou, S.; Liang, Y.; Yu, J.; Dong, H. Thioredoxin Reductase Is Involved in Development and Pathogenicity in Fusarium graminearum. J. Front. Microbiol. 2019, 10, 393. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Fang, Q.; Li, Y.; Zheng, X.; Zhang, Z. SNARE protein FgVam7 controls growth, asexual and sexual development, and plant infection in Fusarium graminearum. J. Mol. Plant Pathol. 2016, 17, 108–119. [Google Scholar] [CrossRef]
- Seong, K.; Hou, Z.; Tracy, M.; Kistler, H.C.; Xu, J.R. Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. J. Phytopathol. 2005, 95, 744–750. [Google Scholar] [CrossRef]
- Wu, A.B.; Li, H.P.; Zhao, C.S.; Liao, Y.C. Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. J. Mycopathol. 2005, 160, 75–83. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Oerke, E.C.; Dehne, H.W.; Steiner, U. Colonization of wheat seedling leaves by Fusarium species as observed in growth chambers: A role as inoculum for head blight infection? J. Fungal. Ecol. 2012, 5, 581–590. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Yin, Y.; Ma, Z. Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum. J. Mol. Plant Pathol. 2013, 14, 71–83. [Google Scholar] [CrossRef]
- Jenczmionka, N.J.; Schäfer, W. The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. J. Curr. Genet. 2005, 47, 29–36. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, H.; Qi, L.; Zhang, S.; Zhou, X.; Zhang, Y.; Xu, J. FgKin1 kinase localizes to the septal pore and plays a role in hyphal growth, ascospore germination, pathogenesis, and localization of Tub1 βtubulins in Fusarium graminearum. J. New Phytol. 2014, 204, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. J. Fungal Genet. Biol. 2009, 46, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.; Weber, J.; Broz, K.; Kistler, H.C. Cellular development associated with induced mycotoxin synthesis in the filamentous fungus Fusarium graminearum. PLoS ONE 2013, 8, e63077. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, P.; Sun, Z.; Zhu, J.; Yu, D.; Tang, Z.; Wang, Z.; Wang, C.; Zheng, H. Sgh1, an SR-like Protein, Is Involved in Fungal Development, Plant Infection, and Pre-mRNA Processing in Fusarium graminearum. J. Fungi 2022, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Cao, S.; Wang, Z.; Xu, H.; Liang, J.; Liu, H.; Wang, G.; Ding, M.; Wang, Q.; Gong, C.; et al. An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. J. Nat. Microbiol. 2019, 4, 1582–1591. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, J.; Wang, Y.; Lin, J.; Fu, Y.; Xie, J.; Jiang, D.; Chen, T.; Liu, H.; Cheng, J. Dicerlike proteins regulate sexual development via the biogenesis of perithecium-specific microRNAs in a plant pathogenic fungus Fusarium graminearum. J. Front. Microbiol. 2018, 9, 818. [Google Scholar]
- Li, B.; Dong, X.; Zhao, R.; Kou, R.; Zheng, X.; Zhang, H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. J. PLoS Pathog. 2019, 15, e1007754. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, S.; Zhou, X.; Wang, C.; Xiang, P.; Zheng, Q.; Xu, J.-R. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. J. PLoS ONE 2012, 7, e49495. [Google Scholar] [CrossRef]
- Lu, P.; Chen, D.; Qi, Z.; Wang, H.; Chen, Y.; Wang, Q.; Jiang, C.; Xu, J.-R.; Liu, H. Landscape and regulation of alternative splicing and alternative polyadenylation in a plant pathogenic fungus. J. New Phytologist. 2022, 235, 674–689. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.; Liu, X.; Zhao, S.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. J. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. J. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Aimin, L.; Junying, Z.; Zhongyin, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar]
- Zheng, Q.; Hou, R.; Zhang, J.; Ma, J.; Wu, Z.; Wang, G.; Wang, C.; Xu, J.-R. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. J. PLoS ONE 2013, 8, e66980. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.H. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, H.J.; Lee, J.; Kim, K.W.; Yun, S.H.; Shim, W.B.; Lee, Y.W. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. J. Curr. Genet. 2009, 55, 449459. [Google Scholar] [CrossRef]
- Xu, Y.B.; Li, H.P.; Zhang, J.B.; Song, B.; Chen, F.F.; Duan, X.J.; Xu, H.Q.; Liao, Y.C. Disruption of the chitin synthase gene CHS1 from Fusarium asiaticum results in an altered structure of cell walls and reduced virulence. J. Fungal Genet. Biol. 2010, 47, 205–215. [Google Scholar] [CrossRef]
- Yun, Y.; Liu, Z.; Zhang, J.; Shim, W.B.; Chen, Y.; Ma, Z. The MAPKK FgMkk1 of Fusarium graminearum regulates vegetative differentiation, multiple stress response, and virulence via the cell wall integrity and high-osmolarity glycerol signaling pathways. J. Environ. Microbiol. 2014, 16, 2023–2037. [Google Scholar] [CrossRef]
- Damveld, R.A.; vanKuyk, P.A.; Arentshorst, M.; Klis, F.M.; van den Hondel, C.A.; Ram, A.F. Expression of agsA, one of five 1,3-a-D-glucan synthase-encoding genes in Aspergillus niger, is induced in response to cell wall stress. J. Fungal Genet. Biol. 2005, 42, 165–177. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Zhang, X.; Song, W.; Zhao, Q.; Dong, Y.; Guo, M.; Zheng, X.; Zhang, Z. A two-component histidine kinase, MoSLN1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. J. Curr. Genet. 2010, 56, 517–528. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Y.; Liu, Y.; Zhang, C.; Ma, Z. The transmembrane protein FgSho1 regulates fungal development and pathogenicity via the MAPK module Ste50-Ste11-Ste7 in Fusarium graminearum. J. New Phytologist. 2015, 206, 315–328. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, C.; Yu, F.; Yin, Y.; Shim, W.B.; Ma, Z. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. J. Environ. Microbiol. 2015, 17, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Tomaru, Y.; Kasukawa, T.; Waki, K.; Nakanishi, M.; Nakamura, M. Antisense transcription in the mammalian transcriptome. Science 2005, 309, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. J. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. J. Mol. Plant Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tong, Q.; Zhang, C.; Ding, K. The transcription factor FgCrz1A is essential for fungal development, virulence, deoxynivalenol biosynthesis and stress responses in Fusarium graminearum. J. Curr. Genet. 2019, 65, 153–166. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wang, L.; Sun, S.; Liu, A.; Liang, Y.; Yu, J.; Dong, H. FgPEX1 and FgPEX10 are required for the maintenance of Woronin bodies and full virulence of Fusarium graminearum. J. Curr. Genet. 2019, 65, 1383–1396. [Google Scholar] [CrossRef]
- Guenther, J.C.; Trail, F. The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. J. Mycologia. 2005, 97, 229–237. [Google Scholar] [CrossRef]
- Trail, F.; Gaffoor, I.; Vogel, S. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). J. Fungal Genet. Biol. 2005, 42, 528–533. [Google Scholar] [CrossRef]
- Trail, F.; Xu, H.; Loranger, R.; Gadoury, D. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). J. Mycologia. 2002, 94, 181–189. [Google Scholar]
- Trail, F. Fungal cannons: Explosive spore discharge in the Ascomycota. J. FEMS Microbiol. Lett. 2007, 276, 12–18. [Google Scholar] [CrossRef]
- Min, K.; Lee, J.; Kim, J.C.; Kim, S.G.; Kim, Y.H.; Vogel, S.; Trail, F.; Lee, Y.-W. A novel gene, ROA, is required for normal morphogenesis and discharge of ascospores in Gibberella zeae. J. Eukaryot. Cell 2010, 9, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Villegas, V.E.; Zaphiropoulos, P.G. Neighboring gene regulation by antisense long non-coding RNAs. Int. J. Mol. Sci. 2015, 16, 3251–3266. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.T.; Chen, X.Y.; Yan, Y.Q.; Huang, J.; Luo, C.; Tom, H.; Zheng, L. Comprehensive transcriptome profiling reveals abundant long non-coding RNAs associated with development of the rice false smut fungus, Ustilaginoidea virens. J. Environ. Microbiol. 2021, 23, 4998–5013. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Chen, Y.; Cai, G.; Peng, H.; Wang, X.; Li, P.; Gu, A.; Li, Y.; Ma, D. An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum. J. Fungi 2024, 10, 692. https://doi.org/10.3390/jof10100692
Fu Z, Chen Y, Cai G, Peng H, Wang X, Li P, Gu A, Li Y, Ma D. An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum. Journal of Fungi. 2024; 10(10):692. https://doi.org/10.3390/jof10100692
Chicago/Turabian StyleFu, Zhizhen, Yanjie Chen, Gaolei Cai, Huijuan Peng, Xiaoyu Wang, Ping Li, Aiguo Gu, Yanli Li, and Dongfang Ma. 2024. "An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum" Journal of Fungi 10, no. 10: 692. https://doi.org/10.3390/jof10100692
APA StyleFu, Z., Chen, Y., Cai, G., Peng, H., Wang, X., Li, P., Gu, A., Li, Y., & Ma, D. (2024). An Antisense Long Non-Coding RNA, LncRsn, Is Involved in Sexual Reproduction and Full Virulence in Fusarium graminearum. Journal of Fungi, 10(10), 692. https://doi.org/10.3390/jof10100692





