Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings
Abstract
1. Introduction
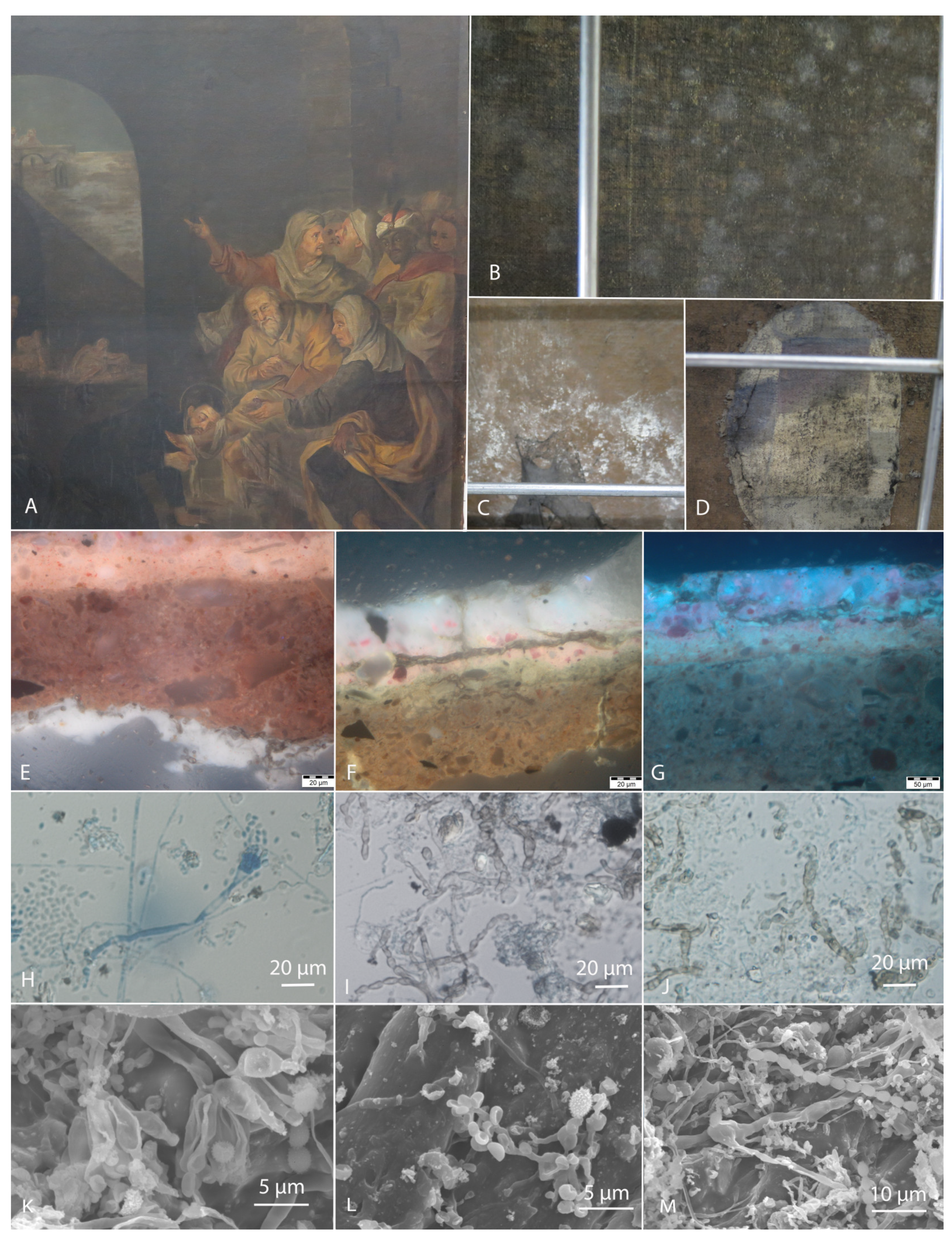
2. Materials and Methods
2.1. Fungal Strains and Inocula Preparation
2.2. Growth at Different Water Activities and Temperatures
2.3. Biodegradation Assays
2.3.1. Excretion of Enzymes
2.3.2. Substrate Acidification
2.3.3. Excretion of Soluble Pigments
2.4. Assimilation of Hydrocarbons
2.5. Siderophore Formation
2.6. Evaluation and Categorization of Fungal Activities
2.7. Statistical Analyses
3. Results
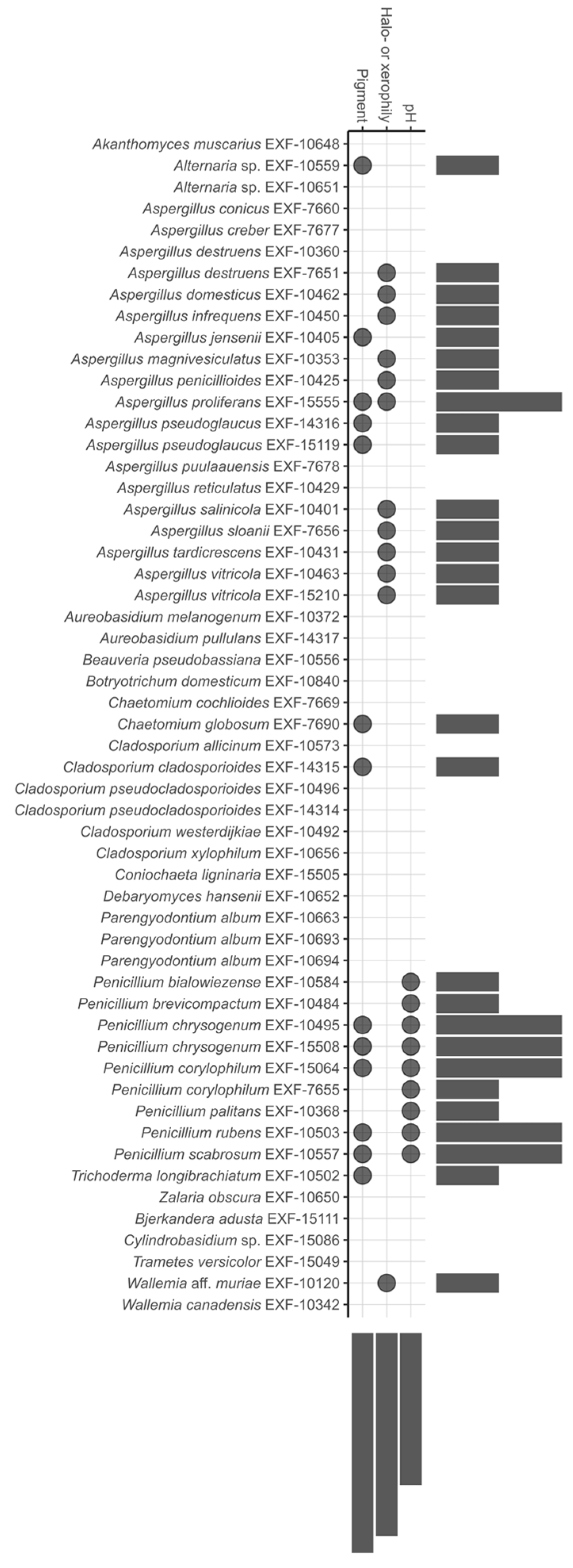
3.1. Physiological Characterisation: Growth at Different Water Activities, Temperatures, and Production of Acids and Pigments
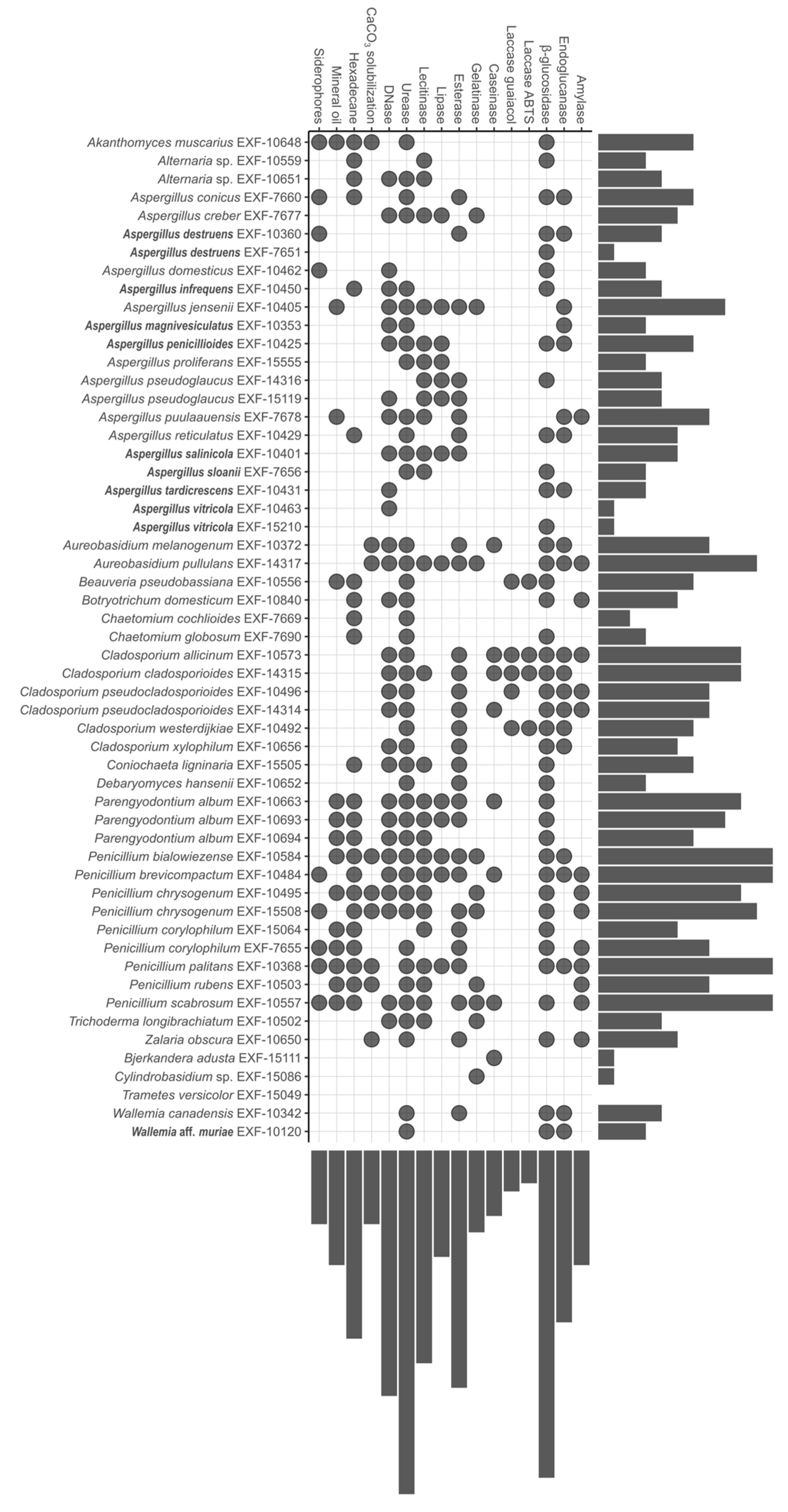
3.2. Biodeteriorative Potential of Fungi: Enzymatic Activities, Ability to Assimilate Hydrocarbons, to Solubilize CaCO3, and Synthesize Siderophores
3.2.1. Enzymatic Activity
3.2.2. Assimilation of Hydrocarbons, Solubilization of CaCO3, and Production of Siderophores
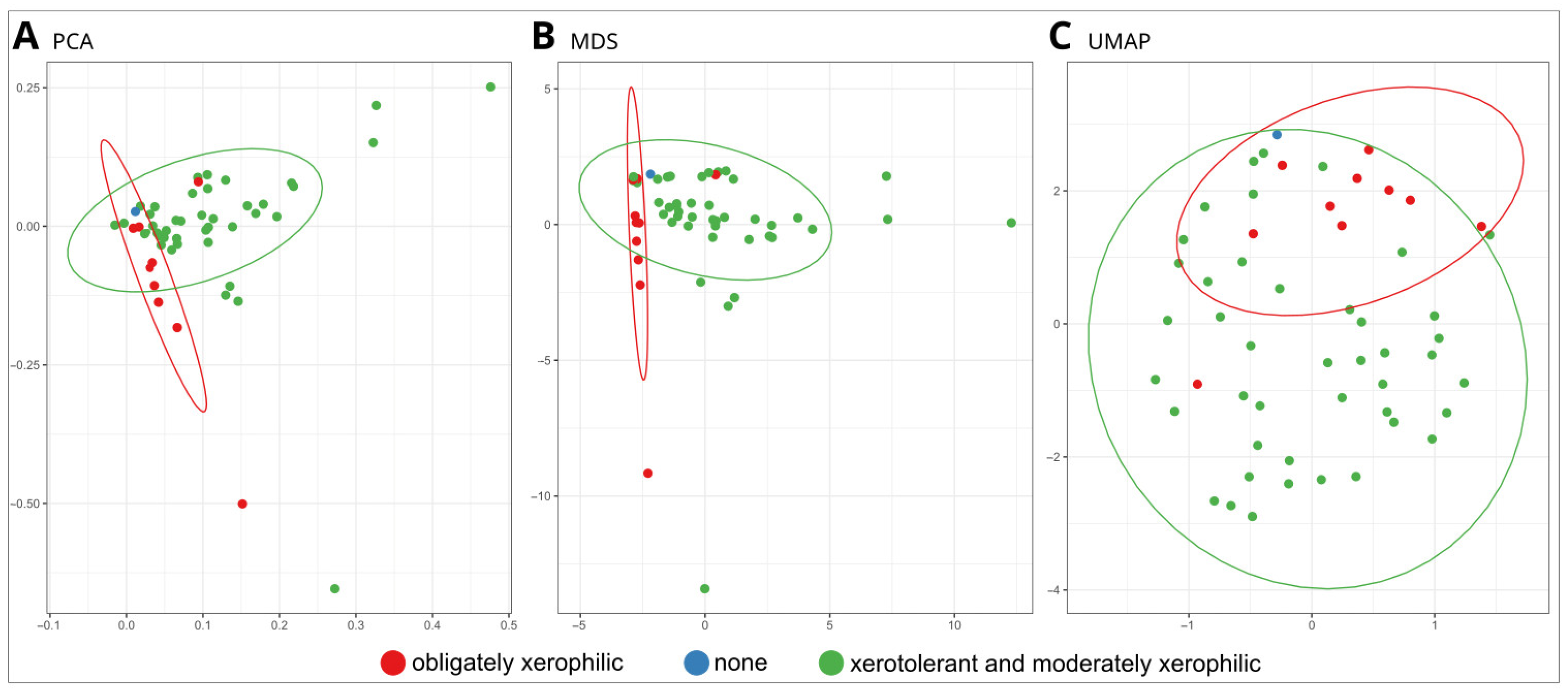
3.2.3. Statistical Analyses
4. Discussion
4.1. Xerophily Is the Crucial Factor That Allows the Biodeterioration of Paintings Indoors
4.2. Testing the Biodeterioration Potential of Obligate Xerophiles by Adapting Culture Media
4.3. Biodeterioration of Canvas and Proteins
4.4. Biodeterioration of Varnish and Wax
4.5. Biodeterioration of Synthetic Polymers
4.6. Novel Tests for Biodeterioration Studies: Degradation of Environmental Debris and Ability to Sequester Fe Ions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciferri, O. Microbial Degradation of Paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K. Fungi: Their Role in Deterioration of Cultural Heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- López-Miras, M.D.M.; Martín-Sánchez, I.; Yebra-Rodríguez, Á.; Romero-Noguera, J.; Bolívar-Galiano, F.; Ettenauer, J.; Sterflinger, K.; Piñar, G. Contribution of the Microbial Communities Detected on an Oil Painting on Canvas to Its Biodeterioration. PLoS ONE 2013, 8, e80198. [Google Scholar] [CrossRef]
- Cappitelli, F.; Sorlini, C. Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage. Appl. Environ. Microbiol. 2008, 74, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Farmakalidis, H.V.; Douvas, A.M.; Karatasios, I.; Sotiropoulou, S.; Boyatzis, S.; Argitis, P.; Chryssoulakis, Y.; Kilikoglou, V. Accelerated Thermal Ageing of Acrylic Copolymers, Cyclohexanone-Based and Urea-Aldehyde Resins Used in Paintings Conservation. Mediterr. Archaeol. Archaeom. 2016, 16, 213–228. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sanmartín, P. Interactions of Microorganisms and Synthetic Polymers in Cultural Heritage Conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
- Poyatos, F.; Morales, F.; Nicholson, A.W.; Giordano, A. Physiology of Biodeterioration on Canvas Paintings. J. Cell. Physiol. 2018, 233, 2741–2751. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Bitossi, G.; Cruz-Cañizares, J.D.; Bolívar-Galiano, F.; López-Miras, M.M.; Romero-Noguera, J.; Martín-Sánchez, I.; Gimeno-Adelantado, J.V.; Doménech-Carbó, A. Study on the Biodeterioration of Alkyd Ah Resin Used As a Binding Medium for Modern Paintings By Pyrolysis-Gas Chromatography-Mass Spectrometry and Ftir Spectroscopy. Arche 2008, 3, 191–196. [Google Scholar]
- Leonardi, R. Nuclear Physics and Painting: Sub-Topic of the Wide and Fascinating Field of Science and Art. Nucl. Phys. A 2005, 752, 659–674. [Google Scholar] [CrossRef]
- López-Miras, M.; Piñar, G.; Romero-Noguera, J.; Bolívar-Galiano, F.C.; Ettenauer, J.; Sterflinger, K.; Martín-Sánchez, I. Microbial Communities Adhering to the Obverse and Reverse Sides of an Oil Painting on Canvas: Identification and Evaluation of Their Biodegradative Potential. Aerobiologia 2013, 29, 301–314. [Google Scholar] [CrossRef]
- Văcar, C.L.; Mircea, C.; Pârvu, M.; Podar, D. Diversity and Metabolic Activity of Fungi Causing Biodeterioration of Canvas Paintings. J. Fungi 2022, 8, 589. [Google Scholar] [CrossRef]
- Ortiz-Miranda, A.S.; Doménech-Carbó, A.; Doménech-Carbó, M.T.; Osete-Cortina, L.; Bolívar-Galiano, F.; Martín-Sánchez, I. Analyzing Chemical Changes in Verdigris Pictorial Specimens upon Bacteria and Fungi Biodeterioration Using Voltammetry of Microparticles. Herit. Sci. 2017, 5, 8. [Google Scholar] [CrossRef]
- Zhgun, A.; Avdanina, D.; Shumikhin, K.; Simonenko, N.; Lyubavskaya, E.; Volkov, I.; Ivanov, V. Detection of Potential Biodeterioration Risks for Tempera Painting in 16th Century Exhibits from State Tretyakov Gallery. PLoS ONE 2020, 15, e0230591. [Google Scholar] [CrossRef] [PubMed]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Ž.; Blagojević, J.; Vukojević, J.; Ljaljević Grbić, M. In Vitro Biodegradation Potential of Airborne Aspergilli and Penicillia. Sci. Nat. 2019, 106, 8. [Google Scholar] [CrossRef] [PubMed]
- Trovão, J.; Portugal, A. Current Knowledge on the Fungal Degradation Abilities Profiled through Biodeteriorative Plate Essays. Appl. Sci. 2021, 11, 4196. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the Fungus Aspergillus a Threat to Cultural Heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, O. Processes of Biodeterioration: General Mechanisms. In Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Getty Publications: Los Angeles, CA, USA, 2008; ISBN 9780892369393. [Google Scholar]
- Sazanova, K.V.; Shchiparev, S.M.; Vlasov, D.Y. Formation of Organic Acids by Fungi Isolated from the Surface of Stone Monuments. Microbiology 2014, 83, 516–522. [Google Scholar] [CrossRef]
- Kumar, S.; Priyanka; Kumar, U. Microbial Community Present on the Reverse Side of a Deteriorated Canvas BT. In Microbial Biotechnology Approaches to Monuments of Cultural Heritage; Yadav, A.N., Rastegari, A.A., Gupta, V.K., Yadav, N., Eds.; Springer: Singapore, 2020; pp. 1–12. ISBN 978-981-15-3401-0. [Google Scholar]
- Berovič, M. Biodeterioration Studies on Pastels and Oil-Based Paintings. In Art, biology, and Conservation: Biodeterioration of Works of Art; Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 50–59. ISBN 9781588391070. [Google Scholar]
- Poyatos Jiménez, F. Procesos de Microbiodeterioro En Pinturas Sobre Lienzo Del Museo de Bellas Artes de Granada Examen Visual y Gráfico. Ph.D. Thesis, University of Granada, Granada, Spain, 2007. [Google Scholar]
- Zalar, P.; Graf Hriberšek, D.; Gostinčar, C.; Breskvar, M.; Džeroski, S.; Matul, M.; Novak Babič, M.; Čremožnik Zupančič, J.; Kujović, A.; Gunde-Cimerman, N.; et al. Xerophilic Fungi Contaminating Historically Valuable Easel Paintings from Slovenia. Front. Microbiol. 2023, 14, e1258670. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and Taxonomy of the Pleomorphic Genus Alternaria. Mycol. Prog. 2016, 15, 3. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but Different: The Expanding Realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef]
- Wang, X.W.; Houbraken, J.; Groenewald, J.Z.; Meijer, M.; Andersen, B.; Nielsen, K.F.; Crous, P.W.; Samson, R.A. Diversity and Taxonomy of Chaetomium and Chaetomium-like Fungi from Indoor Environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef]
- Sklenář, F.; Jurjević; Zalar, P.; Frisvad, J.C.; Visagie, C.M.; Kolařík, M.; Houbraken, J.; Chen, A.J.; Yilmaz, N.; Seifert, K.A.; et al. Phylogeny of Xerophilic Aspergilli (Subgenus Aspergillus) and Taxonomic Revision of Section Restricti. Stud. Mycol. 2017, 88, 161–236. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the Past: “Find the Guilty Bug—Microorganisms Involved in the Biodeterioration of Archeological and Historical Artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Maggi, O.; Nugari, M.P.; Pietrini, A.M.; Piervittori, R.; Ricci, S.; Roccardi, A. The Biological Aerosol as a Factor of Biodeterioration BT. In Cultural Heritage and Aerobiology: Methods and Measurement Techniques for Biodeterioration Monitoring; Mandrioli, P., Caneva, G., Sabbioni, C., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 3–29. ISBN 978-94-017-0185-3. [Google Scholar]
- Stoner Joyce Hill, R.R. (Ed.) Conservation of Easel Paintings, 2nd ed.; Routledge: London, UK; Taylor and Francis: London, UK, 2021; ISBN 9780429680977. [Google Scholar]
- Unković, N.; Dimkić, I.; Stupar, M.; Stanković, S.; Vukojević, J.; Grbić, M.L. Biodegradative Potential of Fungal Isolates from Sacral Ambient: In Vitro Study as Risk Assessment Implication for the Conservation of Wall Paintings. PLoS ONE 2018, 13, e0190922. [Google Scholar] [CrossRef]
- Sabin, A.H. The Industrial and Artistic Technology of Paint Varnish; Wiley: New York, NY, USA, 1927. [Google Scholar]
- Doménech-Carbó, M.T.; Osete-Cortina, L.; De La Cruz Cañizares, J.; Bolívar-Galiano, F.; Romero-Noguera, J.; Fernández-Vivas, M.A.; Martín-Sánchez, I. Study of the Microbiodegradation of Terpenoid Resin-Based Varnishes from Easel Painting Using Pyrolysis-Gas Chromatography-Mass Spectrometry and Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2006, 385, 1265–1280. [Google Scholar] [CrossRef]
- Romero-Noguera, J.; Martín-Sánchez, I.; Doménech-Carbó, M.T.; Osete-Cortina, L.; López-Miras, M.M.; Bolívar-Galiano, F. Analytical Characterisation of the Biodeterioration of Diterpenoid Labdanic Varnishes Used in Pictorial Techniques: Sandarac and Manila Copal. Int. Biodeterior. Biodegrad. 2014, 90, 99–105. [Google Scholar] [CrossRef]
- Romero-Noguera, J.; Bailón-Moreno, R.; Bolívar-Galiano, F. Varnishes with Biocidal Activity: A New Approach to Protecting Artworks. Appl. Sci. 2020, 10, 7319. [Google Scholar] [CrossRef]
- Kavkler, K.; Humar, M.; Kržišnik, D.; Turk, M.; Tavzes, Č.; Gostinčar, C.; Džeroski, S.; Popov, S.; Penko, A.; Gunde-Cimerman, N.; et al. A Multidisciplinary Study of Biodeteriorated Celje Ceiling, a Tempera Painting on Canvas. Int. Biodeterior. Biodegrad. 2022, 170, 105389. [Google Scholar] [CrossRef]
- Bastholm, C.J.; Madsen, A.M.; Andersen, B.; Frisvad, J.C.; Richter, J. The Mysterious Mould Outbreak—A Comprehensive Fungal Colonisation in a Climate-Controlled Museum Repository Challenges the Environmental Guidelines for Heritage Collections. J. Cult. Herit. 2022, 55, 78–87. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Narváez-Zapata, J.; Reyes-Estebanez, M.; Quintana, P.; De la Rosa-García, S.D.C.; Bullen, H.; Gómez-Cornelio, S.; Chan-Bacab, M.J. Bioweathering Potential of Cultivable Fungi Associated with Semi-Arid Surface Microhabitats of Mayan Buildings. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Chovanová, K.; Šimonovičová, A.; Ferianc, P. Investigation of Microbial Community Isolated from Indoor Artworks and Air Environment: Identification, Biodegradative Abilities, and DNA Typing. Can. J. Microbiol. 2009, 55, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, P.; Kumar Singh, A.; Kumar, V.; Chintaman Sonawane, V.; Markandeya; Naresh Bharagava, R.; Raj, A. Decolourisation of Textile Dye by Laccase: Process Evaluation and Assessment of Its Degradation Bioproducts. Bioresour. Technol. 2021, 340, 125591. [Google Scholar] [CrossRef] [PubMed]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current Status on the Biodegradability of Acrylic Polymers: Microorganisms, Enzymes and Metabolic Pathways Involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef]
- Charlang, G.; Ng, B.; Horowitz, N.H.; Horowitz, R.M. Cellular and Extracellular Siderophores of Aspergillus Nidulans and Penicillium Chrysogenum. Mol. Cell. Biol. 1981, 1, 94–100. [Google Scholar] [CrossRef]
- Rapti, S.; Boyatzis, S.C.; Rivers, S.; Pournou, A. Siderophores and Their Applications in Wood, Textile, and Paper Conservation BT. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 301–339. ISBN 978-3-030-69411-1. [Google Scholar]
- Milagres, A.M.; Machuca, A.; Napoleão, D. Detection of Siderophore Production from Several Fungi and Bacteria by a Modification of Chrome Azurol S (CAS) Agar Plate Assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Galloway, L.D.; Burgess, R. Applied Mycology and Bacteriology, 3rd ed.; Leonard Hill: London, UK, 1952. [Google Scholar]
- Hocking, A.D.; Pitt, J.I. Dichloran-Glycerol Medium for Enumeration of Xerophilic Fungi from Low-Moisture Foods. Appl. Environ. Microbiol. 1980, 39, 488–492. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in Archives, Libraries, and Museums: A Review on Paper Conservation and Human Health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef]
- Bridge, R.R.M.P.; Bridge, P.D. Biochemical Techniques for Filamentous Fungi; Wallingford CAB International: Wallingford, UK, 1994. [Google Scholar]
- Brizzio, S.; Turchetti, B.; de García, V.; Libkind, D.; Buzzini, P.; van Broock, M. Extracellular Enzymatic Activities of Basidiomycetous Yeasts Isolated from Glacial and Subglacial Waters of Northwest Patagonia (Argentina). Can. J. Microbiol. 2007, 53, 519–525. [Google Scholar] [CrossRef]
- Lelliott, R.A.; Stead, D.E. Methods for the Diagnosis of Bacterial Diseases of Plants; Wiley–Blackwell: Oxford, UK, 1987; ISBN 978-0632012336. [Google Scholar]
- Cappuccino, J.G.; Sherman, N. Microbiology: A Laboratory Manual; Pearson Education Boston: Boston, MA, USA, 2014; ISBN 9780321864864. [Google Scholar]
- Lima, N.; Teixeira, J.A.; Mota, M. Deep Agar-Diffusion Test for Preliminary Screening of Lipolytic Activity of Fungi. J. Microbiol. Methods 1991, 14, 193–200. [Google Scholar] [CrossRef]
- Christensen, W.B. Urea Decomposition as a Means of Differentiating Proteus and Paracolon Cultures from Each Other and from Salmonella and Shigella Types. J. Bacteriol. 1946, 52, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C.; Narayani, T. Screening and Production of a Potential Extracellular Fungal Laccase from Penicillium Chrysogenum: Media Optimization by Response Surface Methodology (RSM)and Central Composite Rotatable Design (CCRD). Biotechnol. Reports 2019, 23, e00344. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, C.D.; Holtman, D.F.; Guse, D.G. Rapid Method for Determining the Activity of Microorganisms on Nucleic Acids. J. Bacteriol. 1957, 73, 590–591. [Google Scholar] [CrossRef]
- Borrego, S.; Guiamet, P.; Gómez de Saravia, S.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The Quality of Air at Archives and the Biodeterioration of Photographs. Int. Biodeterior. Biodegrad. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Albertano, P.; Urzı, C. Structural Interactions among Epilithic Cyanobacteria and Heterotrophic Microorganisms in Roman Hypogea. Microbiol. Ecol. 1999, 38, 244–252. [Google Scholar] [CrossRef]
- Satow, M.M.; Attili-Angelis, D.; de Hoog, G.S.; Angelis, D.F.; Vicente, V.A. Selective Factors Involved in Oil Flotation Isolation of Black Yeasts from the Environment. Stud. Mycol. 2008, 61, 157–163. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological Control of Postharvest Diseases of Fruits and Vegetables by Microbial Antagonists: A Review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Inkscape. The Inkscape Project; Inkscape: Boston, MA, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- McInnes, L.; Healy, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2023, arXiv:1802.03426. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 9783319242774. [Google Scholar]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and Nomenclature of the Genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Caselli, E.; Pancaldi, S.; Baldisserotto, C.; Petrucci, F.; Impallaria, A.; Volpe, L.; D’Accolti, M.; Soffritti, I.; Coccagna, M.; Sassu, G.; et al. Characterization of Biodegradation in a 17th Century Easel Painting and Potential for a Biological Approach. PLoS ONE 2018, 13, e0207630. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. The Ecology of Fungal Food Spoilage. In Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009. [Google Scholar]
- Paiva de Carvalho, H.; Mesquita, N.; Trovão, J.; Fernández Rodríguez, S.; Pinheiro, A.C.; Gomes, V.; Alcoforado, A.; Gil, F.; Portugal, A. Fungal Contamination of Paintings and Wooden Sculptures inside the Storage Room of a Museum: Are Current Norms and Reference Values Adequate? J. Cult. Herit. 2018, 34, 268–276. [Google Scholar] [CrossRef]
- Caneva, G. La Biología en la Restauración: Arte y Restauración; NEREA: San Sebastian, Spain, 2000. [Google Scholar]
- Camuffo, D. Microclimate for Cultural Heritage, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780080536538. [Google Scholar]
- Stevenson, A.; Hamill, P.G.; O’Kane, C.J.; Kminek, G.; Rummel, J.D.; Voytek, M.A.; Dijksterhuis, J.; Hallsworth, J.E. Aspergillus Penicillioides Differentiation and Cell Division at 0.585 Water Activity. Environ. Microbiol. 2017, 19, 687–697. [Google Scholar] [CrossRef]
- Segers, F.J.J.; Meijer, M.; Houbraken, J.; Samson, R.A.; Wösten, H.A.B.; Dijksterhuis, J. Xerotolerant Cladosporium Sphaerospermum Are Predominant on Indoor Surfaces Compared to Other Cladosporium Species. PLoS ONE 2015, 10, e0145415. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Fang, C.; Kumari, D.; Zhu, X.; Achal, V. Role of Fungal-Mediated Mineralization in Biocementation of Sand and Its Improved Compressive Strength. Int. Biodeterior. Biodegrad. 2018, 133, 216–220. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Liu, Y.; Feng, W. Biodegradation of Hydrocarbons by Purpureocillium Lilacinum and Penicillium Chrysogenum from Heavy Oil Sludge and Their Potential for Bioremediation of Contaminated Soils. Int. Biodeterior. Biodegrad. 2023, 178, 105566. [Google Scholar] [CrossRef]
- Dhar, K.; Dutta, S.; Anwar, M.N. Biodegradation of Petroleum Hydrocarbon by Indigenous Fungi Isolated from Ship Breaking Yards of Bangladesh. Int. Res. J. Biol. Sci. 2014, 3, 22–30. [Google Scholar]
- Federici, F. Extracellular Enzymatic Activities in Aureobasidium pullulans. Mycologia 1982, 74, 738–743. [Google Scholar] [CrossRef]
- Schoeman, M.W.; Dickinson, D.J. Aureobasidium Pullulans Can Utilize Simple Aromatic Compounds as a Sole Source of Carbon in Liquid Culture. Lett. Appl. Microbiol. 1996, 22, 129–131. [Google Scholar] [CrossRef]
- Kanegae, H.; Tomino, N.; Nakamura, Y.; Minakawa, T.; Yaguchi, T.; Izawa, T.; Sano, A.; Itano, E.N.; Ueda, K. Parengyodontium Album Isolated from Cutaneous Lesions of a Pacific White-Sided Dolphin (Lagenorhynchus Obliquidens) During Treatment for Paracoccidioidomycosis Ceti. Mycopathologia 2020, 185, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- González-Abradelo, D.; Pérez-Llano, Y.; Peidro-Guzmán, H.; Sánchez-Carbente, M.D.R.; Folch-Mallol, J.L.; Aranda, E.; Vaidyanathan, V.K.; Cabana, H.; Gunde-Cimerman, N.; Batista-García, R.A. First Demonstration That Ascomycetous Halophilic Fungi (Aspergillus Sydowii and Aspergillus Destruens) Are Useful in Xenobiotic Mycoremediation under High Salinity Conditions. Bioresour. Technol. 2019, 279, 287–296. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation: Organic Consolidants, Adhesives, and Coatings; Butterworths London: London, UK, 1987; Volume 11, p. 281. ISBN 0408015314. [Google Scholar]
- Vieto, S.; Escudero-Leyva, E.; Avendaño, R.; Rechnitzer, N.; Barrantes-Madrigal, M.D.; Conejo-Barboza, G.; Herrera-Sancho, O.A.; Chaverri, P.; Chavarría, M. Biodeterioration and Cellulolytic Activity by Fungi Isolated from a Nineteenth-Century Painting at the National Theatre of Costa Rica. Fungal Biol. 2021, 126, 101–112. [Google Scholar] [CrossRef]
- Šoltys, K.; Planý, M.; Biocca, P.; Vianello, V.; Bučková, M.; Puškárová, A.; Sclocchi, M.C.; Colaizzi, P.; Bicchieri, M.; Pangallo, D.; et al. Lead Soaps Formation and Biodiversity in a XVIII Century Wax Seal Coloured with Minium. Environ. Microbiol. 2020, 22, 1517–1534. [Google Scholar] [CrossRef]
- Szulc, J.; Jablonskaja, I.; Jabłońska, E.; Ruman, T.; Karbowska-Berent, J.; Gutarowska, B. Metabolomics and Metagenomics Characteristic of Historic Beeswax Seals. Int. Biodeterior. Biodegrad. 2020, 152, 105012. [Google Scholar] [CrossRef]
- Pavlović, J.; Sclocchi, M.C.; Planý, M.; Ruggiero, D.; Puškárová, A.; Bučková, M.; Šoltys, K.; Colaizzi, P.; Riccardi, M.L.; Pangallo, D.; et al. The Microbiome of Candle Beeswax Drops on Ancient Manuscripts. Int. Biodeterior. Biodegrad. 2022, 174, 105482. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective Properties and Durability Characteristics of Experimental and Commercial Organic Coatings for the Preservation of Porous Stone. Prog. Org. Coatings 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Sabatini, L.; Sisti, M.; Campana, R. Evaluation of Fungal Community Involved in the Bioderioration Process of Wooden Artworks and Canvases in Montefeltro Area (Marche, Italy). Microbiol. Res. 2018, 207, 203–210. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The Preservation Damage of Hydrophobic Polymer Coating Materials in Conservation of Stone Relics. Prog. Org. Coatings 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Zander, Z.; Newton, D.; Scaglione, H.; Reiber, A.; Agarwal, P. Microbial Susceptibility of Various Polymers and Evaluation of Thermoplastic Elastomers with Antimicrobial Additives. Polym. Eng. Sci. 2021, 61, 3029–3036. [Google Scholar] [CrossRef]
- Cappitelli, F.; Vicini, S.; Piaggio, P.; Abbruscato, P.; Princi, E.; Casadevall, A.; Nosanchuk, J.D.; Zanardini, E. Investigation of Fungal Deterioration of Synthetic Paint Binders Using Vibrational Spectroscopic Techniques. Macromol. Biosci. 2005, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Nosanchuk, J.D.; Casadevall, A.; Toniolo, L.; Brusetti, L.; Florio, S.; Principi, P.; Borin, S.; Sorlini, C. Synthetic Consolidants Attacked by Melanin-Producing Fungi: Case Study of the Biodeterioration of Milan (Italy) Cathedral Marble Treated with Acrylics. Appl. Environ. Microbiol. 2007, 73, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D.; Salvadori, O. Biological Growth on Italian Monuments Restored with Organic or Carbonatic Compounds. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Ciferri, O., Mastromei, G., Tiano, P., Eds.; Plenum Publishers: New York, NY, USA, 1999; pp. 149–154. [Google Scholar]
- Kigawa, R. Evaluation of Mould Resistance of Various Synthetic Resins Used in Conservation of Historic Sites. Sci. Conserv. Hozon Kagaku 2005, 44, 149–156. [Google Scholar]
- Campana, R.; Sabatini, L.; Giorgi, L.; Pettinari, G.; Valentini, L.; Gobbi, P. A Multidisciplinary Approach in Examining the Susceptibility to Microbial Attack of Polyacrylic and Polyurethane Resins Used in Art Restoration. Int. J. Mol. Sci. 2022, 23, 11725. [Google Scholar] [PubMed]
- Scott, G. Polymer and the Environment; RSC Paperbacks: Cambridge, MA, USA, 1999. [Google Scholar]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- do Canto, V.P.; Thompson, C.E.; Netz, P.A. Polyurethanases: Three-Dimensional Structures and Molecular Dynamics Simulations of Enzymes That Degrade Polyurethane. J. Mol. Graph. Model. 2019, 89, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Principi, P.; Pedrazzani, R.; Toniolo, L.; Sorlini, C. Bacterial and Fungal Deterioration of the Milan Cathedral Marble Treated with Protective Synthetic Resins. Sci. Total Environ. 2007, 385, 172–181. [Google Scholar] [CrossRef]
- Abdel-Kareem, O. Microbiological Testing to Assess the Susceptibility of Museum Textiles Conserved with Polymers to Fungal Deterioration. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center S.L.: Badajoz, Spain, 2016. [Google Scholar]
- Crabbe, J.R.; Campbell, J.R.; Thompson, L.; Walz, S.L.; Schultz, W.W. Biodegradation of a Colloidal Ester-Based Polyurethane by Soil Fungi. Int. Biodeterior. Biodegrad. 1994, 33, 103–113. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of Plastic Polymers by Fungi: A Brief Review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Potin, O.; Veignie, E.; Rafin, C. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Cladosporium Sphaerospermum Isolated from an Aged PAH Contaminated Soil. FEMS Microbiol. Ecol. 2004, 51, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.D.A.; Santos, D.; Alvarenga, N.; Garcia, A.C.F.S.; Romão, L.P.C.; Porto, A.L.M. Biodegradation of Anthracene and Several PAHs by the Marine-Derived Fungus Cladosporium Sp. CBMAI 1237. Mar. Pollut. Bull. 2018, 129, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Peidro-Guzmán, H.; Pérez-Llano, Y.; González-Abradelo, D.; Fernández-López, M.G.; Dávila-Ramos, S.; Aranda, E.; Hernández, D.R.O.; García, A.O.; Lira-Ruan, V.; Pliego, O.R.; et al. Transcriptomic Analysis of Polyaromatic Hydrocarbon Degradation by the Halophilic Fungus Aspergillus Sydowii at Hypersaline Conditions. Environ. Microbiol. 2021, 23, 3435–3459. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Trovão, J.; Mesquita, N.; Paiva, D.S.; Paiva de Carvalho, H.; Avelar, L.; Portugal, A. Can Arthropods Act as Vectors of Fungal Dispersion in Heritage Collections? A Case Study on the Archive of the University of Coimbra, Portugal. Int. Biodeterior. Biodegrad. 2013, 79, 49–55. [Google Scholar] [CrossRef]
- Park, H.-J.; Wang, W.; Curlango-Rivera, G.; Xiong, Z.; Lin, Z.; Huskey, D.A.; Hawes, M.C.; VanEtten, H.D.; Turgeon, B.G. A DNase from a Fungal Phytopathogen Is a Virulence Factor Likely Deployed as Counter Defense against Host-Secreted Extracellular DNA. mBio 2019, 10, 02805-18. [Google Scholar] [CrossRef]
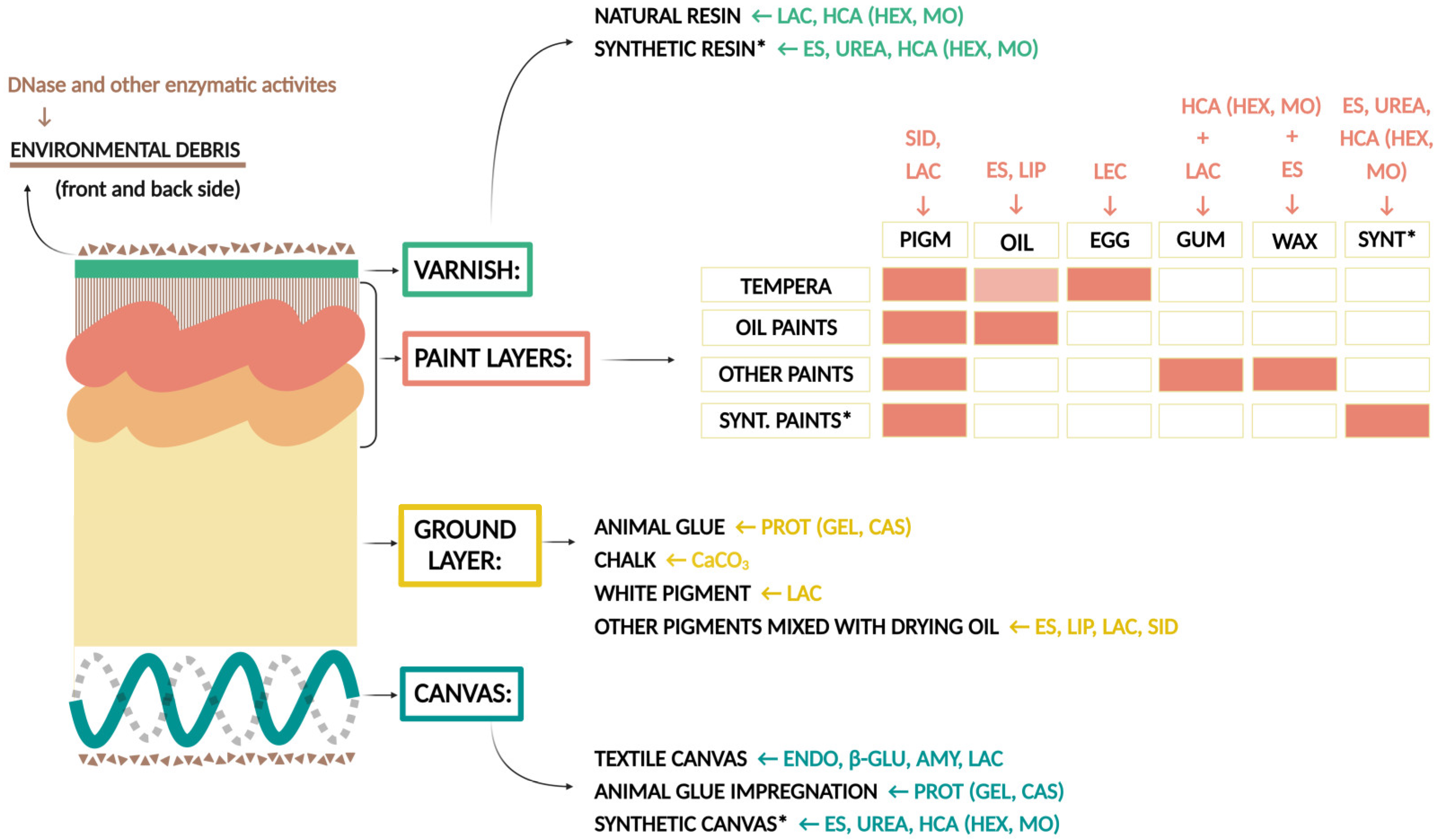
| Incubation Conditions | |||||
|---|---|---|---|---|---|
| Enzymatic Activity | Substrate (Producer) | Temperature [°C] | Time [days] | Positive Reaction | Reference |
| amylase | soluble starch (Sigma-Aldrich) | 24 | 14 | a clear zone around colonies in black/blue stained medium after flooding with iodine solution | [49] |
| β-glucosidase | aesculin (6,7-dihydroxycoumarin 6-glucoside) (Merck) | 24 | 14 | a black zone around colonies in light-yellow medium | [49] |
| endocellulase | carboxymethyl cellulose (Sigma-Aldrich) | 24 | 21 | a yellow clear zone around colonies in red stained medium after flooding with Congo red and rinsing with NaCl solution | [49] |
| gelatinase | gelatine (Sigma-Aldrich) | 24 | 14 | liquefied medium around the colonies in yellow transparent medium | [49] |
| caseinase | skim milk (Merck) | 24 | 14 | a clear zone around colonies in opaque medium | [50] |
| esterase | Tween-80 (Sigma-Aldrich) | 24 | 14 | a purple/blue zone around colonies in yellow medium due to colour change in the indicator; precipitation of calcium salts | [51] |
| lecithinase | egg yolk | 24 | 28 | a white opaque zone around the colonies in yellow medium | [52] |
| lipase | tributyrin (Sigma-Aldrich) | 24 | 28 | a clear zone around the colonies in opaque medium | [53] |
| urease | urea (Merck) | 24 | 14 | change in colour of the medium from yellow to purple | [54] |
| laccase | ABTS (Sigma-Aldrich) | 24 | 14 | a purple halo around the colonies in light yellow transparent medium | [55] |
| laccase | guaiacol (Sigma-Aldrich) | 24 | 14 | an orange/brown halo around the colonies in light yellow transparent medium | [55] |
| DNase | DNase agar (Beckton and Dickinson) | 24 | 7 | a transparent halo around colonies in opaque white medium after flooding with 1 M HCl | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujović, A.; Gostinčar, C.; Kavkler, K.; Govedić, N.; Gunde-Cimerman, N.; Zalar, P. Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings. J. Fungi 2024, 10, 76. https://doi.org/10.3390/jof10010076
Kujović A, Gostinčar C, Kavkler K, Govedić N, Gunde-Cimerman N, Zalar P. Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings. Journal of Fungi. 2024; 10(1):76. https://doi.org/10.3390/jof10010076
Chicago/Turabian StyleKujović, Amela, Cene Gostinčar, Katja Kavkler, Natalija Govedić, Nina Gunde-Cimerman, and Polona Zalar. 2024. "Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings" Journal of Fungi 10, no. 1: 76. https://doi.org/10.3390/jof10010076
APA StyleKujović, A., Gostinčar, C., Kavkler, K., Govedić, N., Gunde-Cimerman, N., & Zalar, P. (2024). Degradation Potential of Xerophilic and Xerotolerant Fungi Contaminating Historic Canvas Paintings. Journal of Fungi, 10(1), 76. https://doi.org/10.3390/jof10010076









